Abstract
Although the phosphatidylinositide 3-kinase (PI3K)/Akt pathway has been reported to contribute to the malignant growth of multiple myeloma (MM), the true relevance of Akt kinases for this disease is still unclear. In particular, functional analyses in primary tumor cells and genetic target validation experiments are missing. Here, we used combined functional and molecular analyses to determine the importance of Akt activity in a large panel of primary MM samples and in MM cell lines. Akt down-regulation with isoform-specific siRNA constructs or with an Akt1/2-specific pharmacologic inhibitor strongly induced apoptosis in approximately half of the primary MM samples analyzed. Sensitivity to Akt inhibition strongly correlated with the activation status of Akt as determined by immunohistochemistry, phospho-Akt–specific flow cytometry, and Western analysis. Additional blockade of the MAPK and the IL-6R/STAT3 pathways was often not sufficient to decrease the viability of MM cells resilient to Akt inhibition. Taken together, these experiments led to the identification of 2 myeloma subgroups: Akt-dependent and Akt-independent MM.
Introduction
Multiple myeloma (MM) is caused by the growth of a malignant plasma cell clone in the bone marrow (BM). This still incurable disease is characterized by a remarkably heterogeneous clinical course and the median survival ranges from 2 to 3 years for high-risk MM to better than 5 years for low-risk patients.1 While this suggests the existence of different biologically defined subgroups, the knowledge about such biologic differences is still limited. Many experimental oberservations support the hypothesis that MM evolves through a multistep transformation process.2 In addition to the BM microenvironment, genetic alterations are supposed to enhance the activity of deregulated signaling pathways.2-5 One of these signaling cascades is the phosphatidylinositide 3-kinase (PI3K)/protein kinase B (PKB) pathway, also known as the Akt pathway.
The PKB/Akt kinase family includes 3 isoforms (PKBα/Akt1, PKBβ/Akt2, and PKBγ/Akt3) that are encoded by separate genes and that are involved in the regulation of apoptosis, proliferation, motility, and energy metabolism.6 Although the different isoforms share high sequence homology, their tissue distribution varies, and functional differences have been reported in healthy tissues and tumors.7-9 For example, human breast and ovarian carcinomas may harbor an activating mutation of Akt1 and often display elevated Akt1 activity.10,11 Amplification and tumorigenicity of Akt2 was found in pancreatic tumors and constitutive activation of Akt2 in ovarian cancer cells sensitized them to PI3K inhibition.12,13 Akt3 enzymatic activity was reported to be elevated in hormone-insensitive breast and prostate tumors.14
Activation of Akt involves phosphorylation of 2 amino-acid residues: threonine 308 and serine 473 (numbers refer to Akt1).6 For a number of human tumors, including various carcinomas, glioblastoma multiforme, and hematologic malignancies, constitutive activation of Akt has been reported and has often been linked to late-stage cancers with poor prognosis.6
The PI3K/Akt pathway has also been studied in MM and Akt was shown to be frequently activated in primary MM specimens.15,16 In addition to stimulation from the BM microenvironment, deletion of the tumor suppressor gene PTEN entails hyperactivation of Akt, and the prominence of PTEN deletions in other human tumors supports a role for Akt in tumor progression.6,17-19 Functionally, the PI3K/Akt pathway is implicated in cell-cycle and apoptosis regulation in MM cells.19-21 A major drawback of previous studies on the role of Akt in MM is that most data were obtained with small compound inhibitors that do not specifically target Akt. Therefore, it is unclear whether their biologic effects are due to Akt inhibition or are the result of off-target effects. Recent advances in the development of specific small-molecule inhibitors of Akt now provide new opportunities to appraise its role in MM. Furthermore, virtually nothing is known about the role of single Akt isoforms and only very limited analyses on the contribution of Akt activity to MM cell survival have been performed with primary material. Consequently, the precise role of Akt for the pathogenesis of MM and its value as a therapeutic target requires further investigation.
Here, we used an siRNA-mediated knockdown approach to selectively deplete the different Akt isoforms and to determine the consequences for the viability of MM cells. This was complemented with an analysis of the biologic and molecular effects of the recently described Akt1- and Akt2-specific inhibitor Akti-1/222-24 on a panel of primary MM cells, and correlated with the activation status of Akt in these samples.
Methods
Cell culture
Human MM cell lines AMO-1, MM.1S, OPM-2, and U266 as well as primary MM cells were maintained in RPMI 1640 medium (PAA Laboratories, Pasching, Austria), supplemented with 10% fetal bovine serum (FBS; Biochrom, Berlin, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin (PAN Biotech, Aidenbach, Germany), GlutaMAX-I (Invitrogen, Karlsruhe, Germany), and 1 mM Na-pyruvate (PAN Biotech). Primary MM cells were cultured either in the presence of 10 ng/mL recombinant human IL-6 or in the presence of bone marrow stromal cells (BMSCs). Cells were grown at 37°C and 5% CO2.
Preparation of primary MM cells and BMSCs
Primary MM cells were obtained from diagnostic BM aspirates of patients with MM and purified as previously described.25 Primary BMSCs were derived from the same source.25 All primary material was collected after informed consent was obtained in accordance with the Declaration of Helsinki and after permission was received from the local ethics committee (Ethik-Kommission der Medizinischen Fakultät der Universität Würzburg; reference number 73/05).
Application of drugs
Drugs were diluted from concentrated stocks to 2 times final concentrations in full RPMI medium and an applicable volume was added to myeloma cell cultures seeded in 96- or 48-well plates. Appropriate controls (cells treated with dimethyl sulfoxide [DMSO] or buffer) were always included.
