Abstract
Integrin α1β1, the major collagen type IV receptor, is expressed by endothelial cells and plays a role in both physiologic and pathologic angiogenesis. Because the molecular mechanisms whereby this collagen IV receptor mediates endothelial cell functions are poorly understood, truncation and point mutants of the integrin α1 subunit cytoplasmic tail (amino acids 1137-1151) were generated and expressed into α1-null endothelial cells. We show that α1-null endothelial cells expressing the α1 subunit, which lacks the entire cytoplasmic tail (mutant α1-1136) or expresses all the amino acids up to the highly conserved GFFKR motif (mutant α1-1143), have a similar phenotype to parental α1-null cells. Pro1144 and Leu1145 were shown to be necessary for α1β1-mediated endothelial cell proliferation; Lys1146 for adhesion, migration, and tubulogenesis and Lys1147 for tubulogenesis. Integrin α1β1–dependent endothelial cell proliferation is primarily mediated by ERK activation, whereas migration and tubulogenesis require both p38 MAPK and PI3K/Akt activation. Thus, distinct amino acids distal to the GFFKR motif of the α1 integrin cytoplasmic tail mediate activation of selective downstream signaling pathways and specific endothelial cell functions.
Introduction
Angiogenesis, the formation of new blood vessels from preexisting vessels, is required for both physiologic and pathologic events, including embryonic development, wound healing, and tumor growth.1,2 Angiogenesis is a multistep process that requires endothelial cell proliferation, migration, adhesion to the vessel basement membrane, and formation of cell-cell junctions. Cell-matrix interactions, which are required for most of these cellular processes, are primarily mediated by integrins, transmembrane receptors for extracellular matrix components.3
Several integrin family members, including αvβ3, αvβ5, α5β1, α1β1, and α2β1, are expressed on endothelial cells and play a role in angiogenesis.4-10 The best studied are the RGD binding αv and α5β1 integrins, and their role in angiogenesis is controversial, as they can be both proangiogenic and antiangiogenic.4,6,11,12 The function of the 2 major collagen binding receptors, integrins α1β1 and α2β1, in the control of endothelial cell functions is less clearly defined. Integrin α1β1 is thought to be proangiogenic as functional blocking antibodies or targeted deletion of the α1 subunit results in a decrease of both vascular endothelial growth factor-mediated and tumor-associated angiogenesis.7,8,13-15 In contrast, integrin α2β1 is proposed to be antiangiogenic, as deletion of the α2 subunit results in increased wound- and tumor-associated vasculature.9,16
The mechanism whereby integrin α1β1 promotes increased angiogenesis is poorly understood. We previously showed that integrin α1–null mice have smaller and less vascularized tumors than their wild-type counterparts. This effect is due in part to increased levels of circulating matrix metalloproteinase-9 in the α1-null mice, which results in increased generation of angiostatin, a potent inhibitor of endothelial cell proliferation, from circulating plasminogen.7,8,14,15 In addition, integrin α1β1 is known to promote cell survival and proliferation on collagenous substrata (key components of the vascular basement membrane) via activation of the Shc/Grb2/ERK pathway,17 suggesting that impaired integrin α1–dependent intracellular signaling in endothelial cells may also contribute to the abnormalities found in integrin α1–null mice.
The extracellular domain of the α integrin subunits is responsible for the specificity of ligand binding, whereas the cytoplasmic and transmembrane domains are important in regulating integrin activation and signaling.18-22 The activation state of integrins is thought to be dependent on interactions between the α and β integrin tails. The highly conserved GFFKR motif in the α tails is proposed to form a salt bridge with a highly conserved sequence HDRRE in the juxtamembrane region of the β tail. This interaction holds the integrin in the inactive state characterized by low ligand-binding affinity.23-27 On binding of intracellular proteins, such as talin or kindlins, to the β tail the juxtamembrane αβ tail interaction is thought to be disrupted, resulting in integrin activation and increased ligand-binding affinity.26,28-33 This mechanism has been primarily studied for the highly modulatable β2 and β3 integrins, where membrane proximal deletions of the GFFKR motif results in a constitutively activated integrin.23,34,35 The role of the GFFKR motif in modulating the activation state of β1-containing integrin is poorly defined.
The cytoplasmic tail is also critical in mediating “outside-in” integrin signaling by interacting with adaptor, cytoskeletal, and signaling molecules.36-39 The importance of the α1 cytoplasmic tail in α1β1 integrin function is demonstrated by the requirement of this domain for cell spreading as well as focal adhesion and stress fiber formation.40,41 The α1 tail has also been shown to interact either directly or indirectly with several signaling molecules, including Shc, T-cell protein tyrosine phosphatase, phospholipase Cγ, FAK, and PRL-3.37,38,40-42 However, little is known about the specific region(s) within the 15 amino acids of the α1 subunit cytoplasmic tail that control cellular function and signaling.
To define which domains of the α1 cytoplasmic tail are required to mediate integrin α1β1–dependent endothelial cell function, truncation and point mutants within the tail were generated and expressed into integrin α1–null endothelial cells. Using these cells, we show that parental α1-null cells, α1-null cells expressing mutants lacking the entire cytoplasmic tail (mutant α1-1136), or expressing all the amino acids up to the GFFKR motif (mutant α1-1143) have a similar phenotype characterized by reduced adhesion, migration, proliferation, and tubulogenic potential on collagen substrata. In addition, we identify the specific residues distal to the GFFKR motif that control different integrin α1β1–dependent cell functions and activation of selective downstream signaling pathways.
Methods
Generation of mutant integrin α1 subunits
Full-length human integrin α1 cDNA in the pLEN vector was a generous gift from Dr E. Marcantonio (Columbia University, New York, NY). The α1 subunit truncation mutants were generated by polymerase chain reaction, using the mature full-length α1 as a template.43
A common sense primer 5′-CGGTACCACCATGTTCAATGTTGATGTGAAAAACTC-3′, containing a Kpn I restriction site and an ATG start codon, was used for all the truncation constructs. The antisense primers containing a Kpn I restriction site and stop codon were as follows: 5′-TAATGGTACCTCATCATTTCTCCATTTTCTTTTTCAG-3′ (full-length α1); 5′-TAATGGTACCTCATCACTCCTCCATTTTCTTTTTCAG-3′ (α1-K1151E); 5′-TAATGGTACCTCATCACTCCATTTTCTTTTTCAGTGGTC-3′ (mutant α1–1150); 5′-TAATGGTACCTCATCATTTCATTTTCTTTTTCAGTGGTC-3′ (mutant α1-E1150K), 5′-TAATGGTACCTCATCACATTTTCTTTTTCAGTGGTCTTTTG-3′ (mutant α1-1149); 5′-TAATGGTACCTCATCATTTCTT-TTTCAGTGGTCTTTTG-3′ (mutant α1-1148), 5′-TAATGGTACCTCAT-CACAGTGGTCTTTTGAAGAATCC-3′ (mutant α1-1145); 5′-TAATGG-TACCTCATCATCTTTTGAAGAATCCAATCTTCC-3′ (mutant α1-1143); 5′-TAATGGTACCTCATCACCACAGTGCTAAAATGAGCAG-3′ (mutant α1-1136).
The single point mutants K1146A, K1147A, and K1148A were generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) with appropriate primers. All mutants were checked by sequencing to verify they were correct. The various constructs were subcloned into the LZRS-GFP vector, modified from the original LZRS vector,44 to allow bicistronic expression of the protein of interest and GFP (a generous gift from Dr A. Reynolds).45 These constructs were transfected into the packaging cell line Phoenix 293 using FuGENE 6 transfection reagent according to the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN).
Cell culture and generation of cell populations
Integrin α1–null pulmonary microvascular endothelial cells were isolated from integrin α1–null mice crossed with the immorto-mouse background, as described.7 Cells were propagated at 33°C in microvascular endothelial-cell medium-2 (EGM-2 MV) containing 5% fetal calf serum (Lonza Walkersville, Walkersville, MD) in the presence of 100 IU/mL of γ-interferon. For experiments, cells were cultured at 37°C in EGM-2 MV medium without γ-interferon for at least 4 days before use as this is the optimal time for the immorto endothelial cells to acquire a phenotype similar to freshly isolated primary endothelial cells.
