Abstract
Through the activity of macrophage-specific matrix metalloproteinase-12 (MMP-12), we found that macrophages dampen the lipopolysaccharide (LPS)-induced influx of polymorphonuclear leukocytes (PMNs)—thus providing a new mechanism for the termination of PMN recruitment in acute inflammation. MMP-12 specifically cleaves human ELR+ CXC chemokines (CXCL1, -2, -3, -5, and -8) at E-LR, the critical receptor-binding motif or, for CXCL6, carboxyl-terminal to it. Murine (m) MMP-12 also cleaves mCXCL1, -2, and -3 at E-LR. MMP-12-cleaved mCXCL2 (macrophage-inflammatory protein-2 [MIP-2]) and mCXCL3 (dendritic cell inflammatory protein-1 [DCIP-1]) lost chemotactic activity. Furthermore, MMP-12 processed and inactivated monocyte chemotactic proteins CCL2, -7, -8, and -13 at position 4-5 generating CCR antagonists. Indeed, PMNs and macrophages in bronchoalveolar lavage fluid were significantly increased 72 hours after intranasal instillation of LPS in Mmp12−/− mice compared with wild type. Specificity occurred at 2 levels. Macrophage MMP-1 and MMP-9 did not cleave in the ELR motif. Second, unlike human ELR+CXC chemokines, mCXCL5 (LPS-induced CXC chemokine [LIX]) was not inactivated. Rather, mMMP-12 cleavage at Ser4-Val5 activated the chemokine, promoting enhanced PMN early infiltration in wild-type mice compared with Mmp12−/− mice 8 hours after LPS challenge in air pouches. We propose that the macrophage, specifically through MMP-12, assists in orchestrating the regulation of acute inflammatory responses by precise proteolysis of ELR+CXC and CC chemokines.
Introduction
Acute inflammation is the host response to tissue injury or infection that is characterized by the production of inflammatory mediators, culminating in the initial but transient recruitment of polymorphonuclear leukocytes (PMNs) that is followed by a prolonged macrophage accumulation.1 However, the underlying, multifactorial mechanisms that shape the extent and kinetics of PMN and macrophage recruitment, apoptosis, and clearance remain unclear. Chemokines are an important class of chemoattractant cytokines produced locally in tissues that provide the directional cues for the movement of blood-derived leukocytes in development, homeostasis, and inflammation.2 These potent chemoattractants are classified according to the position and spacing of their N-terminal cysteine residues, with 46 human chemokines currently divided into 4 families: C, CC, CXC, and CX3C.3
The initial phase of inflammation involves a subset of CXC chemokines, which rapidly attract PMNs.4 These PMN chemoattractants contain a conserved Glu-Leu-Arg (ELR) motif proximal to the CXC sequence, which is critical in cognate receptor binding and activation,5 as well as PMN chemotaxis.6 In humans, there are 7 ELR+ CXC chemokines: CXCL1, -2, and -3, also known as growth-related oncogenes α, β, and γ, respectively; CXCL5/epithelial cell–derived neutrophil activating peptide-78 (ENA-78); CXCL6/granulocyte chemotactic protein-2 (GCP-2); CXCL7/neutrophil-activating peptide-2 (NAP-2); and CXCL8/interleukin-8 (IL-8). All bind the CXC-receptor (CXCR) 1; CXCL6 and -8 also signal through CXCR2.7 Mice lack complete homologs of the 7 human ELR+ chemokines, having only 4: mCXCL1/keratinocyte-derived chemokine (KC); mCXCL2/macrophage-inflammatory protein-2 (MIP-2); the more recently described mCXCL3/dendritic cell inflammatory protein-1 (DCIP-1)8 ; and mCXCL5/lipopolysaccharide (LPS)–induced CXC chemokine (LIX).9 All bind a single receptor that is homologous to human CXCR2.10
After the initial PMN influx, the next stage of inflammation is directed in part by CC chemokines consisting of CCL2/monocyte chemoattractant protein (MCP)-1, CCL7/MCP-3, CCL8/MCP-2, and CCL13/MCP-4, which target multiple leukocyte subsets (monocytes, T lymphocytes, basophils, and eosinophils).11 Monocytes can differentiate into macrophages whose role is to phagocytose and degrade microorganisms and foreign material and to present these antigens to initiate specific immune responses. In addition, macrophages ingest apoptotic PMNs from the inflamed site as a prelude to tissue resolution.12 However, apoptosis alone cannot account for the entirety of the reduction in PMN numbers, because the potential for continued recruitment and replacement.
Resident mast cells, macrophages, and epithelial cells have been proposed to produce the initial signals responsible for the accumulation of PMNs, eosinophils, and mononuclear cells in experimental models of inflammation by secretion of chemokines such as mCXCL1 and mCCL3.13 Modulation of specific ELR+ chemokines by proteolytic processing is critical for amplification of the PMN influx. Matrix metalloproteinase (MMP)–9, a protease common to PMNs, macrophages, and endothelial cells,14 cleaves CXCL1 and increases the chemotactic activity of CXCL8 10-fold by amino-terminal processing.15 More significantly, secretion of neutrophil-specific MMP-8 by PMNs is a nonredundant mechanism, which initiates LPS responsiveness in vivo by N-terminal cleavage of mCXCL5 in mice, and hCXCL5 and hCXCL8 in man.16 However, little is known about the signals or mechanisms that abrogate PMN influx. Is it a passive rundown of chemoattractant signals or an active process to terminate the proinflammatory chemokine signaling, and thus PMN influx?