Transfection of AMO-1 and MM.1S cells by electroporation
Cells were washed with and resuspended in fresh RPMI medium without additives. Electroporations were performed using suspensions of 2 × 107 cells in 500 μL, and 0.4 cm-wide cuvettes (Invitrogen). Pulse settings were 950 μF and either 280 V (AMO-1) or 300 V (MM.1S). Plasmid concentrations were 15 μg/mL for pSUPER-Akt1 (siAkt1) or pSUPER-Akt2 (siAkt2), 20 μg/mL for pSUPER-Akt3 (siAkt3), and 10 μg/mL for pcDNA3.1-EGFP. Cells were collected 24 hours after electroporation, and debris was removed by gradient centrifugation with OptiPrep (Axis-Shield, Oslo, Norway).26 Strongly transfected cells were subsequently purified by fluorescence-activated cell sorting (FACS) of bright enhanced green fluorescent protein (EGFP)–positive cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These were washed with phosphate-buffered saline (PBS), resuspended in full RPMI medium, and used for experiments. The viability of MM cells transfected with control vector was better than 70% in all experiments.
Generation of siRNA expression constructs
Design and construction of pSUPER-based siRNA expression vectors are described in Brummelkamp et al.27 The following sense oligonucleotides were used (sequences derived from the actual genes marked in bold): dGATCCCCACGAGGGGAGTACATCAAGACTTCAAGAGAGTCTTGATGTACTCCCCTCGTTTTTTGGAAA (targeting Akt1; see also Czauderna et al28 ); dGATCCCCGACCTGGAGGCCACGGTACTTCAAGAGAGTACCGTGGCCTCCAGGTCTTTTTGGAAA (targeting Akt2); and dGATCCCCGCTATCCAGGCTGTAGCAGTTCAAGAGACTGCTACAGCCTGGATAGCTTTTTGGAAA (targeting Akt3). The efficacy of individual constructs was confirmed 3 days after electroporation through Western analysis of the respective target. The siRNA expression construct against human signal transducer and activator of transcription 3 (STAT3) is described in Chatterjee et al.26
Apoptosis assay
To identify apoptotic cells, samples were washed with PBS and resuspended in staining buffer as described before.26 Primary MM cells were stained with annexin V–FITC (Bender MedSystems, Vienna, Austria) and propidium iodide (PI). EGFP+ cell populations were stained with annexin V–ATTO 647 (Alexis, Lausen, Switzerland). Experiments with primary MM cells were not evaluated if the absolute survival measured for the DMSO control wells was below 30% (range, 32%-86%; median, 68%).
Phospho-Akt–specific flow cytometry
For phospho-Akt (Ser473) analysis, freshly isolated primary MM cells (5 × 104 cells/well) were kept in 200 μL medium without interleukin-6 (IL-6) and treated in 5 different ways. A total of 2 samples were exposed to either 10 μM Akti-1/2 or to a corresponding amount of DMSO. A third sample (phospho-Akt positive control) was treated for 15 minutes with 25 ng/mL IL-6 and 50 ng/mL insulin-like growth factor-1 (IGF-1) before being harvested. A total of 2 samples were cocultured overnight with BMSCs before 10 μM Akti-1/2 or a corresponding amount of DMSO was added. Cells were harvested after 14 hours of drug treatment and immediately fixed with an equal volume of Fix Buffer I (BD Biosciences, Heidelberg, Germany [BD]) for 20 minutes at 37°C. Cells were spun down, resuspended in ice-cold 90% methanol, and vigorously shaken for 30 minutes at 4°C. After 2 rinses with 3% BSA in PBS (PBS/B), cells were resuspended in 100 μL of anti–phospho-Akt (Ser473) antibody solution (Cell Signaling Technology, Frankfurt am Main, Germany; no. 4058; 1:100 in PBS) and incubated at room temperature for 1 hour. Cells were washed twice with PBS/B, resuspended in 100 μL of Alexa Fluor 647–conjugated secondary antibody solution (Invitrogen; A21244; 1:100 in PBS), and kept at room temperature in the dark for 30 minutes. After 2 rinses with PBS/B, the samples were subjected to flow cytometry. Live cells, which could be clearly distinguished in forward versus sideways scatter plots, were gated and the median fluorescence intensity (MFI) was determined. Fold changes of the MFI were defined as the fraction (MFI of measured sample) divided by (MFI of sample kept in medium and treated with Akti-1/2).29 A change of MFI larger than 1.3-fold was considered indicative for constitutive activation of Akt. Drug treatment alone did not change the autofluorescence of MM cells (Figure S2). Intracellular staining of phospho-STAT3 and phospho-ERK1/2 was performed as described with fixation times of 10 minutes and 50 minutes, respectively. The following antibodies were used: anti–phospho-STAT3–Alexa Fluor 488 (BD; 557814; 1:10 in PBS) and anti–phospho-ERK1/2–Alexa Fluor 647 (BD; 612593; 1:20 in PBS). Incubation with antibodies was for 1 hour at room temperature in the dark.
Histochemical analysis
Giemsa staining and immunohistochemical (IHC) 3-step peroxidase staining were performed with formalin-fixed, paraffin-embedded bone marrow biopsies heavily infiltrated by MM cells as previously described.30 Slides were viewed with equipment from Olympus (Hamburg, Germany; camera BX50/DP-Soft version 5) and processed with Adobe Photoshop version 3.0 (Adobe Systems, San Jose, CA). The anti–phospho-Akt antibody used was from Cell Signaling Technology (no. 3787; dilution 1:50).
Western analysis
Sample preparation for MM cell lines was described before.31 Primary MM cells (2 × 105 cells/well) were treated as detailed in Stühmer et al.25 Antibodies detecting Akt1 (no. 2967), Akt2 (no. 2962), Akt3 (no. 4059), pan-Akt (no. 9272), phospho-Akt (Ser473; no. 4058), phospho-FoxO1/3a (no. 9464), phospho-ERK1/2 (no. 9101), ERK1/2 (no. 9102), phospho-STAT3 (Tyr705; no. 9131), and STAT3 (no. 9132) were purchased from Cell Signaling Technology. Anti–α-tubulin (BZL 03568) was from BIOZOL (Eching, Germany). Secondary antibodies specific for rabbit (NA9310V), mouse (NA9340V), or rat (NA9350V) were from GE Healthcare (Little Chalfont, United Kingdom). Akt isoform specificity of the antibodies was confirmed by Western analysis with recombinant proteins (Figure S3).