For the generation of α1-null endothelial cells expressing the various integrin α1 mutants, cells were infected for 2 weeks with virus contained in the medium of transfected Phoenix 293 cells.
In some experiments, Chinese Hamster Ovary (CHO) cells were stably transfected with PCDNA3.1 or full-length integrin α1, α1-E1150K, and α1-K1151E subcloned into PCDNA3.1. Cells were transfected using Lipofectamine and Plus reagent (Invitrogen, Carlsbad, CA) followed by zeocin selection (Sigma-Aldrich, St Louis, MO) according to the manufacturer's instructions. Stable cell populations of α1-null endothelial cells or CHO cells expressing equal levels of the various mutants were selected by flow cytometry using antibodies recognizing the extracellular I domain of human integrin α1 (MAB1973; Chemicon International, Temecula, CA). Polyclonal cell populations were used to avoid some of the pitfalls associated with isolation of monoclonal cell lines.
Cell morphology
Cells were plated in serum-free medium on chamber slides coated with 10 μg/mL fibronectin (a non–integrin α1β1 ligand, used as positive control) or collagen IV (a specific integrin α1β1 ligand; both from Sigma-Aldrich). After 4 hours, the cells that remained adherent were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with rhodamine-phalloidin (Invitrogen). Cells were subsequently washed with phosphate-buffered saline (PBS) and examined under a fluorescence microscope (Nikon, Tokyo, Japan). Three independent experiments were performed.
Cell adhesion
The cell adhesion assay was performed as previously described.46 Briefly, 96-well plates were coated with fibronectin (10 μg/mL) or collagen IV (at various concentrations) for 1 hour at 37°C. After blocking nonspecific adhesion with 1% bovine serum albumin in PBS, 5 × 104 cells in 100 μL serum-free medium were added to the plates and incubated for 1 hour at 37°C. In some experiments, cells were preincubated with 10 μM PD169316 (a p38 MAPK inhibitor), 10 μM PD98059 (a MEK inhibitor), or 5 μM LY294002 (a PI3K inhibitor; all from Calbiochem) for 30 minutes before the assay. After removing nonadherent cells, the cells were fixed with 4% formaldehyde, stained with 1% crystal violet, solubilized in 20% acetic acid, and the absorbance read at 595 nm. Cell adhesion to 1% bovine serum albumin–coated wells was subtracted from the values obtained on ECM proteins. Four independent experiments were performed in quadruplicates.
Cell migration
Cell migration was assayed in transwells consisting of polyvinylpyrolidone-free polycarbonate filters with 8-μm pores (Costar; Corning, Cambridge, MA) as previously described.46 The bottoms of the filters were coated with collagen IV or fibronectin (10 μg/mL in PBS), and 105 cells were used for each assay. In some experiments, 5 μM LY294002, 10 μM PD98059, or 10 μM PD169316 were added to both top and bottom wells. Cells were allowed to migrate for either 6 (CHO cells) or 16 (endothelial cells) hours at 37°C, after which they were fixed in 4% formaldehyde, stained with 1% crystal violet, and 5 randomly chosen fields counted at 200× magnification. Four independent experiments were performed in duplicates.
Tubulogenesis
Capillary-like formation on collagen and fibrin gels was analyzed as described.46 For collagen gels, 50 μL gel composed of 1 mg/mL rat tail collagen I (BD Biosciences, San Jose, CA), 30 μg/mL collagen IV, and Dulbecco minimal essential media containing 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.2) were allowed to polymerize in 96-well plates. For fibrin gels, fibrinogen (2.5 mg/mL) was dissolved in PBS and clotting was started by the addition of thrombin (0.250 U/mL); 50 μL of the mixture was added to 96-well plates and allowed to polymerize for 10 minutes at 37°C. Endothelial cells (1.5 × 104 in 150 μL serum-free medium) were then plated on solidified gels in the presence or absence of the kinase inhibitors indicated in “Cell adhesion.” After 12 hours, the cells were fixed with 4% formaldehyde in PBS, and phase-contrast photomicrographs were taken using a Nikon inverted microscope. To quantify capillary-linked network formation, cellular nodes were defined as junctions linking at least 3 cells, and they were counted from digital images. Four independent experiments were performed with a total of 20 images analyzed per cell population.
Cell proliferation
Endothelial cells (5 × 103/well) or CHO cells (103/well) were plated in low serum (1% fetal calf serum) onto 96-well plates coated with 10 μg/mL collagen IV or 10 μg/mL fibronectin. Under these plating conditions, all cell populations, independent of the genotype or construct expressed, adhere and spread on both matrices. After 4 hours, the cells were gently washed and incubated with serum-free medium (thus allowing integrin-mediated signaling to control cell proliferation) with or without 5 μM LY294002, 10 μM PD98059, or 10 μM PD169316 in the presence of [3H]thymidine (0.5 μCi/well). After 48 hours, the cells were collected and the amount of incorporated [3H]thymidine analyzed as previously described.7 Four independent experiments were performed in quadruplicates.
Western blot analysis
To determine the activation of intracellular downstream signaling, serum-starved endothelial cells were embedded for different amount of times in 30 μL collagen I + IV gels, prepared as described in “Tubulogenesis” (3.5 × 104 cells/gel); or plated for different amount of times onto dishes coated with 10 μg/mL fibronectin (5 × 105 cells/10-cm dishes). In some experiments, human anti–integrin α1 or mouse anti–integrin α2 antibodies were used at the final concentration of 30 μg/mL. For cells in the gels, equal volumes of Laemmli buffer containing β-mercaptoethanol were then added to the gels. Samples were then sonicated, boiled, and run onto a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and subsequently transferred to nitrocellulose membranes. For cells on fibronectin, adherent cells were scraped and lysed, and equal amounts of total cell lysates (20 μg/lane) were run onto a 10% SDS-PAGE. Membranes were incubated with antiphospho-ERK, antiphospho-p38 MAPK, antiphospho-Akt, anti-ERK, anti-p38 MAPK, or anti-Akt antibodies (all from Cell Signaling Technology, Danvers, MA) followed by the appropriate horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were identified using enhanced chemiluminescence according to the manufacturer's instructions. Four independent experiments were performed. Phosphorylated and total ERK, p38 MAPK, and Akt bands were quantified by densitometry analysis, and the levels of phosphorylated signal were expressed as phosphorylated kinase/total kinase ratio. Values were expressed as the mean plus or minus SD of 3 experiments.
Immunoprecipitation assay
The different endothelial cell populations were lysed in a lysis buffer consisting of 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 200 mM NaCl, 5% glycerol, 1% NP-40, 0.05% Tween-20, 1 mM NaV, 1 mM NaF, and 1× proteinase inhibitor. The lysates were centrifuged at 12 000g for 10 minutes, and the supernatants were used for immunoprecipitation; 1 mg total cell lysates were precleaned with 20 μL packed protein-G Sepharose beads (GE Healthcare, Little Chalfont, United Kingdom) and subsequently incubated with 5 μg anti–human integrin α1 antibodies (TS 2/7; Santa Cruz Biotechnology, Santa Cruz, CA) and 30 μL packed protein-G Sepharose and incubated overnight at 4°C. Beads were washed 3 times with lysis buffer, and bound proteins were eluted by boiling the beads in SDS-PAGE sample buffer under reducing conditions. Samples were separated onto a 10% SDS gel and subsequently transferred to nitrocellulose membranes. Membranes were then cut at the level of the 150-kDa marker. The upper part of the membranes was incubated with an antihuman integrin α1 antibody (MAB1973; Chemicon International), whereas the lower part of the membranes was incubated with an antimouse integrin β1 antibody (Chemicon International) followed by the appropriate horseradish peroxidase-conjugated secondary antibodies; 40 μg total cell lysates was analyzed for ERK levels to verify loading.
Statistical analysis
The Student t test was used for comparisons between 2 groups, and analysis of variance using Sigma-Stat software for statistical difference between multiple groups. A value of P less than .05 was considered statistically significant.