Matrix metalloproteinases are a family of endopeptidases that dogmatically are thought to collectively degrade all components of the extracellular matrix.17 However, it has been shown recently that MMPs have a much wider substrate repertoire, or degradome, with specific processing of bioactive molecules rather than matrix protein degradation to completion being their most important in vivo role.18-22 In addition to MMP-8 and -9 modulating ELR+ CXC chemokine activity, gelatinase A (MMP-2) was the first MMP shown to regulate chemokine activity by N-terminally truncating CCL7 and so dampening inflammation.22 Subsequently, we reported that all MCPs (CCL2, -7, -8, and -13) were processed by multiple MMPs to generate potent antagonists,11 and that CXCL12 was inactivated by specific MMP cleavage at position 4-5,23 leading to a switch in receptor recognition.24 The membrane-associated CX3CLI chemokine (fractalkine) is also shed by MMP-2 as a soluble receptor antagonist.19 Together these studies highlight both proinflammatory,15,16,20 and importantly the anti-inflammatory roles for MMPs,11,22 as well as recent evidence for their role in proteolytic regulation of Th1 lymphocyte chemoattraction through processing of CXCL11.25
Macrophage metalloelastase (MMP-12) is a 54-kDa elastinolytic protease mainly expressed by macrophages and is associated with numerous pathologic conditions, such as aortic aneurysm formation,26 atherosclerosis,27 emphysema,28 and rheumatoid arthritis.29 The enzyme rapidly loses its hemopexin C-domain by autolysis shortly after activation without loss of its elastin-degrading function.30 It is noteworthy that the hemopexin C-domain has antimicrobial activity in the macrophage.31 In addition, MMP-12 has potent extracellular matrix remodeling properties32 but may also participate in the inflammatory response through the activation of tumor necrosis factor-α.33 Other proinflammatory properties of MMP-12 include the ability to induce neutrophil recruitment,34,35 and cytokine and chemokine production,34 but the substrates and mechanistic aspects of these important biologic functions are unknown.
Here we characterize the proteolytic processing of chemokines by MMP-12 identifying both activating and inactivating cleavages of every human and murine ELR+ CXC and human CC macrophage selective chemokines. Using 2 mouse models of acute inflammation, we demonstrate both an early proinflammatory function of MMP-12 in the recruitment of PMNs and a late anti-inflammatory property that abrogates both the PMN and macrophage influx. Hence, MMP-12 is heavily involved in orchestrating the regulation of the cellular activity of the acute inflammatory response independent of any matrix degradative activity.
Methods
Recombinant human MMP-12 protein expression and purification
The pET 5b vector containing full-length human MMP12 cDNA was a kind gift from Dr S. Shapiro (University of Pittsburgh, PA). It was transformed into Escherichia coli strain BL21 Gold and cultures for protein production were grown in Lennox Broth and ampicillin (100 μg/mL) at 37°C. High-level expression of human MMP-12 was induced with 1 mmol/L isopropyl-β-d-thiogalactopyranoside when the culture OD595 reached 0.5 nm and then the cells were harvested by centrifugation (7000g, 10 min-utes at 4°C) 4 hours after induction.
The resuspended cell pellet (50 mmol/L Tris, pH 8.0, 10 mmol/L CaCl2, and 150 mmol/L NaCl) was lysed by 5 × 10-second sonication (VibraCell; Sonics & Materials, Danbury, CT) on ice and after centrifugation (10 000g, 10 minutes at 4°C) MMP-12 was localized in inclusion bodies. These were solubilized in 8 mol/L urea, 50 mmol/L Tris, pH 8.0, 10 mmol/L CaCl2, and 30 mmol/L NaCl by continuous stirring overnight at 4°C. The supernatant was collected after centrifugation (32 000g, 60 minutes at 4°C) and dialyzed with continuous aeration of the buffer, against 3 mol/L urea in Tris buffer (50 mmol/L Tris, pH 8.0, 10 mmol/L CaCl2, and 30 mmol/L NaCl).
Human MMP-12 activity in the dialyzed supernatant was confirmed by cleavage of a quenched fluorescent synthetic MMP peptide substrate (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-[3-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl]-Ala-Arg-NH2. MMP-12 containing samples were applied to Q-Sepharose ion exchange chromatography (GE Healthcare, Chalfont St Giles, United Kingdom) after incubation at 4°C for 3 days. The flow-through was applied to Chelating-Sepharose (GE Healthcare) charged with ZnCl2, and bound protein was eluted with 50 mmol/L imidazole/phosphate-buffered saline (PBS). Eluates were desalted into 50 mmol/L Tris, pH 8.0, 5 mmol/L CaCl2, and 0.05% (w/v) Brij by the use of PD-10 desalting columns (GE Healthcare). 10% Tris-glycine sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) and silver staining confirmed purity of human MMP-12. The identity and molecular mass of the protein was confirmed by Western blotting (MAB919; R&D Systems), Edman sequencing, and matrix-assisted laser desorption ionization/time-of-flight (MALDI-TOF) mass spectrometry (MS) on a Voyager-DE STR Biospectrometry Workstation (Applied Biosystems, Foster City, CA). Concentration of pure MMP-12 was determined by active-site titration against tissue inhibitor of metalloproteinases (TIMP)-2 and the BCA protein assay (Pierce, Rockford, IL).
Recombinant murine MMP-12 protein expression and purification
The pUC9 cloning vector containing full-length murine MMP-12 cDNA was a kind gift from Dr S. Shapiro (University of Pittsburgh, PA). A single-point mutation C948T was introduced into the MMP-12 cDNA by site-directed mutagenesis to remove the NdeI restriction site and the full-length murine Mmp12 gene (1-1389 bp) cloned into the pET 5b vector with the addition of 5′ NheI and 3′ BamHI restriction sites.
Expression and purification of murine MMP-12 was similar to that of human MMP-12. However, additional purification steps were required after Q-Sepharose ion exchange chromatography. Flow-through from the Q-Sepharose column was diluted to a final protein concentration of 0.25 mg/mL with 3 mol/L urea in Tris buffer, and loaded onto a HiTRAP SP-Sepharose ion exchange column (GE Healthcare). Sequential 10-column-volume washes of Tris buffer containing 3, 2, 1, and 0 mol/L urea were applied and MMP-12 was eluted in 0.3 mol/L NaCl, 50 mmol/L Tris, pH 8.0, 5 mmol/L CaCl2, and 0.05% (w/v) Brij. Western blotting (AF3467; R&D Systems) and MALDI-TOF MS confirmed the identity and molecular mass of the protein.