Reagents
Akti-1/2 (124018) and PD98059 (513001) were purchased from Merck (Darmstadt, Germany) and phorbol myristate acetate (PMA) was purchased from Alexis. Sant7 was produced in our laboratory as described before.32 Recombinant human IL-6 and IGF-1 proteins were obtained from ImmunoTools (Friesoythe, Germany).
Statistical analysis
For statistical analysis of viability assays, a 1-tailed unpaired Student t test was used.
Results
siRNA-mediated knockdown of Akt induces apoptosis in MM.1S but not in AMO-1 cells
To directly test the necessity of Akt signaling for the viability of MM cells and to appraise the importance of the different Akt isoforms for cell survival, we performed transfection experiments with siRNA expression vectors directed selectively against Akt1, Akt2, or Akt3. For this approach, 2 electroporatable MM cell lines were used that in culture either do (MM.1S) or do not (AMO-1) display constitutive phosphorylation of Akt (phosphorylation of Ser473 used as readout for activity; Figures 1A,2A). Each siRNA expression construct led to specific down-regulation of its intended target without affecting protein levels of the other 2 Akt isoforms (Figure 1A). Knockdown of Akt1 and to a lesser extent of Akt2 in MM.1S cells also produced a sizeable decrease of total phosphorylated Akt, whereas down-regulation of Akt3 did not, indicating that phosphorylation of Akt3 may not significantly contribute to the phospho-Akt pool. Knockdown of Akt3 was restricted to MM.1S because AMO-1 cells were devoid of Akt3 protein as judged by Western analysis (Figure 1A). Next, combined down-regulation of Akt1 and Akt2 or of all 3 Akt isoforms (only in MM.1S) was performed to abolish Akt signaling (Figure 1B). Knockdown of Akt1 and Akt2 was sufficient to strongly deplete AMO-1 and MM.1S cells of total Akt protein (Figure 1B). In MM.1S cells, this treatment led to strong reductions of phospho-Akt and of phospho-FoxO1/3a, a substrate of Akt. Additional down-regulation of Akt3 in MM.1S cells did not increase these effects. The viability of the transfected cells was determined 6 days after transfection (Figure 1C). Knockdown of Akt1 strongly induced apoptosis in MM.1S cells (16% survival compared with cells transfected with empty pSUPER vector) and, although less pronounced, a similar result was obtained by down-regulation of Akt2 (41% survival). Combined knockdown of both Akt1 and Akt2 did not produce any benefit over Akt1 knockdown alone (20% survival). Depletion of Akt3 had rather minor effects on the viability of MM.1S cells (72% survival). The viability of AMO-1 cells, which do not display constitutive Akt phosphorylation, was completely unaffected by siRNA-mediated single knockdown of either Akt1 or Akt2 (Figure 1C). Likewise, knockdown of both Akt isoforms did not induce cell death (Figure 1C) or impair proliferation (A.Z., personal observations, June 2007).
SiRNA knockdown of Akt isoforms in MM cell lines. (A) Specificity of Akt isoform knockdown in MM cell lines AMO-1 and MM.1S as determined by Western analysis 3 days after electroporation with pSUPER-derived siRNA expression vectors (siAkt1, siAkt2, and siAkt3; pSU = empty vector) and subsequent enrichment of strongly transfected cells. MM.1S cells display a constitutive phospho-Akt signal, whereas AMO-1 cells do not. AMO-1 cells are devoid of Akt3 protein. Staining of α-tubulin was used as loading control. (B) Western analysis of the molecular effects of combined knockdown of Akt1 and Akt2 or of all 3 isoforms in AMO-1 and MM.1S cells. Shown is the down-regulation of single Akt isoforms, its effect on the total level of Akt protein (pan-Akt), on the level of activated Akt protein (phospho-Akt [Ser473]), and on the amount of phosphorylated Akt substrate FoxO1/3a. (C) Viability of MM cells transfected with (combinations of) Akt knockdown constructs. Purified populations of transfected cells were cocultured with BMSCs and their survival determined by annexin V–ATTO 647/PI staining 6 days after electroporation. The graph comprises the results from 3 independent experiments. The bars display the percentage of viable cells for the respective Akt knockdown constructs compared with identically treated empty vector controls. Error bars mark standard deviations. P values were determined by 1-tailed unpaired Student t tests (*P < .01; **P = .04).
SiRNA knockdown of Akt isoforms in MM cell lines. (A) Specificity of Akt isoform knockdown in MM cell lines AMO-1 and MM.1S as determined by Western analysis 3 days after electroporation with pSUPER-derived siRNA expression vectors (siAkt1, siAkt2, and siAkt3; pSU = empty vector) and subsequent enrichment of strongly transfected cells. MM.1S cells display a constitutive phospho-Akt signal, whereas AMO-1 cells do not. AMO-1 cells are devoid of Akt3 protein. Staining of α-tubulin was used as loading control. (B) Western analysis of the molecular effects of combined knockdown of Akt1 and Akt2 or of all 3 isoforms in AMO-1 and MM.1S cells. Shown is the down-regulation of single Akt isoforms, its effect on the total level of Akt protein (pan-Akt), on the level of activated Akt protein (phospho-Akt [Ser473]), and on the amount of phosphorylated Akt substrate FoxO1/3a. (C) Viability of MM cells transfected with (combinations of) Akt knockdown constructs. Purified populations of transfected cells were cocultured with BMSCs and their survival determined by annexin V–ATTO 647/PI staining 6 days after electroporation. The graph comprises the results from 3 independent experiments. The bars display the percentage of viable cells for the respective Akt knockdown constructs compared with identically treated empty vector controls. Error bars mark standard deviations. P values were determined by 1-tailed unpaired Student t tests (*P < .01; **P = .04).