Results
Specific amino acids of the integrin α1 tail mediate endothelial cell spreading, adhesion, migration, proliferation, and tubulogenesis
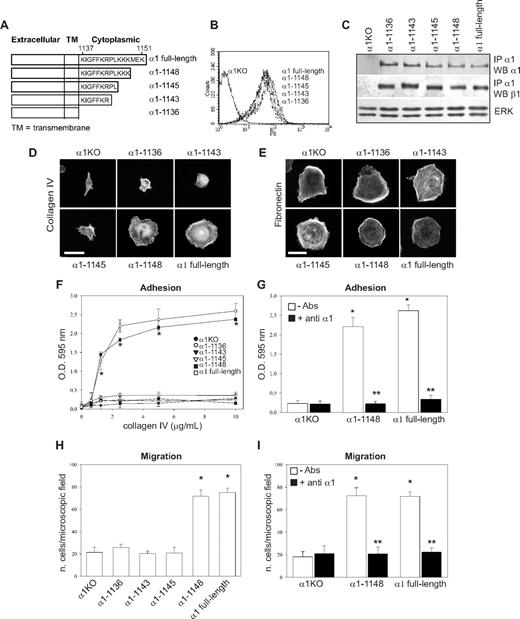
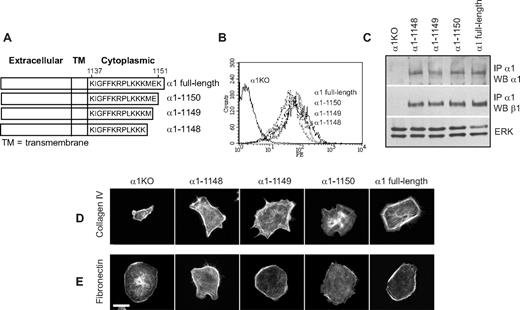
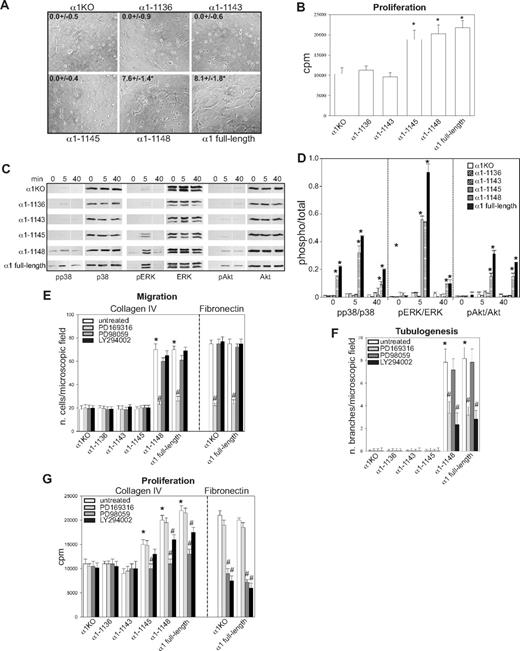
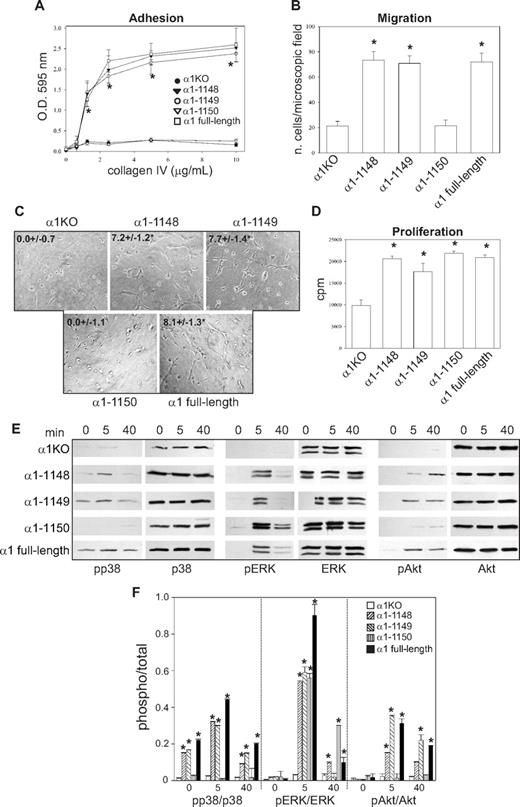
To identify the amino acids in the α1 integrin subunit cytoplasmic domain that regulate endothelial cell proliferation, migration, and tubulogenesis, truncation mutants of the human integrin α1 cDNA were generated (Figure 1A). Full-length or mutated human α1 integrin subunits were expressed into mouse α1-null (α1KO) endothelial cells, and cell populations expressing similar levels of this subunit were isolated by fluorescence-activated cell sorter (FACS; Figure 1B). Formation of chimeric dimers between the human integrin α1 and the mouse β1 subunits was confirmed by immunoprecipitation assays (Figure 1C). Thus, the human integrin α1 subunit forms a complex with the mouse β1 subunit, as previously suggested for other human α subunits expressed in mouse cells.46-48
Amino acids 1146-1148 of the integrin α1 tail control cell spreading, adhesion, and migration. (A) Schematic representation of the cytoplasmic truncation mutants of the human integrin α1 cDNA. (B) α1-null endothelial cells were transduced with either empty vector (α1KO) or the human integrin α1 mutant cDNAs indicated in panel A, and cell populations with equal levels of expression of the integrin α1 subunits were sorted by FACS using anti–human integrin α1 antibodies. (C) One millligram of total cell lysates of the cell populations indicated was immunoprecipitated with anti–human integrin α1 antibodies, subjected to SDS-PAGE, and immunoblotted with antibodies to either human integrin α1 or mouse β1 subunits. Equal loading was confirmed by analyzing the levels of total ERK in 40 μg total cell lysates. (D,E) Integrin α1KO endothelial cells expressing either the empty vector or cytoplasmic tail deletion mutants were plated in serum-free medium on 10 μg/mL collagen IV (D) or fibronectin (E). After 4 hours, the cells were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm. (F,G) The cell populations were plated in serum-free medium on collagen IV at the concentrations indicated (F) or 10 μg/mL collagen IV with or without anti–human integrin α1 antibodies (10 μg/mL) (G) for 1 hour and their adhesion determined as described in “Cell adhesion.” Values are mean plus or minus SD of one representative experiment performed in quadruplicate. (H,I) The cell populations were plated on serum-free medium transwells coated with 10 μg/mL collagen IV with (▬) or without (▭) anti–human integrin α1 antibodies (10 μg/mL), and migration was evaluated 16 hours after plating. Values are mean plus or minus SD of one representative experiment performed in duplicate (5 fields/transwell were analyzed). Differences between α1KO and α1 mutant–expressing cells (*) and antibody-untreated versus-treated α1 mutant–expressing cells (**) were significant with P < .05.
Amino acids 1146-1148 of the integrin α1 tail control cell spreading, adhesion, and migration. (A) Schematic representation of the cytoplasmic truncation mutants of the human integrin α1 cDNA. (B) α1-null endothelial cells were transduced with either empty vector (α1KO) or the human integrin α1 mutant cDNAs indicated in panel A, and cell populations with equal levels of expression of the integrin α1 subunits were sorted by FACS using anti–human integrin α1 antibodies. (C) One millligram of total cell lysates of the cell populations indicated was immunoprecipitated with anti–human integrin α1 antibodies, subjected to SDS-PAGE, and immunoblotted with antibodies to either human integrin α1 or mouse β1 subunits. Equal loading was confirmed by analyzing the levels of total ERK in 40 μg total cell lysates. (D,E) Integrin α1KO endothelial cells expressing either the empty vector or cytoplasmic tail deletion mutants were plated in serum-free medium on 10 μg/mL collagen IV (D) or fibronectin (E). After 4 hours, the cells were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm. (F,G) The cell populations were plated in serum-free medium on collagen IV at the concentrations indicated (F) or 10 μg/mL collagen IV with or without anti–human integrin α1 antibodies (10 μg/mL) (G) for 1 hour and their adhesion determined as described in “Cell adhesion.” Values are mean plus or minus SD of one representative experiment performed in quadruplicate. (H,I) The cell populations were plated on serum-free medium transwells coated with 10 μg/mL collagen IV with (▬) or without (▭) anti–human integrin α1 antibodies (10 μg/mL), and migration was evaluated 16 hours after plating. Values are mean plus or minus SD of one representative experiment performed in duplicate (5 fields/transwell were analyzed). Differences between α1KO and α1 mutant–expressing cells (*) and antibody-untreated versus-treated α1 mutant–expressing cells (**) were significant with P < .05.