Chemokines and proteinases
Human CCL2/MCP-1, CCL8/MCP-3, CCL7/MCP-2, CCL13/MCP-4, CXCL1/Groα, CXCL2/Groβ, CXCL3/Groγ, CXCL5/ENA-78, CXCL6/GCP-2, CXCL7/NAP-2, and CXCL8/IL-8 and murine CXCL1/KC, CXCL2/MIP-2, CXCL3/DCIP-1, CXCL3(7-73), and CXCL5/LIX were chemically synthesized, purified by high-performance liquid chromatography, and analyzed by mass spectrometry.36 Recombinant human MMP-1 and -9 were expressed and purified as described previously.22
Chemokine cleavage assays
The concentration of active MMP-1, -9, and -12 after p-aminophenylmercuric acetate (APMA) activation (1 mmol/L, 15 minutes) was determined by active-site titration against TIMP-1 or TIMP-2. APMA-activated MMP was incubated with the chemokine at an enzyme/chemokine ratio of 1:10 (w/w), with or without the synthetic hydroxymate MMP inhibitor prinomastat (final concentration, 1 μmol/L), in 50 mmol/L Tris-HCl, pH 7.4, 200 mmol/L NaCl, 5 mmol/L CaCl2, and 0.025% NaN3 for 16 hours, 37°C. Reaction products were analyzed by Tris-Tricine SDS-PAGE and silver stained. The mass of each cleavage product was determined after MALDI-TOF MS. Mass spectrometry data were deconvoluted to identify the substrate cleavage sites.11
PMN isolation
Murine PMNs were isolated from bone marrow as described previously.16 Human PMNs were purified from the pooled buffy coats of blood obtained from healthy volunteers (blood collection protocol approved by the Clinical Research Ethics Board of the University of British Columbia) by a density gradient composed of Histopaque 1077 layered on top of Histopaque 1119 according to manufacturer's instructions (Sigma-Aldrich, St Louis, MO). Contaminating erythrocytes were lysed with one volume hypotonic PBS (3.9 mmol/L KH2PO4 and 4.89 mmol/L Na2HPO4, pH 7.2) for 45 seconds and neutralized with 1 volume of hypertonic PBS (460 mmol/L NaCl, 3.9 mmol/L KH2PO4, and 4.89 mmol/L Na2HPO4, pH 7.2). Lysed erythrocytes were removed by centrifugation (250g, 5 minutes) and PMNs were washed twice with Ca2+/Mg2+-free Hanks balanced salt solution (Sigma-Aldrich).
In vitro chemotaxis assay
Chemotactic assays were performed in 48-well Boyden chambers (NeuroProbe, Gaithersburg, MD) using polycarbonate filters with 3-μm pores. Into the upper chambers were plated human or murine isolated PMNs (4 ×106 cells/mL in RPMI 1640 medium, 20 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], and 0.5% bovine serum albumin [BSA]) and a range of concentrations of the chemokine (0-100 nmol/L in RPMI 1640 medium, 20 mmol/L HEPES, and 0.5% BSA) added to the lower chambers. After incubation at 37°C for 75 minutes, the migrated cells were removed from the lower chambers and lysed by freeze-thawing, and total cell numbers quantified against a standard curve of PMNs using the CyQuant assay according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
Animals
Mmp12−/− mice on a 129/SvEv background37 and wild-type 129/SvEv mice were kindly provided by Dr V. Wee Yong (University of Calgary, Calgary, AB). The Animal Care Committee of the University of British Columbia approved animal breeding and experimental procedures. Six- to 9-week-old mice, segregated according to sex, were used for all experiments.
Subcutaneous PMN chemotaxis in response to LPS in vivo
The air pouch model of PMN chemotaxis was used as described previously.16 Air pouches were raised on the dorsum of mice by subcutaneous injection of 3 mL of sterile air on days 1 and 3. On day 5, 1 mL of LPS (1 μg/mL) or its diluent (PBS) was injected into the air pouch. After 8 hours, the mice were killed by asphyxiation using CO2, and air pouches were lavaged with 2 mL sterile PBS. Total leukocytes in the lavage were counted using a hematocytometer, and the remaining cell suspension lysed with 0.1% Triton X-100 and freeze-thawed. PMN content was determined by myeloperoxidase (MPO) activity using isolated PMN cells as a standard.
Transalveolar chemotaxis of leukocytes in response to LPS in vivo
Mice were anesthetized by isoflurane (Baxter Corporation, Toronto, ON), and 35 μL of LPS (10 μg) dissolved in saline was administered intranasally. At 0, 8, 24, 48, and 72 hours, the mice were killed, the trachea cannulated, and the lungs lavaged 6 times with 0.5 mL of PBS. Contaminating erythrocytes in the bronchoalveolar lavage fluid (BALF) were lysed with 1 volume hypotonic PBS (3.9 mmol/L KH2PO4, 4.89 mmol/L Na2HPO4, pH 7.2) for 30 s and neutralized with 1 volume hypertonic PBS (460 mmol/L NaCl, 3.9 mmol/L KH2PO4, 4.89 mmol/L Na2HPO4, pH 7.2). Lysed erythrocytes were removed by centrifugation (250g, 5 minutes) and total cell counts measured in the BALF with a hematocytometer. Cell differential counts were determined after centrifugation (150g, 5 minutes) (Cytospin 2; Thermo Fisher Scientific, Waltham, MA) and staining with Diff-Quik. The remaining cell suspension was lysed with 0.1% Triton X-100 and freeze-thawed. The PMN content was determined by MPO activity using isolated PMN cells as a standard.
Results
Purification of human and murine MMP-12
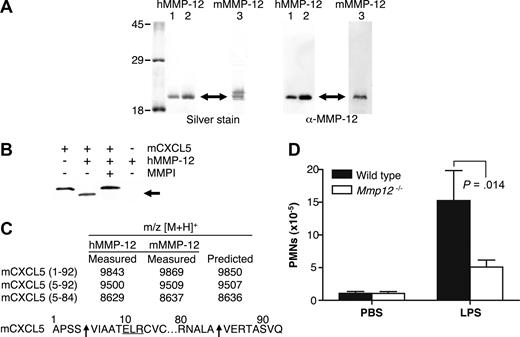
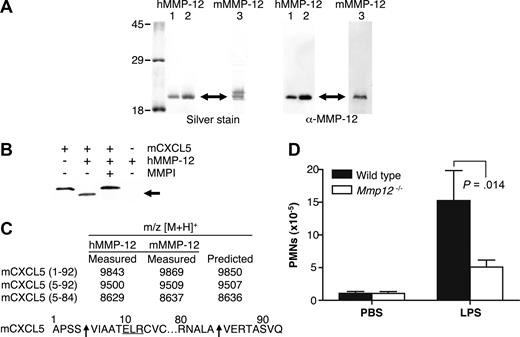
Recombinant human MMP-12 was expressed in E coli with essentially all of the MMP-12 protein present in inclusion bodies. After solubilization with 8 mol/L urea and dialysis into 3 mol/L urea, several bands were detected by Western blot analysis by using anti-human MMP-12 antibody specific for the catalytic domain. These bands are the processed forms of active MMP-12 corresponding to active MMP-12 lacking its propeptide (45 kDa) and autolytically processed active MMP-12 lacking its hemopexin C-domain (19 kDa)30 (data not shown). MMP-12 activity was confirmed by cleavage of a quenched fluorescent synthetic MMP peptide substrate. Incubation of the dialyzed supernatant at 4°C for 3 days increased purity and specific activity of the sample as many E coli proteins were degraded by the active protease. After incubation, only one band was detected on a silver-stained 10% SDS-PAGE gel as a result of further MMP-12 autolytic processing to a stable ∼19 kDa form (Figure 1A).