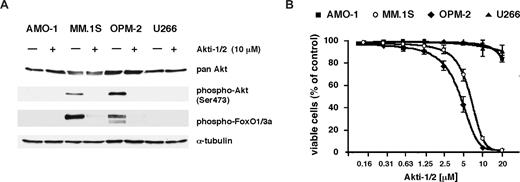
Treatment with Akt1/2 inhibitor Akti-1/2 is preferentially toxic for phospho-Akt–positive MM cells. (A) Western analysis of AMO-1, MM.1S, OPM-2, and U266 cells treated overnight with either 10 μM Akti-1/2 or a corresponding amount of solvent (DMSO). Whereas the levels of total Akt protein remained unaffected, phosphorylated forms of Akt and its substrate FoxO1/3a were strongly diminished in MM.1S and OPM-2 cells. (B) Viability of AMO-1, MM.1S, OPM-2, and U266 cells in coculture with BMSCs after 5 days of treatment with different concentrations of Akti-1/2. Survival was determined by annexin V–FITC/PI staining and expressed in relation to the viability of DMSO-treated control cells. The graph displays the results from 3 independent experiments; error bars mark standard deviations.
Treatment with Akt1/2 inhibitor Akti-1/2 is preferentially toxic for phospho-Akt–positive MM cells. (A) Western analysis of AMO-1, MM.1S, OPM-2, and U266 cells treated overnight with either 10 μM Akti-1/2 or a corresponding amount of solvent (DMSO). Whereas the levels of total Akt protein remained unaffected, phosphorylated forms of Akt and its substrate FoxO1/3a were strongly diminished in MM.1S and OPM-2 cells. (B) Viability of AMO-1, MM.1S, OPM-2, and U266 cells in coculture with BMSCs after 5 days of treatment with different concentrations of Akti-1/2. Survival was determined by annexin V–FITC/PI staining and expressed in relation to the viability of DMSO-treated control cells. The graph displays the results from 3 independent experiments; error bars mark standard deviations.
Pharmacologic inhibition of Akt1 and Akt2 mimics the apoptotic effects of siRNA-mediated Akt knockdown in MM cells
For technical reasons the analysis of Akt signaling in primary MM samples relies on pharmacologic inhibitors. We therefore explored the utility and biologic consequences of Akt inhibition with Akti-1/2, a novel small-molecule inhibitor selective for Akt1 and Akt2, in 4 MM cell lines, including the 2 cell lines for which siRNA-mediated Akt knockdown had been performed. Akti-1/2 is an allosteric inhibitor specific for Akt1 and Akt2 that down-regulates phosphorylation and activation of these isozymes in cultured cells and in mice.23 It is assumed that binding of Akti-1/2 leads to the formation of an inactive conformation where Akt cannot be phosphorylated by upstream kinases, and that this process is pleckstrin homology domain dependent.22,23 Treatment with 10 μM Akti-1/2 abolished phosphorylation of Akt and of FoxO1/3a in both cell lines that displayed a constitutive phospho-Akt signal (MM.1S and OPM-2; Figure 2A), and induced cell death with half-maximally effective concentrations of 7 μM (MM.1S) and 4 μM (OPM-2), respectively. Conversely, the viability of AMO-1 and U266 cells was largely unaffected at concentrations of up to 20 μM (Figure 2B). These results mirror the effects of siRNA-mediated Akt knockdown in AMO-1 and MM.1S cells and strongly support the notion that the biologic effects of Akti-1/2 on MM cells are indeed due to its specificity for Akt1/2.
Treatment with the Akt-inhibitor Akti-1/2 down-regulates Akt activation in primary MM cells and induces apoptosis
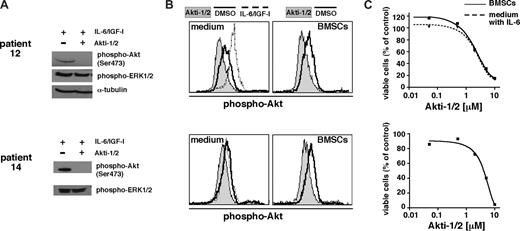
Next, we analyzed Akt activation in primary MM cells and the effect of Akti-1/2 on their viability. Freshly isolated CD138+ cells were treated with 10 μM Akti-1/2 and survival was determined after 5 days. Effectivity of Akti-1/2 to block Akt signaling was tested by Western analysis of primary cells stimulated for 15 minutes with IL-6 and IGF-1 to generate a maximal phospho-Akt signal (n = 4). In addition, differences in the relative levels of phospho-Akt were determined by phospho-Akt–specific flow cytometry of primary MM cells kept either with or without the presence of BMSCs (n = 20). Western analyses confirmed that IL-6– and IGF-1–mediated activation of Akt was blocked by the inhibitor (Figure 3A). We observed that Akti-1/2 inhibited Akt phosphorylation in primary MM cells in the absence or presence of BMSCs (Figure 3B). Akti-1/2 thus effectively abrogated Akt activity in different culture settings and also in the presence of stimulating cytokines. Both patient tumor samples shown in Figure 3 underwent apoptosis after Akti-1/2 treatment. Coculture with BMSCs did not protect Akti-1/2–sensitive primary MM cells from drug-induced apoptosis (n = 4).
Akti-1/2 inhibits Akt phosphorylation in primary MM cells and induces apoptosis in the presence or absence of BMSCs. Shown are 2 examples of biochemical analysis of the effects of Akti-1/2 on primary MM cells. (A) Western analysis of freshly purified CD138+ MM cells (200 000 per lane) treated overnight with either DMSO or Akti-1/2 (10 μM). At 15 minutes before harvest, cells were stimulated with a combination of IL-6 and IGF-1 to show inducibility of activated Akt and lack of this effect in cells treated with the Akt inhibitor. Phospho-ERK1/2, which was always unchanged by Akti-1/2 treatment in primary MM cells or in MM cell lines, serves as surrogate loading control for the blot for patient 14. (B) Phospho-Akt–specific flow cytometry for the same primary samples as in panel A. A total of 50 000 cells per well were kept for a total of 24 hours in medium alone or in coculture with BMSCs. They were then treated overnight with Akti-1/2 (10 μM) or DMSO. Primary MM cells stimulated with IL-6 and IGF-1 for 15 minutes served as positive control. (C) Cytotoxic effect of Akti-1/2 treatment on the same primary MM cells as shown in panels A and B. Freshly purified MM cells were kept overnight either in medium supplemented with 2 ng/mL IL-6 or in the presence of BMSCs. They were then exposed to different concentrations of Akti-1/2 and their viability was measured after 5 days by annexin V–FITC/PI staining and flow cytometry.