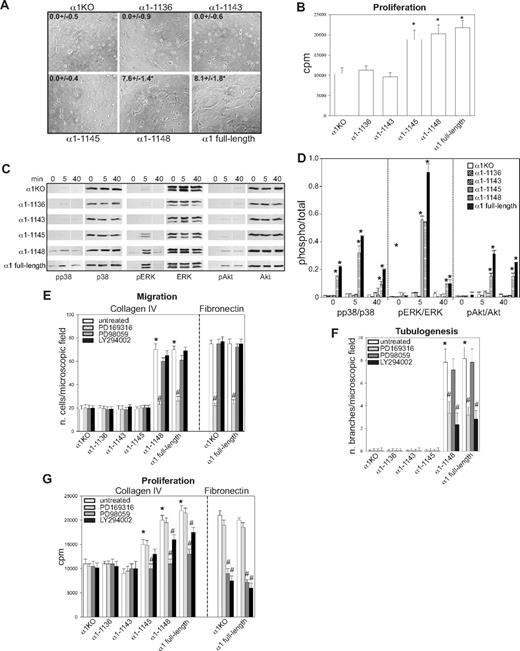
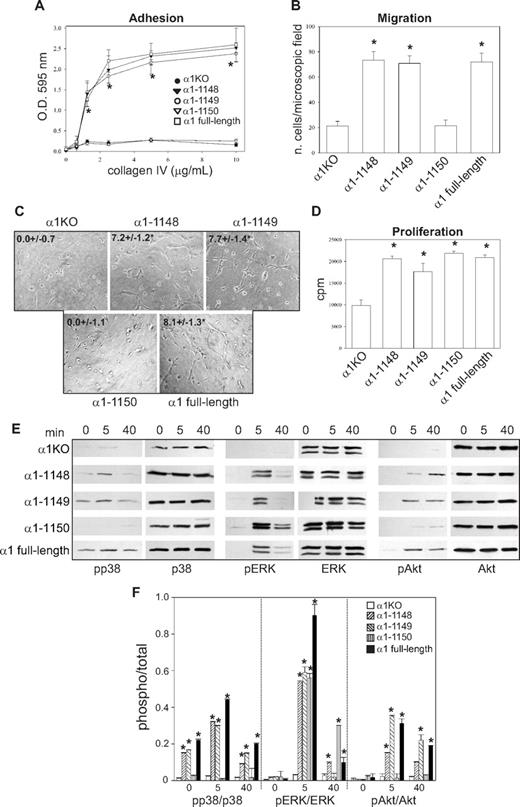
To investigate functionality of the mutants, cells were plated in serum-free medium on either collagen IV (the major integrin α1β1 binding ligand) or fibronectin (an integrin α1β1–independent ligand) and their morphology evaluated after 4 hours. As previously described for fibroblasts,49 approximately 5% to 10% of the α1KO endothelial cells adhered to the collagen IV under these conditions but failed to spread (Figure 1D). This phenotype was reversed by expression of the full-length α1 subunit (Figure 1D). Like the α1KO cells, approximately 5% to 10% of the of the endothelial cells expressing the α1-1136, α1-1143, or α1-1145 mutants adhered to collagen IV but failed to spread (Figure 1D). In contrast, endothelial cells transfected with the α1-1148 mutant spread to a similar extent as cells reconstituted with full-length α1 (Figure 1D). No differences in spreading among the different mutants were observed on fibronectin (Figure 1E), confirming that the phenotypes observed were the result of alterations in integrin α1β1 interactions with collagen IV. Similar to cell spreading, only cells transfected with either full-length α1 or the α1-1148 mutants adhered in a dose-dependent manner and migrated on collagen IV (Figure 1F,H). Both cell adhesion and migration on collagen IV were inhibited by antihuman integrin α1 antibodies, proving these cell functions were integrin α1β1 dependent (Figure 1G,I). Finally, only cells expressing either full-length α1 or the α1-1148 mutants underwent tubulogenesis on collagen I + IV gels (Figure 2A), and this event was inhibited by antihuman integrin α1 antibodies (not shown). Interestingly, both the α1-1145– and α1-1148–expressing cells proliferated on collagen IV to the same degree as cells reconstituted with full-length α1 (Figure 2B). Thus, Pro1144 and Leu1145 within the α1 cytoplasmic tail are required for proliferation, whereas the triple lysine motif (amino acids 1146-1148) is required for α1β1-dependent cell spreading, adhesion, migration, and tubulogenesis on collagen IV.
Integrin α1 cytoplasmic tail mutants activate distinct signaling pathways in endothelial cells. (A) The cell populations were plated in serum-free medium on solidified collagen I + IV gels. Tubulogenesis was quantified 16 hours after plating as described in “Tubulogenesis.” Values are mean plus or minus SD of 4 independent experiments. Gels were viewed with a Nikon Diaphot inverted research microscope (Nikon, Tokyo, Japan) using a lens at 20×/0.2 Ph2 LD 0.4. Images were acquired using a Canon PowerShot S5 IS camera (Canon USA, Lake Success, NY) and were processed with Adobe Photoshop version 9.0 software (Adobe Systems, San Jose, CA). (B) The cell populations were plated in 96-well plates coated with 10 μg/mL collagen IV. Four hours later, the cells were incubated with serum-free medium containing 3H-thymidine (0.5 μCi/well) for a further 48 hours, and proliferation was then evaluated as described in “Cell proliferation.” Values are mean plus or minus SD of one representative experiment performed in quadruplicate. *Statistically significant differences (P < .05) between the α1KO cells and α1 mutant–expressing cells. (C) The cell populations were serum-starved for 24 hours and embedded in collagen I + IV gels for the time indicated. The gels were sonicated and run on an SDS-PAGE gel to detect levels of activated and total p38 MAPK, ERK, and Akt. Images are representative of 3 independent experiments. Vertical line(s) have been inserted to indicate a repositioned gel lane. (D) Phosphorylated and total kinase bands were quantified by densitometry analysis, and the phosphorylated signal was expressed as phosphorylated kinase/total kinase ratio. Values are the mean plus orf minus SD of 3 independent experiments. * indicates significant differences (P < .05) relative to α1KO cells. (E-G) The cell populations indicated were subjected to migration (E), tubulogenesis (F), and proliferation (G) assays on collagen IV or fibronectin in the presence or absence of 10 μM PD169316, 10 μM PD98059, or 5 μM LY294002. Values are the mean plus or minus SD of one representative experiment. Differences between untreated α1KO and α1 mutant–expressing cells (*) and inhibitor untreated versus treated α1 mutant–expressing cells (#) were significant with P < .05.
Integrin α1 cytoplasmic tail mutants activate distinct signaling pathways in endothelial cells. (A) The cell populations were plated in serum-free medium on solidified collagen I + IV gels. Tubulogenesis was quantified 16 hours after plating as described in “Tubulogenesis.” Values are mean plus or minus SD of 4 independent experiments. Gels were viewed with a Nikon Diaphot inverted research microscope (Nikon, Tokyo, Japan) using a lens at 20×/0.2 Ph2 LD 0.4. Images were acquired using a Canon PowerShot S5 IS camera (Canon USA, Lake Success, NY) and were processed with Adobe Photoshop version 9.0 software (Adobe Systems, San Jose, CA). (B) The cell populations were plated in 96-well plates coated with 10 μg/mL collagen IV. Four hours later, the cells were incubated with serum-free medium containing 3H-thymidine (0.5 μCi/well) for a further 48 hours, and proliferation was then evaluated as described in “Cell proliferation.” Values are mean plus or minus SD of one representative experiment performed in quadruplicate. *Statistically significant differences (P < .05) between the α1KO cells and α1 mutant–expressing cells. (C) The cell populations were serum-starved for 24 hours and embedded in collagen I + IV gels for the time indicated. The gels were sonicated and run on an SDS-PAGE gel to detect levels of activated and total p38 MAPK, ERK, and Akt. Images are representative of 3 independent experiments. Vertical line(s) have been inserted to indicate a repositioned gel lane. (D) Phosphorylated and total kinase bands were quantified by densitometry analysis, and the phosphorylated signal was expressed as phosphorylated kinase/total kinase ratio. Values are the mean plus orf minus SD of 3 independent experiments. * indicates significant differences (P < .05) relative to α1KO cells. (E-G) The cell populations indicated were subjected to migration (E), tubulogenesis (F), and proliferation (G) assays on collagen IV or fibronectin in the presence or absence of 10 μM PD169316, 10 μM PD98059, or 5 μM LY294002. Values are the mean plus or minus SD of one representative experiment. Differences between untreated α1KO and α1 mutant–expressing cells (*) and inhibitor untreated versus treated α1 mutant–expressing cells (#) were significant with P < .05.