MMP-12 cleaves mCXCL5 and impairs PMN recruitment to LPS in Mmp12−/− mice. (A) E coli was used to express 2 batches of active human MMP-12 (hMMP-12; lanes 1 and 2) and one batch of murine MMP-12 (mMMP-12; lane 3). Protease integrity and purity was verified on silver-stained 10% SDS-PAGE gels and Western blotted using antibodies directed to the human and murine MMP-12 catalytic domain as appropriate. Arrow indicates active MMP-12. Molecular mass markers (× 10−3 Da) are shown. (B) Analysis of MMP-12 proteolysis of murine CXCL5 incubated with or without the MMP inhibitor 1 μmol/L prinomastat (MMPI) as a control on a silver-stained 15% Tris-Tricine SDS-PAGE gel with arrow indicating cleaved mCXCL5. (C) Identification of human and murine MMP-12 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that MMP-12 cleaved mCXCL5 (LIX) in the same position as MMP-8.16 Arrow indicates the cleavage site of human and murine MMP-12. (D) PMN responsiveness to LPS (n = 8) or PBS vehicle control (n = 6) injected into dorsal air pouches of Mmp12−/− or wild-type mice was measured 8 hours after injection by myeloperoxidase activity in the lavage fluids. Standard error bars are shown. P values calculated with Mann-Whitney nonparametric test.
MMP-12 cleaves mCXCL5 and impairs PMN recruitment to LPS in Mmp12−/− mice. (A) E coli was used to express 2 batches of active human MMP-12 (hMMP-12; lanes 1 and 2) and one batch of murine MMP-12 (mMMP-12; lane 3). Protease integrity and purity was verified on silver-stained 10% SDS-PAGE gels and Western blotted using antibodies directed to the human and murine MMP-12 catalytic domain as appropriate. Arrow indicates active MMP-12. Molecular mass markers (× 10−3 Da) are shown. (B) Analysis of MMP-12 proteolysis of murine CXCL5 incubated with or without the MMP inhibitor 1 μmol/L prinomastat (MMPI) as a control on a silver-stained 15% Tris-Tricine SDS-PAGE gel with arrow indicating cleaved mCXCL5. (C) Identification of human and murine MMP-12 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that MMP-12 cleaved mCXCL5 (LIX) in the same position as MMP-8.16 Arrow indicates the cleavage site of human and murine MMP-12. (D) PMN responsiveness to LPS (n = 8) or PBS vehicle control (n = 6) injected into dorsal air pouches of Mmp12−/− or wild-type mice was measured 8 hours after injection by myeloperoxidase activity in the lavage fluids. Standard error bars are shown. P values calculated with Mann-Whitney nonparametric test.
Q-Sepharose and Zn chelating-Sepharose chromatography were used to further purify and concentrate the active MMP-12 protein with Western blot analysis verifying the identity of the purified protein to be human MMP-12 catalytic domain (Figure 1A). Edman sequencing identified the N terminus of the protein to be FREMPG, which corresponds to the start of the catalytic domain (Phe100) after propeptide removal. The molecular mass determined by MALDI-TOF MS was 18 802 Da corresponding to the human MMP-12 catalytic domain Phe100-Asn269. Murine MMP-12 was purified essentially as for human MMP-12 with an additional SP-Sepharose chromatography step. Integrity and purity of the active protein was verified on a silver-stained 10% SDS-PAGE gel with 2 bands detected; however, only the lower mass band was recognized by Western blotting using an antibody directed to the murine MMP-12 catalytic domain (Figure 1A). The second, higher mass band might be the proteolyzed MMP-12 hemopexin domain shed during the purification procedure as described by Shapiro et al.27 The molecular masses of the 2 bands determined by MALDI-TOF MS were 18 562 and 18 177 Da.
MMP-12 processing of the murine CXCL5 chemokine
Infiltrating monocytes, macrophages, and tissue-resident activated macrophages are the primary sources of MMP-12. MMPs have recently been found to modulate inflammatory and immune responses by chemokine processing,11,16,19,20,22,38,39 but MMP-12 has not been characterized in its ability to proteolyze these inflammatory mediators. First, we investigated whether MMP-12 could process the cognate ELR+ ligands of mCXCR2, which specifically induce the migration of murine neutrophils.