Akti-1/2 inhibits Akt phosphorylation in primary MM cells and induces apoptosis in the presence or absence of BMSCs. Shown are 2 examples of biochemical analysis of the effects of Akti-1/2 on primary MM cells. (A) Western analysis of freshly purified CD138+ MM cells (200 000 per lane) treated overnight with either DMSO or Akti-1/2 (10 μM). At 15 minutes before harvest, cells were stimulated with a combination of IL-6 and IGF-1 to show inducibility of activated Akt and lack of this effect in cells treated with the Akt inhibitor. Phospho-ERK1/2, which was always unchanged by Akti-1/2 treatment in primary MM cells or in MM cell lines, serves as surrogate loading control for the blot for patient 14. (B) Phospho-Akt–specific flow cytometry for the same primary samples as in panel A. A total of 50 000 cells per well were kept for a total of 24 hours in medium alone or in coculture with BMSCs. They were then treated overnight with Akti-1/2 (10 μM) or DMSO. Primary MM cells stimulated with IL-6 and IGF-1 for 15 minutes served as positive control. (C) Cytotoxic effect of Akti-1/2 treatment on the same primary MM cells as shown in panels A and B. Freshly purified MM cells were kept overnight either in medium supplemented with 2 ng/mL IL-6 or in the presence of BMSCs. They were then exposed to different concentrations of Akti-1/2 and their viability was measured after 5 days by annexin V–FITC/PI staining and flow cytometry.
Primary MM cells that display constitutive Akt activation are more sensitive to Akti-1/2 treatment
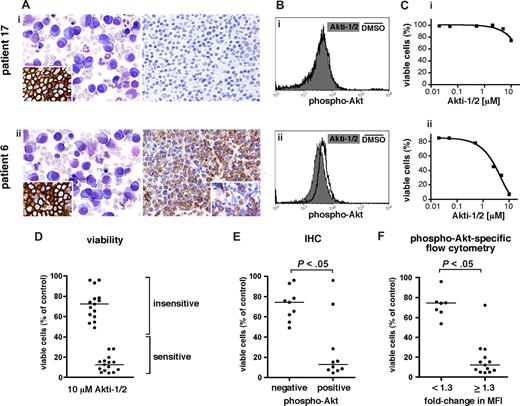
For several primary myeloma samples, we investigated the effects of Akti-1/2–mediated Akt inhibition in freshly isolated tumor cells in relation to the presence of Akt phosphorylation in situ as detected by IHC in formaldehyde-fixed, paraffin-embedded bone marrow biopsies, or Akt phosphorylation in vitro as determined by phospho-Akt–specific flow cytometry. The results for 2 different MM cases are exemplarily depicted in Figure 4A through C. Both samples displayed similar prominence of abundant atypical plasma cells with strong CD138 positivity (Figure 4A). However, only one of these samples was positive for phospho-Akt, with strong staining in confluent plasmacytoma infiltrates (Figure 4A; patient 6) and in scattered intermixed myeloma cells (inset). No phospho-Akt signal was detected for patient 17. Phospho-Akt–specific flow cytometry to probe shifts of Akt activity (Figure 4B) was performed with MM cells isolated from both patients. Tumor cells were treated overnight with 10 μM Akti-1/2 or with DMSO. Treatment with the inhibitor led to a prominent downward MFI shift for phospho-Akt in cells from patient 6, whereas no such shift was detectable in cells from patient 17, confirming that Akt phosphorylation was present only in the former. Consistent with these results, treatment with 10 μM Akti-1/2 for 5 days was toxic for tumor cells from patient 6 but had little effect on the viability of cells from patient 17.
Akt is activated in a subset of primary MM samples and this determines their vulnerability to pharmacologic Akt inhibition. (A-C) IHC, phospho-Akt–specific flow cytometry, and susceptibility to pharmacologic Akt inhibition in 2 exemplary primary MM samples. (Ai,ii) Immunohistochemical detection of phospho-Akt in bone marrow biopsies heavily infiltrated by MM cells (right panels). Giemsa stained overview and IHC staining for plasma-cell marker CD138 (inset) shown to the left. Original magnifications, 400× (left) and 200× (right and insets). (Bi,ii) Phospho-Akt–specific flow cytometry in freshly purified CD138+ MM cells from the same patients as in panel A. A total of 50 000 cells per well were kept for a total of 24 hours in medium alone. They were then treated overnight with Akti-1/2 (10 μM) or DMSO. (Ci,ii) Viability of freshly purified CD138+ MM cells from the same patients as in panels A and B after treatment with Akti-1/2 for 5 days. Cells were cocultured with BMSCs and survival was determined by annexin V–FITC/PI staining and flow cytometry. The percentage of viable cells reflects comparison to DMSO-treated controls. Of note, whereas the Akti-1/2 resistant sample (patient 17) did not display Akt phosphorylation, the Akti-1/2–sensitive case (patient 6) showed strong phospho-Akt signals. (D) Effect plot of all primary MM samples tested (n = 30) for their susceptibility to treatment with 10 μM Akti-1/2. Viability determined as described in panel C. The medians for the strongly sensitive and the more resilient/resistant groups are indicated. (E) Correlation of Akt activation in BM biopsies with viability of MM cells from the corresponding patient after treatment with Akti-1/2 (n = 19). Viability was determined as described in panel C. (F) Correlation of the fold change in MFI for phospho-Akt in primary MM cells with their viability after treatment with Akti-1/2 (n = 20). Measurements performed as described in panels B and C. Horizontal bars mark the medians.