Distinct cellular functions are mediated by activation of different α1 integrin subunit-dependent signaling pathways
Although we previously showed that integrin α1β1 confers the ability of fibroblasts to proliferate on collagenous substrata via activation of the Shc/Grb2/ERK pathway,17 little is known about other pathways regulated by this collagen receptor. Because p38 MAPK, ERK, and Akt activation is required for the regulation of different endothelial cell functions,50 the various mutant expressing cells were embedded in collagen I + IV gels and activation of these 3 kinases analyzed at different time points. Significant basal and collagen I + IV-mediated p38 MAPK activation was only seen in the α1–1148- and full-length α1-expressing cells (Figure 2C,D), and collagen I + IV–induced Akt phosphorylation was detected in the same cell populations (Figure 2C,D). Interestingly, collagen I + IV–mediated ERK activation was seen in the α1-1145–, α1-1148–, and full-length α1-expressing cells (Figure 2C,D). When α1KO and full-length α1-expressing endothelial cells were plated on fibronectin, both cell types activated all 3 kinases equally within 10 minutes of plating (Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article), indicating that loss and/or reexpression of the integrin α1 subunit only affects collagen-mediated signaling. Altogether, these results suggest that, in a collagenous milieu, ERK activation appears to correlate with mutants able to support cell proliferation (Figure 2B), whereas activation of p38 MAPK and Akt correlated with mutants able to support migration and tubulogenesis (Figures 1H, 2A).
To determine whether integrin α1β1–mediated signaling correlated with the ability of this receptor to induce cell migration, tubulogenesis, and proliferation, these assays were performed in the presence of selective kinase inhibitors. The p38 MAPK inhibitor significantly decreased the ability of cells expressing α1-1148 and full-length α1 to migrate on collagen IV, whereas the MEK and PI3K inhibitors had no effect (Figure 2E). When migration on fibronectin was analyzed, α1KO- and full-length α1-expressing endothelial cells showed comparable migration rates, which was inhibited to a similar extent by the p38 MAPK inhibitor (Figure 2E). Both the p38 MAPK and PI3K inhibitors inhibited the ability of the α1-1148 and full-length α1-expressing cells to form tubes on collagen I + IV gels (Figure 2F). When α1KO- and full-length α1-expressing endothelial cells were plated onto fibrin gels, both cell types underwent tubulogenesis that was equally inhibited by the p38 MAPK and PI3K inhibitors (Figure S1C,D). Proliferation of the α1-1145–, α1-1148–, and full-length α1-expressing cells on collagen IV was predominantly inhibited by the MEK inhibitor and, to a lesser extent, by the PI3K inhibitor (Figure 2G). In contrast, proliferation of the α1KO- and full-length α1-expressing endothelial cells on fibronectin was inhibited to a greater extent than cells grown on collagen IV by both MEK and PI3K inhibitors (Figure 2G).
These results suggest that (1) integrin-dependent migration of endothelial cells, including that mediated by α1β1, requires the p38 MAPK pathway; (2) the p38 MAPK and PI3K signaling pathways are required for both α1β1-dependent and -independent tubulogenesis; (3) ERK, and to a lesser extent PI3K, plays a role in integrin α1β1-mediated proliferation; and (4) both the ERK and PI3K pathways play an equally important role in regulating endothelial cell proliferation on fibronectin.
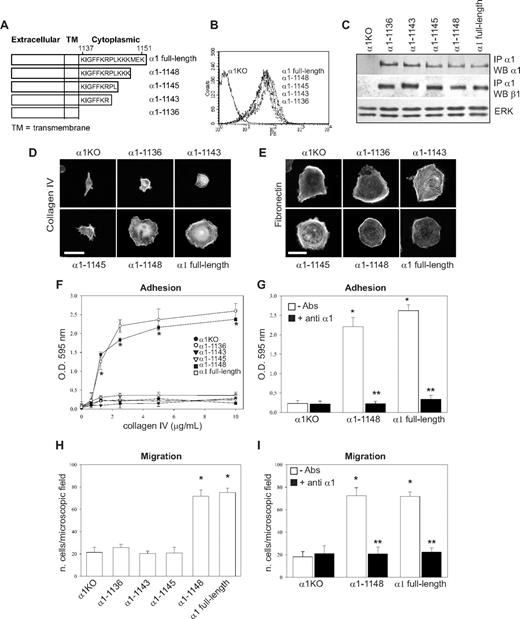
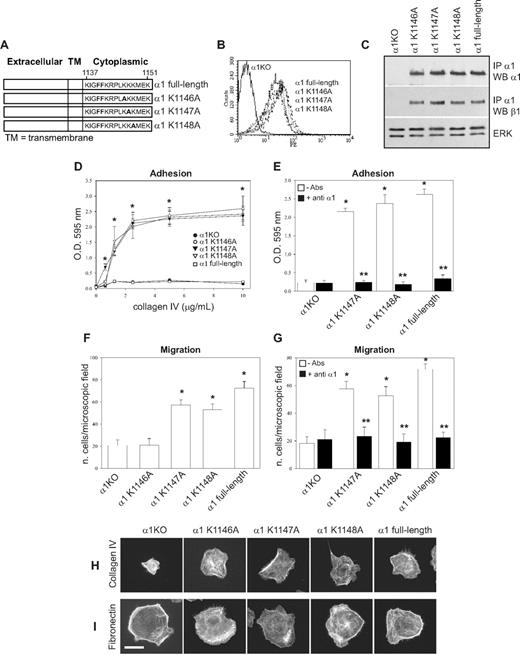
Lys1146 is required for integrin α1β1-dependent endothelial cell adhesion, migration and tubulogenesis, whereas Lys1147 is only required for tubule formation
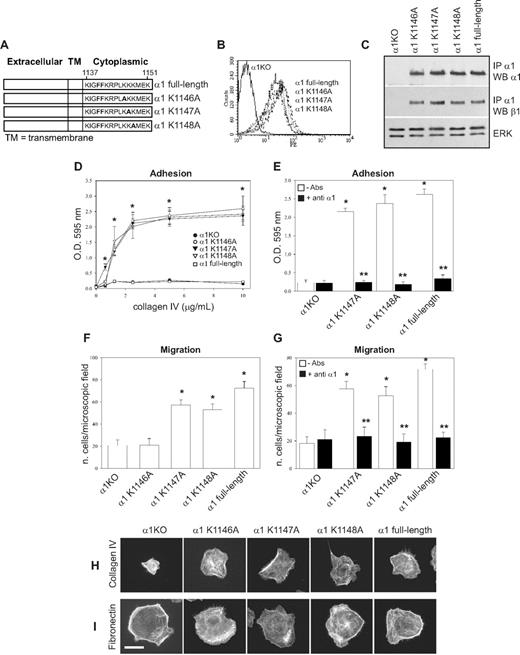
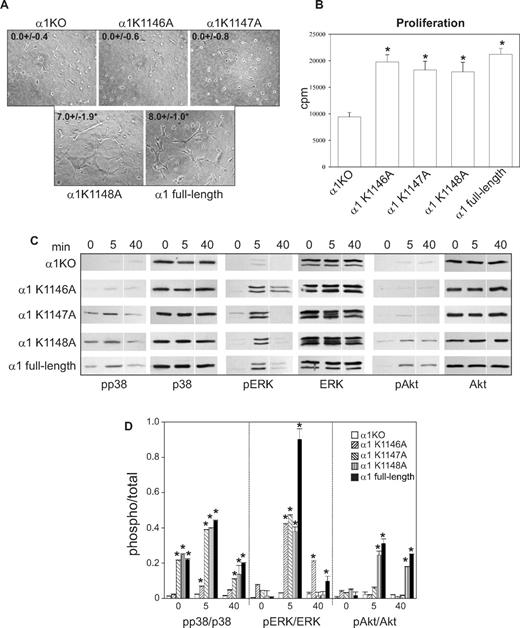
The 6 COOH-terminal amino acids of the integrin α1 cytoplasmic tail (KKKMEK) were required for cell spreading, adhesion, migration, and tubulogenesis (Figures 1D-I, 2A); however, the last 3 amino acids (MEK) were dispensable, allowing us to hypothesize that Lys1146-1148 were required to mediate these cell functions. For this reason, we mutated each lysine individually as well as all 3 lysines to alanines (Figure 3A) and attempted to express the mutants into α1KO endothelial cells. Whereas generation of cell populations expressing the single mutants was successful (Figure 3B,C), we were unable to obtain surface expression of the triple mutant. The K1146A-expressing cells failed to adhere, migrate, or undergo tubulogenesis on collagen IV (Figures 3D,F, 4A). In contrast, the K1147A mutant cells adhered and migrated to a similar extent as endothelial cells reconstituted with full-length α1 (Figure 3D,F) but were unable to undergo tubulogenesis on collagen IV (Figure 4A). No differences in adhesion, migration, and tubulogenesis were observed between the K1148A and full-length α1-expressing cells (Figures 3D,F, 4A). The observation that adhesion and migration of K1147A and K1148A mutant cells were inhibited by antihuman integrin α1 antibodies (Figure 3E,G) confirmed that these effects were indeed integrin α1β1–dependent.