mCXCL5 was the first chemokine to be screened because we had recently reported its susceptibility to cleavage by a variety of MMPs.16 MMP-12 processed mCXCL5 (Figure 1B) with MALDI-TOF mass spectrometry analysis of the cleavage products revealing that both murine and human MMP-12 cleaved mCXCL5 at Ser4-Val5, designated mCXCL5(5-92), and at Ala84-Val85, designated mCXCL5(5-84) (Figure 1C). The generation of mCXCL5(5-92) by MMP-12 is the same cleavage product reported for MMP-8 that we have shown to promote PMN influx in response to LPS in vitro and in vivo.16
Subcutaneous PMN infiltration in response to LPS in vivo
To ascertain whether MMP-12 cleavage of mCXCL5, also known as LIX, had a role in PMN cell migration in vivo, Mmp12−/− mice were compared with wild-type mice in response to LPS. The infiltration of PMNs toward LPS in a dorsal skin air pouch was significantly reduced after 8 hours (P = .014) in female Mmp12−/− mice (n = 8) compared with wild-type female controls (Figure 1D). The effect was also observed in male mice, although the PMN infiltrate was less (data not shown), a sex difference similar to that observed with Mmp8−/− mice.16
MMP-12 processing of murine ELR+ CXC chemokines
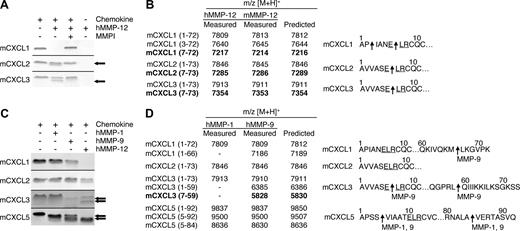
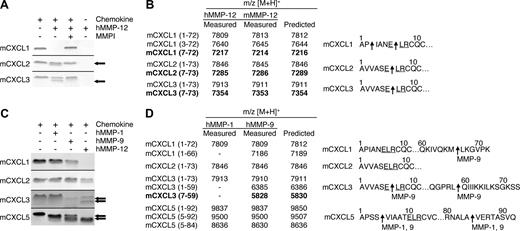
Proteolytic screening of the 3 other murine ELR+ CXC chemokines mCXCL1, -2, and -3 showed that all were susceptible to MMP-12 processing (Figure 2A). MALDI-TOF mass spectrometry analysis of the human and murine MMP-12–generated cleavage products revealed that mCXCL2 and -3 were cleaved within the ELR motif at Glu6-Leu7, designated mCXCL2(7-73) and mCXCL3(7-73), respectively, whereas mCXCL1 was cleaved at Pro2-Ile3 as well as Glu6-Leu7, designated mCXCL2(3-72) and mCXCL2(7-72), respectively (Figure 2B). Because the ELR motif is critical to receptor activation,5 these MMP-12 processing events are predicted to be inactivating cleavages. It is noteworthy that mCXCL5 was the only murine ELR+ CXC chemokine not to be processed by MMP-12 within the ELR motif. Instead, mCXCL5 was cleaved at position 4-5 as discussed above, a site previously shown to be an activation event (Figure 1).16
MMP-1, -9, and -12 processing of murine ELR+ CXC chemokines. (A) Analysis of MMP-12 proteolysis of murine CXCL1, 2, and 3 with or without 1 μmol/L prinomastat (MMPI) as a control on a silver-stained 15% Tris-Tricine SDS-PAGE gel with arrows indicating cleaved chemokine. Black horizontal lines separate individual gels. (B) Identification of human and murine MMP-12 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that MMP-12 cleaved 3 murine ELR+ CXC chemokines in the ELR motif. Cleavage products generated by proteolysis within the ELR motif are highlighted in bold. Arrows indicate human and murine MMP-12 cleavage sites. (C) Tris-Tricine 15% SDS-PAGE gel analysis of MMP-1 and -9 cleavage of murine CXCL1, -2, -3, and -5 with arrows indicating cleaved chemokine. MMP-12 cleavage products are shown for comparison. Black horizontal lines separate individual gels. (D) Identification of human MMP-1 and -9 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that only MMP-9 cleaved one murine ELR+ CXC chemokine in the ELR motif. Cleavage products generated by proteolysis within the ELR motif are highlighted in bold. Arrows indicate MMP-1 or -9 cleavage sites.
MMP-1, -9, and -12 processing of murine ELR+ CXC chemokines. (A) Analysis of MMP-12 proteolysis of murine CXCL1, 2, and 3 with or without 1 μmol/L prinomastat (MMPI) as a control on a silver-stained 15% Tris-Tricine SDS-PAGE gel with arrows indicating cleaved chemokine. Black horizontal lines separate individual gels. (B) Identification of human and murine MMP-12 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that MMP-12 cleaved 3 murine ELR+ CXC chemokines in the ELR motif. Cleavage products generated by proteolysis within the ELR motif are highlighted in bold. Arrows indicate human and murine MMP-12 cleavage sites. (C) Tris-Tricine 15% SDS-PAGE gel analysis of MMP-1 and -9 cleavage of murine CXCL1, -2, -3, and -5 with arrows indicating cleaved chemokine. MMP-12 cleavage products are shown for comparison. Black horizontal lines separate individual gels. (D) Identification of human MMP-1 and -9 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that only MMP-9 cleaved one murine ELR+ CXC chemokine in the ELR motif. Cleavage products generated by proteolysis within the ELR motif are highlighted in bold. Arrows indicate MMP-1 or -9 cleavage sites.
MMP-1 and -9 processing of murine ELR+ CXC chemokines
To investigate whether the chemokine processing by MMP-12 was protease-specific, we tested human MMP-1 and -9, 2 other metalloproteinases expressed by macrophages.40 It is noteworthy that mCXCL2 was not cleaved by either MMP-1 or -9, whereas mCXCL1 was processed by MMP-9 at Met66-Leu67, designated mCXCL1(1-66) (Figure 2C,D). MMP-9 also cleaved mCXCL3 in the C-terminal tail at Leu59-Gln60, designated mCXCL3(1-59) as well as in the ELR motif to generate mCXCL3(7-59). Both MMP-1 and -9 cleaved mCXCL5 in the same positions as MMP-12 (Figure 1), generating mCXCL5(5-92) and mCXCL5(5-84) In summary, MMP-9 cleaved only one mCXC chemokine in the ELR motif, revealing the unique specificity of MMP-12 for this motif in the murine ELR+ CXC chemokines.