Akt is activated in a subset of primary MM samples and this determines their vulnerability to pharmacologic Akt inhibition. (A-C) IHC, phospho-Akt–specific flow cytometry, and susceptibility to pharmacologic Akt inhibition in 2 exemplary primary MM samples. (Ai,ii) Immunohistochemical detection of phospho-Akt in bone marrow biopsies heavily infiltrated by MM cells (right panels). Giemsa stained overview and IHC staining for plasma-cell marker CD138 (inset) shown to the left. Original magnifications, 400× (left) and 200× (right and insets). (Bi,ii) Phospho-Akt–specific flow cytometry in freshly purified CD138+ MM cells from the same patients as in panel A. A total of 50 000 cells per well were kept for a total of 24 hours in medium alone. They were then treated overnight with Akti-1/2 (10 μM) or DMSO. (Ci,ii) Viability of freshly purified CD138+ MM cells from the same patients as in panels A and B after treatment with Akti-1/2 for 5 days. Cells were cocultured with BMSCs and survival was determined by annexin V–FITC/PI staining and flow cytometry. The percentage of viable cells reflects comparison to DMSO-treated controls. Of note, whereas the Akti-1/2 resistant sample (patient 17) did not display Akt phosphorylation, the Akti-1/2–sensitive case (patient 6) showed strong phospho-Akt signals. (D) Effect plot of all primary MM samples tested (n = 30) for their susceptibility to treatment with 10 μM Akti-1/2. Viability determined as described in panel C. The medians for the strongly sensitive and the more resilient/resistant groups are indicated. (E) Correlation of Akt activation in BM biopsies with viability of MM cells from the corresponding patient after treatment with Akti-1/2 (n = 19). Viability was determined as described in panel C. (F) Correlation of the fold change in MFI for phospho-Akt in primary MM cells with their viability after treatment with Akti-1/2 (n = 20). Measurements performed as described in panels B and C. Horizontal bars mark the medians.
In total, apoptosis measurements after Akti-1/2 treatment were performed for 30 different primary samples. They segregated into 2 groups of approximately equal numbers that were either very sensitive to Akti-1/2 treatment (less than 40% survival compared with DMSO-treated controls) or more resilient or even resistant to Akt inhibition (Figure 4D). For 26 of these samples, IHC staining for phospho-Akt (n = 19; Figure 4E), and/or phospho-Akt–specific flow cytometry (n = 20; Figure 4F), was performed. Correlation of IHC positivity for phospho-Akt with sensitivity to treatment with Akti-1/2 showed that of the 10 samples that displayed phospho-Akt staining, 8 underwent strong apoptosis after Akt inhibition (Figure 4E). Conversely, all primary samples that were IHC-negative for Akt phosphorylation fell into the Akti-1/2 resilient/resistant subgroup.
Phospho-Akt–specific flow cytometry showed presence of phosphorylated Akt in 13 of the 20 primary samples thus analyzed (Figure 4F). Of these samples, 12 were sensitive to Akti-1/2 treatment, whereas all of the 7 samples devoid of Akt phosphorylation displayed Akti-1/2 resistance. In summary, these results showed that 15 (58%) of 26 primary samples displayed activated Akt as assessed by IHC and/or phospho-Akt–specific flow cytometry, and that 13 of these were strongly sensitive to pharmacologic inhibition of Akt. Samples without detectable levels of phospho-Akt were invariably less sensitive or even insensitive to treatment with Akti-1/2.
MM cells resilient to Akt inhibition are often unaffected by additional inhibition of STAT3 and MAPK
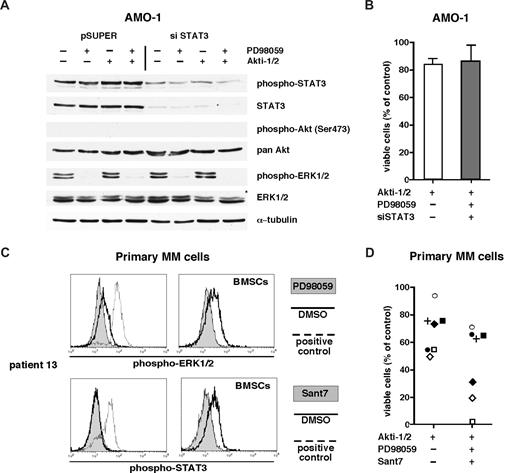
In addition to the PI3K/Akt pathway, the STAT3 and MAPK pathways have been implicated in MM cell survival. We therefore tested whether the combined blockade of all 3 pathways would lead to an increase in apoptosis of MM cells resistant to Akt inhibition. Knockdown of STAT3 in AMO-1 cells was combined with pharmacologic inhibition of MEK1/2 with PD98059 and of Akt with Akti-1/2 (Figure 5A). The viability of the cells was not affected by this treatment (Figure 5B). A total of 7 Akt resilient/resistant primary MM samples were also subjected to the triple pathway blockade. Treatment with Sant7 or PD98059 effectively down-regulated the levels of phospho-STAT3 and phospho-ERK1/2 in primary MM cells cocultured with BMSCs (exemplary depiction of 1 primary sample in Figure 5C), but in 4 of 7 samples the combined inhibition of Akt, MEK, and STAT3 hardly increased apoptosis beyond the level of Akti1/2 treatment alone (Figure 5D). Thus, there appear to be patients with MM who are quite resistant even to the combined blockade of 3 major signaling pathways commonly implicated in the growth and survival of MM cells.