K1146A point mutation of the integrin α1 cytoplasmic tail leads to decreased endothelial cell adhesion and migration. (A) Schematic representation of single point mutants (K/A) generated from the full-length integrin α1 subunit. (B) α1KO endothelial cells were transduced with either empty vector or the integrin α1 mutant cDNAs indicated in panel A, and cell populations were sorted by FACS using antihuman integrin α1 antibodies. (C) Total cell lysates of the cell populations were used to detect the levels of full-length and mutant human integrin α1 as well as mouse β1 subunits as described in detail in Figure 1C. (D-G) Cell adhesion on different concentrations of collagen IV (D) or in the presence of anti–human integrin α1 antibodies (E) as well as migration in the absence (F) and presence (G) of anti–human integrin α1 antibodies were determined as described in Figure 1. Note that only cells expressing K1146A mutant fail to adhere and migrate on collagen IV. * indicates statistically significant differences (P < .05) between the α1KO and the α1 mutant–expressing endothelial cells; * indicates statistically significant differences (P < .05) between untreated and antibody-treated α1 mutant–expressing cells. (H,I) The endothelial cells indicated were plated on 10 μg/mL collagen IV or fibronectin. After 4 hours, the cells were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm.
K1146A point mutation of the integrin α1 cytoplasmic tail leads to decreased endothelial cell adhesion and migration. (A) Schematic representation of single point mutants (K/A) generated from the full-length integrin α1 subunit. (B) α1KO endothelial cells were transduced with either empty vector or the integrin α1 mutant cDNAs indicated in panel A, and cell populations were sorted by FACS using antihuman integrin α1 antibodies. (C) Total cell lysates of the cell populations were used to detect the levels of full-length and mutant human integrin α1 as well as mouse β1 subunits as described in detail in Figure 1C. (D-G) Cell adhesion on different concentrations of collagen IV (D) or in the presence of anti–human integrin α1 antibodies (E) as well as migration in the absence (F) and presence (G) of anti–human integrin α1 antibodies were determined as described in Figure 1. Note that only cells expressing K1146A mutant fail to adhere and migrate on collagen IV. * indicates statistically significant differences (P < .05) between the α1KO and the α1 mutant–expressing endothelial cells; * indicates statistically significant differences (P < .05) between untreated and antibody-treated α1 mutant–expressing cells. (H,I) The endothelial cells indicated were plated on 10 μg/mL collagen IV or fibronectin. After 4 hours, the cells were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm.
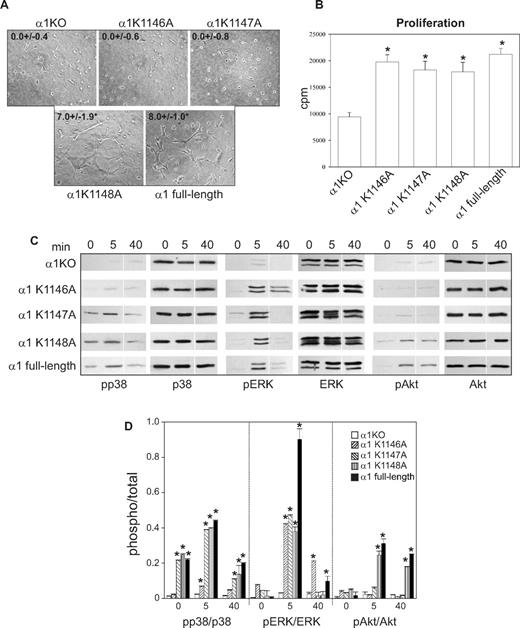
K1146A and K1147A point mutations of the integrin α1 cytoplasmic tail lead to decreased tubulogenesis. Tubulogenesis (A), cell proliferation (B), cell signaling (C), and densitometry analysis (D) were determined as described in Figure 2. Images of cells undergoing tubulogenesis were taken as described in Figure 2A. *Statistically significant differences (P < .05) between the α1KO and the α1 mutant–expressing endothelial cells. Vertical line(s) in panel C have been inserted to indicate a repositioned gel lane.
K1146A and K1147A point mutations of the integrin α1 cytoplasmic tail lead to decreased tubulogenesis. Tubulogenesis (A), cell proliferation (B), cell signaling (C), and densitometry analysis (D) were determined as described in Figure 2. Images of cells undergoing tubulogenesis were taken as described in Figure 2A. *Statistically significant differences (P < .05) between the α1KO and the α1 mutant–expressing endothelial cells. Vertical line(s) in panel C have been inserted to indicate a repositioned gel lane.
When cell morphology was assessed, only 5% to 10% of the K1146A mutant cells adhered on collagen IV; however, these adherent cells spread in a manner similar to K1147A-, K1148A-, and full-length α1-expressing cells (Figure 3H). As expected, none of the single K/A substitutions affected cell spreading on fibronectin (Figure 3I). These results suggest that Lys1146 is necessary for cell adhesion, migration, and tubulogenesis, whereas Lys1147 is required primarily for tubulogenesis. Finally, the single mutant-expressing cells proliferated to levels observed in full-length α1-expressing cells (Figure 4B), supporting the finding that cell proliferation is primarily mediated by the Pro1144 and Lys1145 NH2 terminal to these 3 lysine residues.
When the ability of the K/A point mutants to activate p38 MAPK, Akt, and ERK was determined, the K1147A-, K1148A-, and full-length α1-expressing cells activated p38 MAPK to the same degree both at baseline and after collagen stimulation (Figure 4C,D). Only the K1148A mutant cells activated Akt to the same degree as cells expressing full-length α1 after collagen stimulation (Figure 4C,D). ERK activation in all mutants was the same as full-length α1-expressing cells (Figure 4C,D). Thus, the ability of these mutants to activate p38 MAPK correlated with endothelial cell migration, whereas Akt activity correlated with the ability of endothelial cell to undergo tubule formation.