MMP-12 processing of human ELR+ CXC chemokines
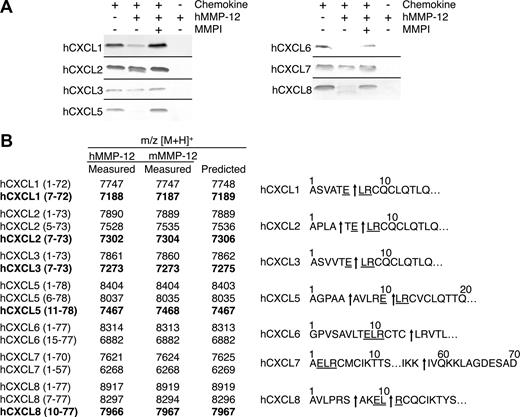
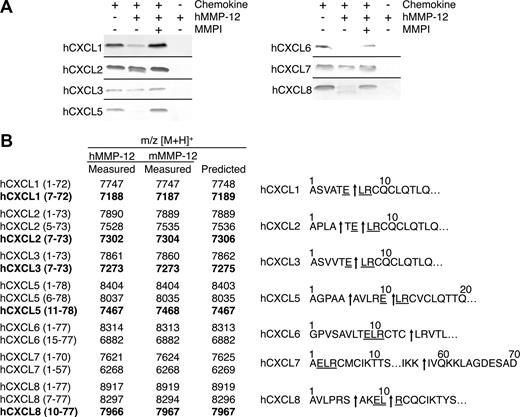
All 7 of the human ELR+ CXC chemokines tested were processed by human MMP-12 either within the ELR motif or just C-terminal to it, a cleavage that also would result in a truncated chemokine lacking an intact ELR motif (Figure 3A), and in the C-terminal tail. MALDI-TOF mass spectrometry analysis of the MMP-12 cleavage products revealed that hCXCL1, -2, and -3 were cleaved within the ELR sequence at Glu6-Leu7, designated hCXCL1(7-72), hCXCL2(7-73), and hCXCL3(7-73), respectively. Human CXCL2 was also processed at Ala4-Thr5, designated hCXCL2(5-73) (Figure 3B). hCXCL5 and hCXCL8 were both processed at 2 sites, one upstream of the ELR motif at Ala5-Ala6 and Ser6-Ala7, designated hCXCL5(6-78) and hCXCL8(7-77), respectively, as well as within the ELR motif Glu10-Leu11, designated hCXCL5(11-78), and Leu9-Arg10, designated hCXCL8(10-77). hCXCL6 was cleaved not in the ELR motif but 3 residues downstream, at an unusual site, Cys14-Leu15, designated hCXCL6(15-77). The only ELR+ CXC chemokine tested that was cleaved at the C-terminal tail was hCXCL7, having been processed at Lys57-Ile58 designated hCXCL7(1-57). All cleavage products generated by human MMP-12 were identical when assayed with murine MMP-12 (Figure 3B). In summary, 6 of the 7 human ELR+ CXC chemokines tested were cleaved by MMP-12 within, or a few residues downstream of, the ELR motif.
MMP-12 processing of human ELR+ CXC chemokines. (A) Analysis of MMP-12 proteolysis of human CXCL1, -2, -3, -5, -6, -7, and -8 incubated with or without 1 μmol/L prinomastat (MMPI) as a control on silver-stained 15% Tris-Tricine SDS-PAGE gel. Black horizontal lines separate individual gels. (B) Identification of human and murine MMP-12 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that MMP-12 cleaved 6 human ELR+ CXC chemokines in, or downstream of, the ELR motif. Cleavage products generated by proteolysis within the ELR motif are highlighted in bold. Arrows indicate human and murine MMP-12 cleavage sites.
MMP-12 processing of human ELR+ CXC chemokines. (A) Analysis of MMP-12 proteolysis of human CXCL1, -2, -3, -5, -6, -7, and -8 incubated with or without 1 μmol/L prinomastat (MMPI) as a control on silver-stained 15% Tris-Tricine SDS-PAGE gel. Black horizontal lines separate individual gels. (B) Identification of human and murine MMP-12 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that MMP-12 cleaved 6 human ELR+ CXC chemokines in, or downstream of, the ELR motif. Cleavage products generated by proteolysis within the ELR motif are highlighted in bold. Arrows indicate human and murine MMP-12 cleavage sites.
MMP-1 and -9 processing of human ELR+ CXC chemokines
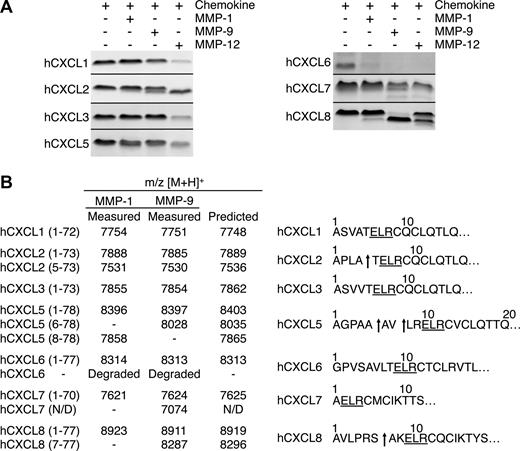
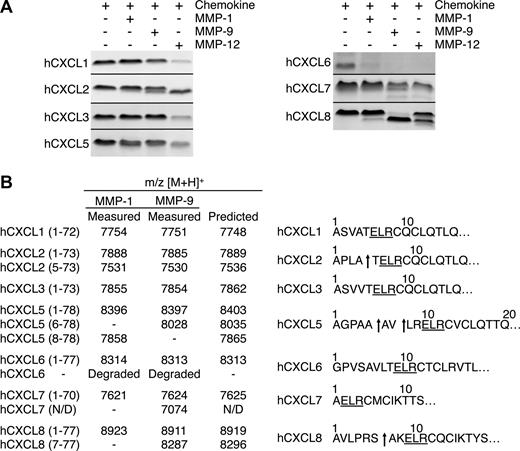
The ELR motif in ELR+ CXC chemokines is crucial in cognate receptor binding and activation.5 Therefore, cleavage here is likely to be biologically very significant. To investigate whether the processing within this motif was protease-specific we tested the other macrophage MMPs, MMP-1 and -9. Five of the 7 chemokines tested were processed by MMP-1 and -9 (Figure 4A). However, none were cleaved within the ELR motif, revealing the exquisite MMP-12 substrate specificity for this cleavage site. Outside of the ELR motif, MMP-1 and -9 also generated hCXCL2(5-73), hCXCL7(1-57), and hCXCL8(7-77), similar to MMP-12. However, only MMP-1 cleaved hCXCL5 at Val7-Leu8, designated hCXCL5(8-78). Human CXCL6 was completely degraded, whereas MALDI-TOF mass spectrometry analysis revealed that hCXCL1 and hCXCL3 were not processed by MMP-1 or -9 (Figure 4B), even after prolonged incubation times or high enzyme to substrate ratios (data not shown).
Proteolysis of human ELR+ CXC chemokines by MMP-1 and -9. (A) Tris-Tricine 15% SDS-PAGE gel analysis of MMP-1 and -9 cleavage of human CXCL1, -2, -3, -5, -6, -7, and -8. MMP-12 cleavage products are shown for comparison. Black horizontal lines separate individual gels. (B) Identification of human MMP-1 and -9 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that neither MMP-1 nor MMP-9 cleaved any of the human ELR+ CXC chemokines in the ELR motif. Arrows indicate MMP-1 or -9 cleavage sites. N/D, not determined.