MM cells resistant to Akt inhibition can also be unresponsive to blockade of other survival pathways. (A) Western analysis of signaling pathway components in AMO-1 cells after knockdown of STAT3 and additional inhibition of the Ras/MAPK pathway with PD98059 (50 μM) and of Akt with Akti-1/2 (10 μM) (pSUPER = control vector, siSTAT3 = siRNA expression construct26 ). Transfected cells were purified 1 day after electroporation, cultured together with BMSCs, and treated with PD98059, Akti-1/2, or both. The next day, cells were harvested for Western blotting, and the same material was used to run 2 different gels to permit unequivocal staining of phosphorylated versus total protein. Only 1 of 2 identical tubulin controls is shown for clarity. (B) Viability of pSUPER-transfected AMO-1 cells after treatment with 10 μM Akti-1/2 and of siSTAT3-transfected cells additionally treated with 10 μM Akti-1/2 and 50 μM PD98059. Viability was assayed at day 5 of drug treatment; survival is calculated as the percentage of live cells compared with the pSUPER control sample. Cells were cocultured with BMSCs, and the graph displays the results from 3 independent experiments. Error bars mark standard deviations. (C) Signaling analysis of primary MM cells after treatment with Sant7 and PD98059. A total of 5 × 104 primary MM cells were kept in medium alone or in coculture with BMSCs for 24 hours. They were then treated overnight with Sant7 (50 μg/mL) or with an equivalent amount of buffer and changes in their phospho-STAT3 level were measured. Primary MM cells stimulated with 25 ng/mL IL-6 for 15 minutes served as positive control. Similarly, phospho-ERK1/2 signals were blocked with PD98059 (50 μM) and induced with PMA. (D) Viability of Akti-1/2–resistant/resilient primary MM cells (n = 7; individually coded) after additional blockade of the IL-6R/STAT3 and Ras/MAPK pathways. Freshly isolated MM cells were cocultured with BMSCs and incubated with Akti-1/2 (10 μM) or, in addition, with PD98059 (50 μM) and Sant7 (50 μg/mL). Viability was measured on day 5. Cells treated with DMSO served as control.
MM cells resistant to Akt inhibition can also be unresponsive to blockade of other survival pathways. (A) Western analysis of signaling pathway components in AMO-1 cells after knockdown of STAT3 and additional inhibition of the Ras/MAPK pathway with PD98059 (50 μM) and of Akt with Akti-1/2 (10 μM) (pSUPER = control vector, siSTAT3 = siRNA expression construct26 ). Transfected cells were purified 1 day after electroporation, cultured together with BMSCs, and treated with PD98059, Akti-1/2, or both. The next day, cells were harvested for Western blotting, and the same material was used to run 2 different gels to permit unequivocal staining of phosphorylated versus total protein. Only 1 of 2 identical tubulin controls is shown for clarity. (B) Viability of pSUPER-transfected AMO-1 cells after treatment with 10 μM Akti-1/2 and of siSTAT3-transfected cells additionally treated with 10 μM Akti-1/2 and 50 μM PD98059. Viability was assayed at day 5 of drug treatment; survival is calculated as the percentage of live cells compared with the pSUPER control sample. Cells were cocultured with BMSCs, and the graph displays the results from 3 independent experiments. Error bars mark standard deviations. (C) Signaling analysis of primary MM cells after treatment with Sant7 and PD98059. A total of 5 × 104 primary MM cells were kept in medium alone or in coculture with BMSCs for 24 hours. They were then treated overnight with Sant7 (50 μg/mL) or with an equivalent amount of buffer and changes in their phospho-STAT3 level were measured. Primary MM cells stimulated with 25 ng/mL IL-6 for 15 minutes served as positive control. Similarly, phospho-ERK1/2 signals were blocked with PD98059 (50 μM) and induced with PMA. (D) Viability of Akti-1/2–resistant/resilient primary MM cells (n = 7; individually coded) after additional blockade of the IL-6R/STAT3 and Ras/MAPK pathways. Freshly isolated MM cells were cocultured with BMSCs and incubated with Akti-1/2 (10 μM) or, in addition, with PD98059 (50 μM) and Sant7 (50 μg/mL). Viability was measured on day 5. Cells treated with DMSO served as control.
Discussion
Previous studies have suggested a role for the PI3K/Akt pathway for malignant growth and survival of MM cells and pharmacologic intervention with this pathway induced cell death in MM cell lines and primary tumor samples.16,20,21 In BM biopsies from patients with MM phosphorylated Akt has been observed, and this activation may result from BM microenvironment–derived cytokines, such as IL-6 or IGF-1, or from monoallelic deletion of the gene for the Akt-inhibitory phosphatase PTEN.17-19 However, most of the latter data has been accrued through the use of small-molecule inhibitors that are known not to target Akt directly. Thus, the use of PI3K inhibitors abrogates all signaling downstream of PI3K, Akt included, and likewise, a class of novel alkylphospholipids (eg, perifosine) blocks Akt phosphorylation in conjunction with several other effects.33-35 Accordingly, it is still unclear to what extent Akt contributes to the viability of MM cells.
We therefore conducted siRNA-mediated knockdown of Akt1, Akt2, and Akt3 and assessed their contribution to the viability of MM cells that constitutively display Akt phosphorylation (cell line MM.1S) or that are devoid of activated Akt (cell line AMO-1). Whereas AMO-1 cells, which did not contain detectable levels of Akt3, remained completely unaffected by any combination of Akt-isoform depletion, survival of MM.1S cells was strongly impaired by knockdown of either Akt1 or Akt2, and was slightly compromised by knockdown of Akt3. Akt3 depletion did not notably affect the total level of phospho-Akt in MM.1S. A prosurvival role for Akt3 has been reported in some tumor entities, but this was associated with strong protein expression.14 These results imply that in MM Akt1 and Akt2 may well be the most important isoforms.
We therefore used Akti-1/2, a novel allosteric small-molecule inhibitor specific for Akt1 and Akt2 to perform functional analyses with MM cell lines and primary tumor cells.22-24 Isozyme-specific inhibitors might help to identify therapy-relevant Akt isoforms and thus to explore less-toxic routes to the clinical use of Akt blockade.36
MM cell lines with constitutive Akt phosphorylation (MM.1S, OPM-2) were efficiently driven into apoptosis by Akti-1/2, whereas those devoid of phospho-Akt (AMO-1, U266) were insensitive. These effects match the Akt1 and Akt2 knockdown results in the transfectable cell lines AMO-1 and MM.1S and confirm that the antimyeloma activity of Akti-1/2 in cell lines and, by implication, in primary MM cells, appears to be a true consequence of Akt1 and/or Akt2 blockade.