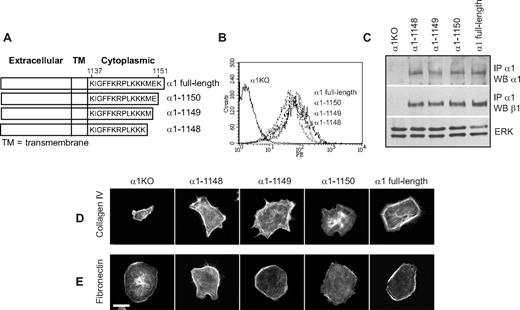
Deletion of Lys1151 of the integrin α1 cytoplasmic tail impairs endothelial cell adhesion, migration, and tubulogenesis
As endothelial cells expressing the α1-1148 mutant displayed comparable functions to cells expressing full-length α1, we hypothesized that deleting both Glu1150 and Lys1151 (mutant α1-1149) or only Lys1151 (mutant α1-1150) (Figure 5A) would not alter integrin α1β1–mediated function. Cell populations expressing comparable levels of both α1-1149 and α1-1150 mutants (Figure 5B,C) spread on collagen IV and fibronectin to a similar extent as α1-1148 or α1 full-length expressing cells within 4 hours from plating (Figure 5D,E). The α1-1149 cells also adhered, migrated, and formed tubules, such as α1-1148 or α1 full-length expressing cells (Figure 6A-C), and these events were integrin α1β1–dependent (not shown). Despite their ability to spread, the α1-1150 cells adhered, migrated, and formed tubules significantly less than α1-1148 or α1 full-length expressing cells (Figure 6A-C). No differences in cell proliferation were observed between these 2 mutants and α1 full-length expressing cells (Figure 6D). As expected, the α1-1149 cells activated p38 MAPK, ERK, and Akt in response to collagen stimulation, whereas the α1-1150 cells only activated ERK (Figure 6E,F).
Generation of integrin α1-null endothelial cells expressing deletion mutants lacking Glu1150 and/or Lys1151 of the integrin α1 cytoplasmic tail. (A) Schematic representation of the cytoplasmic truncation mutants of the human integrin α1 cDNA. (B) α1KO endothelial cells were transduced with either empty vector or the integrin α1 mutant cDNAs indicated in panel A, and cell populations expressing the integrin α1 constructs were sorted by FACS using anti–human integrin α1 antibodies. (C) Total cell lysates of the cell populations were used to detect the levels of full-length and mutated human integrin α1 as well as mouse β1 subunits as described in detail in Figure 1C. (D,E) Integrin α1KO endothelial cells expressing vector alone or the truncation mutants were plated on 10 μg/mL collagen IV or fibronectin. After 4 hours, the cells were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm.
Generation of integrin α1-null endothelial cells expressing deletion mutants lacking Glu1150 and/or Lys1151 of the integrin α1 cytoplasmic tail. (A) Schematic representation of the cytoplasmic truncation mutants of the human integrin α1 cDNA. (B) α1KO endothelial cells were transduced with either empty vector or the integrin α1 mutant cDNAs indicated in panel A, and cell populations expressing the integrin α1 constructs were sorted by FACS using anti–human integrin α1 antibodies. (C) Total cell lysates of the cell populations were used to detect the levels of full-length and mutated human integrin α1 as well as mouse β1 subunits as described in detail in Figure 1C. (D,E) Integrin α1KO endothelial cells expressing vector alone or the truncation mutants were plated on 10 μg/mL collagen IV or fibronectin. After 4 hours, the cells were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm.
Lys1151 within the integrin α1 tail is required for endothelial cell adhesion, migration, and tubulogenesis, but not proliferation. Cell adhesion (A), migration (B), tubulogenesis (C), and proliferation (D) were determined as described in Figures 1 and 2. (E,F) Integrin α1β1–dependent signaling and densitometry analysis were determined as described in Figure 2C,D. * indicates statistically significant differences (P < .05) between the α1KO and the α1 mutant–expressing endothelial cells.
Lys1151 within the integrin α1 tail is required for endothelial cell adhesion, migration, and tubulogenesis, but not proliferation. Cell adhesion (A), migration (B), tubulogenesis (C), and proliferation (D) were determined as described in Figures 1 and 2. (E,F) Integrin α1β1–dependent signaling and densitometry analysis were determined as described in Figure 2C,D. * indicates statistically significant differences (P < .05) between the α1KO and the α1 mutant–expressing endothelial cells.
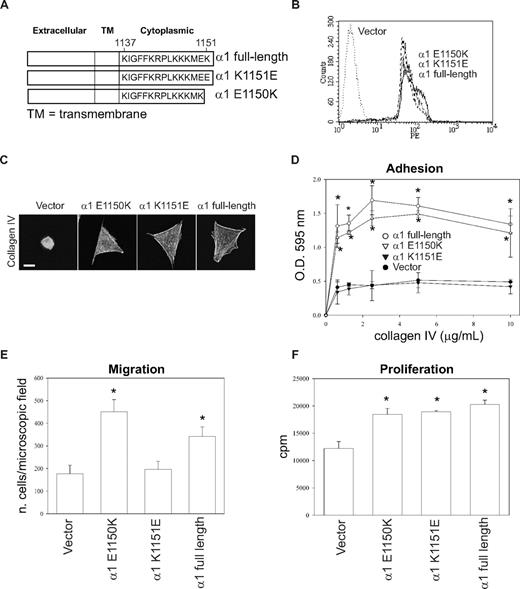
Substitution of Lys1151 with a negatively charged amino acid impairs cell adhesion and migration
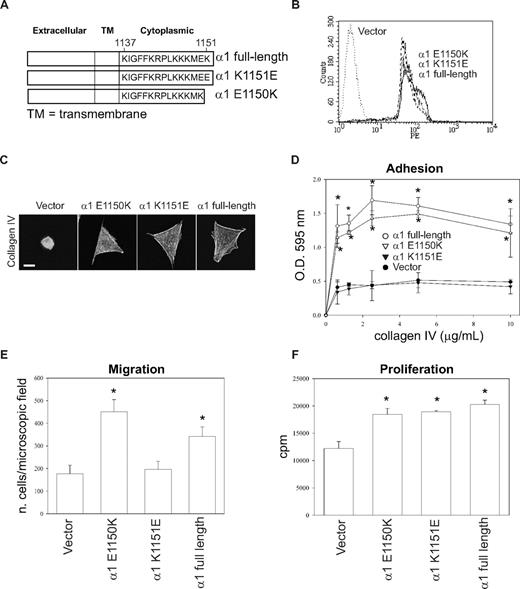
These results suggest that the presence of a negatively charged amino acid at the COOH terminus of the integrin α1 tail impairs cell adhesion, migration, and tubulogenesis. To test this hypothesis, we generated populations of CHO cells expressing equal levels of the full-length integrin α1 subunit, the point mutant α1-K1151E, and the truncation mutant α1-E1150K (Figure 7A,B). CHO cells were used for this experiment because (1) making stable endothelial cell populations is extremely difficult and time-consuming; (2) similar to integrin α1–null endothelial cells, CHO cells show low baseline spreading, adhesion, migration, and proliferation on collagen IV (Figure 7)51 ; and (3) most importantly, they have been successfully used to analyze the role of the human integrin α1 subunit in cell functions.40,51
Negatively charged amino acids at the COOH-terminus of the integrin α1 tail inhibit cell adhesion and migration, but not proliferation. (A) Schematic representation of mutants generated from the full-length integrin α1 subunit. (B) CHO cells were transfected with either empty vector or the integrin α1 mutant cDNAs indicated in panel A, and cell populations were sorted by FACS using anti–human integrin α1 antibodies. (C) The CHO cell populations indicated were plated on 10 μg/mL collagen IV and after 1 hour they were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm. (D-F) Cell adhesion (D), migration (E), and proliferation (F) were determined as described in Figures 1 and 2. * indicates statistically significant differences (P < .05) between the vector transfected and α1 mutant–expressing CHO cells.
Negatively charged amino acids at the COOH-terminus of the integrin α1 tail inhibit cell adhesion and migration, but not proliferation. (A) Schematic representation of mutants generated from the full-length integrin α1 subunit. (B) CHO cells were transfected with either empty vector or the integrin α1 mutant cDNAs indicated in panel A, and cell populations were sorted by FACS using anti–human integrin α1 antibodies. (C) The CHO cell populations indicated were plated on 10 μg/mL collagen IV and after 1 hour they were fixed and stained with rhodamine-phalloidin. A representative cell is shown for each cell population. Bar represents 10 μm. (D-F) Cell adhesion (D), migration (E), and proliferation (F) were determined as described in Figures 1 and 2. * indicates statistically significant differences (P < .05) between the vector transfected and α1 mutant–expressing CHO cells.