Proteolysis of human ELR+ CXC chemokines by MMP-1 and -9. (A) Tris-Tricine 15% SDS-PAGE gel analysis of MMP-1 and -9 cleavage of human CXCL1, -2, -3, -5, -6, -7, and -8. MMP-12 cleavage products are shown for comparison. Black horizontal lines separate individual gels. (B) Identification of human MMP-1 and -9 cleavage products by MALDI-TOF MS. Deconvolution of mass spectrometry data revealed that neither MMP-1 nor MMP-9 cleaved any of the human ELR+ CXC chemokines in the ELR motif. Arrows indicate MMP-1 or -9 cleavage sites. N/D, not determined.
In vitro chemotaxis toward MMP-12–proteolyzed mCXCL2 and mCXCL3
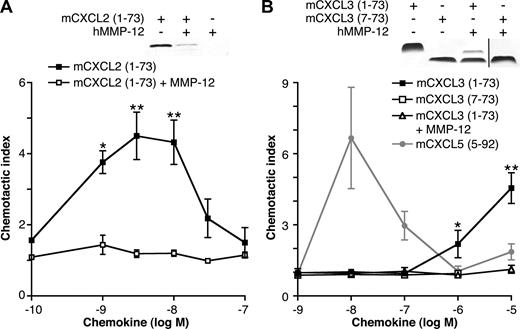
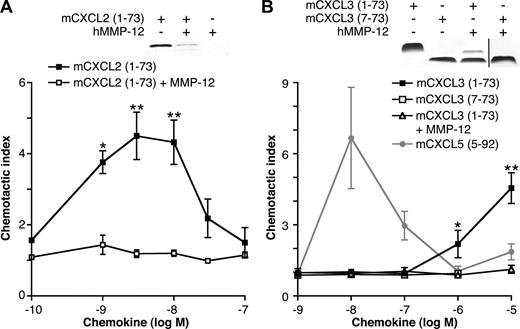
The ELR motif in hCXCL8 (IL-8) is essential for CXCR binding,5 activation, and PMN chemotaxis.6 Therefore, we hypothesized that cleavage within the ELR motif of ELR+ CXC chemokines by MMP-12 would be inactivating and therefore functionally extremely important. mCXCL2 or mCXCL3 incubated with MMP-12 for 24 hours was tested for chemotactic properties. Mass spectrometry revealed cleavage of mCXCL2 by MMP-12 in the ELR motif (Figure 2B); however, gel analysis showed degradation of the chemokine, which not surprisingly led to a loss in chemotactic ability (Figure 5A). Compared with full-length mCXCL3(1-73), removal of 6 N-terminal amino acids (including the Glu of the Glu-Leu-Arg motif) of mCXCL3 by MMP-12 abolished the ability of the chemokine to direct murine (Figure 5B) or human neutrophil chemotaxis (data not shown), as did a synthetic analog of MMP-12–proteolyzed mCXCL3(7-73) (Figure 5B).
The effect of MMP-12 cleavage on mCXCL2 and 3 in vitro chemotactic activity. Silver-stained 15% Tris-Tricine SDS-PAGE gels of the appropriate full-length or cleaved chemokines used in the chemotaxis assay are shown. Black vertical line indicates part of gel removed for presentation purposes. PMNs isolated from murine bone marrow chemoattracted toward full-length mCXCL2(1-73) (A) and mCXCL3(1-73) (B) in a transwell cell migration assay. However, mCXCL2(1-73) and mCXCL3(1-73) incubated with MMP-12 overnight lost all chemotactic activity toward PMNs, as did the synthetic analog of MMP-12-proteolyzed mCXCL3(7-73). This demonstrates that the MMP-12 cleavage in the CXC-receptor binding ELR motif is an inactivating one. However, using a synthetic analog of MMP-12 cleaved mCXCL5(5-92) as a positive control, we also demonstrate enhancement of PMN chemotaxis after MMP-12 activity on this chemokine alone. Data are presented as the ratio of cells migrated toward chemokine compared with buffer control (chemotactic index); standard error bars are shown. *, **, Student t test values (P < .05 and .01, respectively).
The effect of MMP-12 cleavage on mCXCL2 and 3 in vitro chemotactic activity. Silver-stained 15% Tris-Tricine SDS-PAGE gels of the appropriate full-length or cleaved chemokines used in the chemotaxis assay are shown. Black vertical line indicates part of gel removed for presentation purposes. PMNs isolated from murine bone marrow chemoattracted toward full-length mCXCL2(1-73) (A) and mCXCL3(1-73) (B) in a transwell cell migration assay. However, mCXCL2(1-73) and mCXCL3(1-73) incubated with MMP-12 overnight lost all chemotactic activity toward PMNs, as did the synthetic analog of MMP-12-proteolyzed mCXCL3(7-73). This demonstrates that the MMP-12 cleavage in the CXC-receptor binding ELR motif is an inactivating one. However, using a synthetic analog of MMP-12 cleaved mCXCL5(5-92) as a positive control, we also demonstrate enhancement of PMN chemotaxis after MMP-12 activity on this chemokine alone. Data are presented as the ratio of cells migrated toward chemokine compared with buffer control (chemotactic index); standard error bars are shown. *, **, Student t test values (P < .05 and .01, respectively).