Treatment of primary MM samples with Akti-1/2 showed that a substantial measure of heterogeneity exists in the sensitivity of myeloma cells toward Akt inhibition. Whereas the drug was essentially lethal for approximately half of the primary MM samples tested, the remainder was clearly more resilient or even resistant. Because we attempted to elucidate the role of Akt activation in primary MM cells, samples (cell numbers permitting) were simultaneously analyzed for cell death and probed for the presence of constitutive Akt activation by phospho-Akt–specific flow cytometry. When available, bone marrow biopsies of the respective patients were immunohistochemically stained for phospho-Akt. A strong correlation was found between the phospho-Akt status in situ and presence of a phospho-Akt signal in vitro. MM samples for which a phospho-Akt signal could be confirmed were usually very sensitive for Akti-1/2–mediated apoptosis, whereas phospho-Akt–negative samples were always resilient or resistant. Lack of a clearly detectable phospho-Akt signal therefore appears to be predictive for resistance to pharmacologic Akt inhibition. Furthermore, our analysis suggests the existence of 2 functional myeloma subgroups: those with silent Akt, that do not depend on activated Akt for survival; and those with activated Akt, in which pharmacologic blockade leads to cell death.
Several genetic lesions have been described that can lead to increased activity of Akt. Thus, monoallelic deletion of PTEN causes higher levels of phosphatidylinositol (3,4,5)-trisphosphate, which is important for the placement of Akt near its upstream kinase(s). PTEN deletions are found in 20% of MM cell lines (eg, in OPM-2) or plasma cell leukemias, but are less frequent in patients with intramedullary MM (5%),37 and therefore seem to be rare events in MM. We did not detect PTEN deletions in 8 primary samples tested, 4 of which displayed constitutively activated Akt (Table S1), or in the cell line MM.1S. PTEN deficiency is therefore unlikely to account for the high proportion of phospho-Akt–positive primary samples.
Because other signaling pathways also play a role for the survival and growth of MM cells, we tested if additional blockade of the IL-6R/STAT3 and the Ras/MAPK pathway leads to apoptosis of Akt-independent myeloma cells. Single or combined disruption of these pathways in AMO-1 cells did not appreciably compromise their survival, and 4 of 7 primary samples resilient to the Akt inhibitor were not further affected by additional pathway blockade. Thus, survival of some MM cells was unaffected even by the combined blockade of 3 prominent oncogenic pathways. This heterogeneity in tumor cell signaling might reflect the genetic variability of the disease and underscores that different signaling events are involved in the pathogenesis and in disease progression of MM.
The presence of Akt-dependent and Akt-independent MM could have important implications for the development of future therapies. One important aspect is that functional signaling analyses might help to guide the treatment of patients with novel molecular (targeted) therapies. Another issue is to appraise the status of phospho-Akt as a prognostic marker and to find out if this might help to predict whether or not a patient will benefit from a certain therapy. Thus, MM subgroups defined by functional signaling analysis could become novel prognostic markers for a better stratification of disease management.
In summary, this paper represents the first comprehensive signaling analysis for the Akt pathway in MM and identifies novel functionally defined MM subgroups. Future studies are intended to elucidate the genetic lesions that promote constitutive activation of the Akt pathway. Better knowledge of the molecular characteristics of relevant oncogenic pathways and a more detailed understanding of tumor heterogeneity should then provide a rational basis for the development of targeted therapies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Novartis-Stiftung für Therapeutische Forschung (Nürnberg, Germany) and the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB-TR17).
Authorship
Contribution: A.Z. performed and analyzed experiments and wrote the paper; T.S. analyzed results and wrote the paper; M.C. analyzed results; S.G. and M.A. performed IHC analysis; E.H. performed FISH analysis; H.-K.M.-H. and A.G. contributed to experimental designs and data analysis; C.W., J.C.R., and H.E. provided patient samples; and R.C.B. initiated, designed, and supervised research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thorsten Stühmer, University Hospital of Würzburg, Department of Internal Medicine II, Josef-Schneider-Straße 2, 97080 Würzburg, Germany; e-mail: stuehmer_t@medizin.uni-wuerzburg.de.

![Figure 1. SiRNA knockdown of Akt isoforms in MM cell lines. (A) Specificity of Akt isoform knockdown in MM cell lines AMO-1 and MM.1S as determined by Western analysis 3 days after electroporation with pSUPER-derived siRNA expression vectors (siAkt1, siAkt2, and siAkt3; pSU = empty vector) and subsequent enrichment of strongly transfected cells. MM.1S cells display a constitutive phospho-Akt signal, whereas AMO-1 cells do not. AMO-1 cells are devoid of Akt3 protein. Staining of α-tubulin was used as loading control. (B) Western analysis of the molecular effects of combined knockdown of Akt1 and Akt2 or of all 3 isoforms in AMO-1 and MM.1S cells. Shown is the down-regulation of single Akt isoforms, its effect on the total level of Akt protein (pan-Akt), on the level of activated Akt protein (phospho-Akt [Ser473]), and on the amount of phosphorylated Akt substrate FoxO1/3a. (C) Viability of MM cells transfected with (combinations of) Akt knockdown constructs. Purified populations of transfected cells were cocultured with BMSCs and their survival determined by annexin V–ATTO 647/PI staining 6 days after electroporation. The graph comprises the results from 3 independent experiments. The bars display the percentage of viable cells for the respective Akt knockdown constructs compared with identically treated empty vector controls. Error bars mark standard deviations. P values were determined by 1-tailed unpaired Student t tests (*P < .01; **P = .04).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/8/10.1182_blood-2007-11-119362/4/m_zh80190824850001.jpeg?Expires=1766325506&Signature=tp6r1-jjSoLdDBwBR03lSSdk6eY7hbJY8O9niXrXvEjIPRkZvYavjwyqGwZAeN1lfAk0VPJBs1WbdQ6jrOUOMUzS4i3AswbtpuKfVhi5f9QbIkApBbzo4IL3s-dwKRIsHwO2xT60yizfjE0SlLZBUJ9jIxrZwDloCuOML6IdRtLagDeJAl9A3O3fq2ZZh0~41YMzYv3dqpGtw0F3VNoMZu4Ze5G49VHmsJBE3XBeet6iftbBVgViafpcV24AdgR4YNiw3rJ-quOGZl2c1W8L3ysXBfA6FHq2SChATR9fj0wVbg67czO6Ndky9-yPzgQQ9TVrZunZdMbjQ6HWrJbihA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)