As previously reported,51 vector-transfected CHO cells failed to spread on collagen IV, whereas CHO cells transfected with mutants α1-E1150K, α1-K1151E, or α1 full-length spread equally on this substrate (Figure 7C). The CHO cells expressing either full-length or the mutant α1-E1150K adhered and migrated on collagen IV significantly more than vector or α1-K1151E transfected CHO cells (Figure 7D,E). Finally, all the CHO cells expressing the different integrin α1 constructs proliferated significantly more than vector-transfected CHO cells on collagen IV (Figure 7F). These results strongly suggest that a positively charged amino acid at the COOH-terminus of the integrin α1 tail mediates integrin α1β1–dependent cell adhesion and migration.
Discussion
We previously showed that integrin α1β1 plays a role in pathologic angiogenesis,7,8,14,15 but the specific mechanism(s) by which the α1 subunit controls endothelial cell functions is unknown. Using a mutagenesis approach, we determined the critical residues within the α1 cytoplasmic tail, as well as the signaling pathways required for specific integrin α1β1–dependent endothelial cell functions. We show that: (1) mutants with deletion of the entire cytoplasmic tail (α1-1136) or just distal to the GFFKR motif (α1-1143) do not restore endothelial cell adhesion, migration, proliferation and tubulogenesis on collagen IV; (2) Pro1144 and Leu1145 are required for integrin α1β1–dependent endothelial cell proliferation; (3) Lys1146 is required for adhesion, migration, and tubulogenesis, whereas Lys1147 is only required for tubulogenesis; (4) a positively charged amino acid is most probably required at the COOH-terminus of the α1 tail to mediate cell adhesion, migration, and tubulogenesis; and (5) integrin α1β1–dependent cell migration requires p38 MAPK activation; tubulogenesis requires both p38 MAPK and PI3K activation, whereas proliferation is predominantly dependent on ERK activation. Thus, specific amino acids distal to the GFFKR motif are critical for activation of distinct signaling pathways and α1β1-dependent cell functions.
The highly conserved GFFKR region in the juxtamembrane domain of the α integrins has been proposed to bind to the β tail and keep the integrin in an inactive state.26,52 In this context, deletion of this region in the integrin αIIb tail or mutations that disrupt integrin αIIb-β3 interactions activates this receptor.26,52 In our study, α1KO endothelial cells expressing both α1-1136 (which lacks the GFFKR motif) and α1-1143 (which contains the GFFKR motif) have a similar phenotype to parental α1KO cells, suggesting that the highly conserved GFFKR motif might not play a role in regulating integrin α1 activation. This possibility is supported by the finding of Czuchra et al53 that mice carrying a D759A mutation in the integrin β1 tail HDRRE motif, which is thought to interact with the GFFKR of the α subunits, had no obvious phenotype in vivo. Moreover, keratinocytes isolated from these mice showed normal adhesion, spreading, and migration in vitro.53 It is possible that the GFFKR in the α1 subunit does not play a role in integrin activation but is simply required for hetero- or homo-dimerization of the integrin α and β subunits resulting in integrin clustering,54 which is critical for cell adhesion. This hypothesis is supported by the finding that CHO cells expressing an integrin α1 subunit lacking the cytoplasmic tail adhered but did not migrate on collagen IV, whereas a mutant containing the GFFKR motif induced cell migration and stress fiber formation.55 Although we attempted to perform binding assays in cell suspension to determine the role of different α1 mutants on α1β1 affinity for collagen IV, we were unable to identify a fragment that bound efficiently and specifically to the extracellular domain of this integrin.
We show that the addition of Pro1144 and Leu1145 to the GFFKR motif is sufficient to support integrin α1β1–dependent endothelial cell proliferation and induce ERK activation. The inhibitor studies show this kinase is critical for α1β1-dependent cell proliferation. This result parallels our previous finding in fibroblasts that integrin α1β1 activates the Shc/Grb2/ERK pathway to promote proliferation on collagenous substrata.17 However, our observation that the first 9 amino acids of the α1 tail are required for ERK activation contrasts with the previous finding that the integrin α1 transmembrane, but not cytoplasmic domain, is required for integrin α1–mediated recruitment of Shc and consequent ERK activation.38 Our study suggests that a Shc-independent pathway might be responsible for integrin α1β1-mediated activation of ERK in endothelial cells and that Pro1144 and Leu1145 might serve as a binding site for an adaptor molecule(s) able to promote ERK activation in endothelial cells. Thus, it is possible that activation of ERK by integrin α1β1 is cell type–specific.
We demonstrate that Lys1146 is required for cell adhesion, migration, and tubulogenesis, whereas Lys1147 is only required for tubulogenesis. These differences in cell function correlate with the finding that the K1146A mutation results in loss of both p38 MAPK and Akt activation, whereas the K1147A mutation only results in loss of Akt activation. Thus, these 2 lysines promote particular cell functions by activating specific signaling pathways.
The observation that integrin α1–mediated p38 MAPK activation is required for endothelial cell migration and tubulogenesis agrees with the finding that activation of this kinase promotes endothelial cell migration.56,57 However, these data contrast with the findings that integrin α2 subunit is required for collagen-mediated p38 MAPK activation.47,58 The integrin α1KO endothelial cells (despite expressing endogenous integrin α2β1) cannot activate p38 MAPK when embedded in collagen gels, whereas reexpression of the integrin α1 subunit results in significant collagen-mediated p38 MAPK activation. Interestingly, incubation of full-length α1 reconstituted endothelial cells with either antihuman α1 or antimouse α2 antibodies significantly decreases the collagen-mediated p38 MAPK activation (Figure S2A,B). Together, these data suggest that in endothelial cells both integrins α1β1 and α2β1 are required to promote collagen-mediated p38 MAPK activation, and blocking either integrin decreases p38 MAPK activation to the levels observed in α1KO cells.
We demonstrate that integrin α1β1–mediated Akt activation plays a role in regulating endothelial cell tubulogenesis. The K1147A expressing cells, which cannot activate Akt or form tubules, show normal adhesion and migration, suggesting that tubulogenesis is regulated by cellular events other than cell migration. One possible mechanism might be cell polarization because PI3K inhibitors block cell polarization and directional migration of leukocytes and breast cancer cells.59,60 Moreover, inhibition of PI3K in endothelial cells prevents growth factor-induced branching or tubule formation without affecting other cell functions.61,62
One of the most surprising results in the study was that deletion of the terminal Lys1151 (mutant α1-1150) or substitution of this lysine with a glutamic acid (mutant α1-K1151E) resulted in the loss of integrin α1β1–dependent cell adhesion, migration, and tubulogenesis, whereas cells expressing mutant α1-1149 (where both Lys1151 and Glu1150 are deleted) or mutant α1-E1150K behaved like cells expressing full-length α1. A possible explanation for this result is that the presence of a positive or neutral (ie, α1-1150 mutant) charge at the COOH-terminus of the α1 tail determines how the tail is oriented relative to the β tail and/or to the plasma membrane, thus influencing the binding of signaling molecules to the COOH-terminus of the α1 cytoplasmic tail.
In conclusion, we demonstrate that specific regions of the integrin α1 cytoplasmic tail are responsible for the activation of selective downstream signaling pathways that regulate specific endothelial cell functions. As integrin α1β1 plays a key role in angiogenesis, identification of the molecular mechanisms whereby this collagen binding receptor controls endothelial cell functions might have implications in determining whether this integrin is a good drug target for diseases characterized by uncontrolled new blood vessel formation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cathy Alford at the Department of Veterans Affairs for help with the flow cytometric analysis.
This work was supported by R01-CA94849 (A.P.), RO1-DK 69 921 and R01-DK 075594 (R.Z.), P01 DK65123 (R.Z., A.P.), and a Merit award from the Department of Veterans Affairs (R.Z.).
National Institutes of Health
Authorship
Contribution: T.D.A. performed experiments and made figures; N.B. performed experiments; C.B. contributed to the generation of reagents; M.S. contributed analytical tools and analyzed data; R.Z. analyzed data and wrote the paper; and A.P. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ambra Pozzi, Department of Medicine, Division of Nephrology, Medical Center North, B3109, Vanderbilt University, Nashville, TN 37232; e-mail: ambra.pozzi@vanderbilt.edu.