Transalveolar migration of leukocytes in response to LPS in vivo
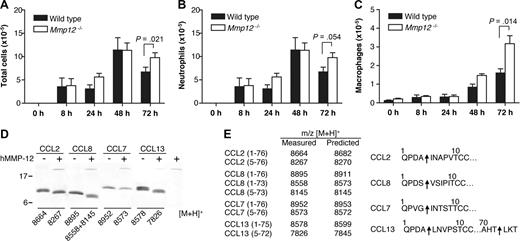
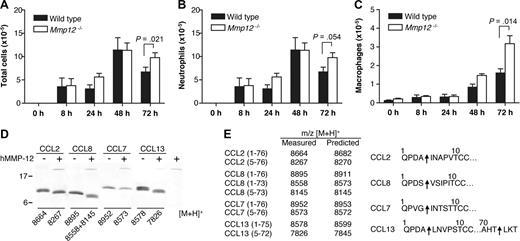
The lungs contain the highest proportion of tissue-resident macrophages.41 To confirm the role of MMP-12 in regulating leukocyte migration, Mmp12−/− and wild-type mice were challenged intranasally with LPS, and then the bronchoalveolar lavage fluid (BALF) leukocytes were counted. As shown in Figure 6A, at 72 hours, there was a significant increase (P = .021) in total cell numbers in the BALF from Mmp12−/− mice compared with wild-type (Figure 6A). Differential leukocyte counts of the BALF showed that the increase in total cells observed in the Mmp12−/− mice was due to an accumulation of both neutrophils (P = .054; Figure 6B) and macrophages (P = .014; Figure 6C). Secondary validation of the neutrophil recruitment was confirmed by MPO assay (P = .045) (data not shown). It is noteworthy that there was a sex difference in that the female Mmp12−/− mice did not show this change (data not shown). Male and female mice are known to differ in their response to LPS in a sex-dependent manner.42
Transalveolar migration of leukocytes in response to LPS in vivo. Time course of leukocyte recruitment in BALF from male Mmp12−/− and wild-type mice instilled intranasally with LPS (10 μg). Total number of leukocytes (A), neutrophils (B), and macrophages (C) in BALF are shown. Results are expressed as mean of 2 biological replicates (n = 2 animals per experiment) for 0-, 8-, 24-, and 48-hour time points and mean of 4 biological replicates (n = 2 animals per experiment) for 72-hour time point. Standard error bars are shown. P values calculated with Mann-Whitney nonparametric test. Secondary validation of the neutrophil influx was confirmed by MPO assay (P = .045; data not shown). (D) Tris-Tricine 15% SDS-PAGE gel analysis of MMP-12 cleavage of human monocyte chemoattractant proteins CCL2, -7, -8, and -13 with masses (m/z) obtained by MALDI-TOF MS. (E) Deconvolution of mass spectrometry data revealed that MMP-12 cleaved all 4 CC chemokines at position 4-5, a known inactivating cleavage.11,22 Arrows indicate MMP-12 cleavage sites.
Transalveolar migration of leukocytes in response to LPS in vivo. Time course of leukocyte recruitment in BALF from male Mmp12−/− and wild-type mice instilled intranasally with LPS (10 μg). Total number of leukocytes (A), neutrophils (B), and macrophages (C) in BALF are shown. Results are expressed as mean of 2 biological replicates (n = 2 animals per experiment) for 0-, 8-, 24-, and 48-hour time points and mean of 4 biological replicates (n = 2 animals per experiment) for 72-hour time point. Standard error bars are shown. P values calculated with Mann-Whitney nonparametric test. Secondary validation of the neutrophil influx was confirmed by MPO assay (P = .045; data not shown). (D) Tris-Tricine 15% SDS-PAGE gel analysis of MMP-12 cleavage of human monocyte chemoattractant proteins CCL2, -7, -8, and -13 with masses (m/z) obtained by MALDI-TOF MS. (E) Deconvolution of mass spectrometry data revealed that MMP-12 cleaved all 4 CC chemokines at position 4-5, a known inactivating cleavage.11,22 Arrows indicate MMP-12 cleavage sites.
MMP-12 proteolysis of monocyte chemoattractant proteins
We reported previously that MMP proteolysis of monocyte chemotactic proteins CCL2, -7, -8, and -13 generates antagonists.11,22 To investigate whether the accumulation of macrophages in the BALF from Mmp12−/− mice might be due to the lack of inactivating cleavages of these chemokines, we analyzed the cleavage of CCL2, -7, -8, and -13 by MMP-12. We found that MMP-12 processes all 4 CC chemokines at position 4-5 (Figure 6D,E), a truncation identical to that observed for other MMPs and one that was characterized to generate receptor antagonists.11,23
Discussion
Our studies lead us to propose a novel macrophage-driven negative feedback mechanism for terminating the PMN and macrophage influx in acute inflammation. Resident and infiltrating macrophages, via the precise activity of macrophage-specific MMP-12, but not the other macrophage MMPs, MMP-1 and -9, cleave and inactivate human ELR+ CXC chemokines consistent with a role in controlling the duration of the inflammatory cellular infiltrate of PMNs. The integrity of these factors is critical for their functional activity in regulating neutrophil and macrophage recruitment, chemotaxis, and cellular activation in inflammation.2 So, compared with a passive rundown of PMN recruitment signals and clearance of apoptotic PMNs in the infiltrate by macrophages,12 the cellular regulation of the inflammatory cell infiltrate imparts a higher degree of active control of these critical host responses. The “off” signal is as important as the “on” signal.
MMP-12 is a monocyte/macrophage-specific protease long thought to be primarily involved in elastin degradation.30 Hence, considerable attention has been focused over the past 10 years on its role in chronic inflammation, such as in cigarette smoke-induced chronic obstructive pulmonary disease,28 yet its role in an acute inflammatory response remained unclear. We found that MMP-12 processed all but one of the 7 human ELR+ CXC chemokines either in the critical ELR activation motif or carboxyl-terminal to it. It is noteworthy that cleavage in the ELR motif was protease-specific, because the other macrophage-secreted MMPs, MMP-1 and -9,40 failed to cleave the same peptide bond. MMP-12 also cleaved 3 of the 4 murine ELR+ CXC chemokines within the ELR motif at Glu-Leu, and was again protease-specific, because neither MMP-1 nor -9 cleaved here. Cleavage in the ELR sequence was not apparent in mCXCL5, which might be due to its extended 9-amino acid N terminus upstream from the Glu-Leu-Arg residues compared with mCXCL1, -2, and -3.
A broad-spectrum peptide library43 and insoluble elastin44 have both been used to demonstrate that MMP-12 has a strong proteolytic preference for Leu/Ile at the P1′ residue on the carboxyl-terminal side of the scissile bond. This has further been confirmed by MMP-12 hydrolysis of 2 insulin β-chain bonds Ala14-Leu15 and Tyr16-Leu17,45 and tissue factor pathway inhibitor at Lys20-Leu21 and Arg83-Ile84.46 Therefore, proteolysis of the ELR+ CXC chemokines by MMP-12 between the Glu-Leu bond of the ELR motif is at a preferred and natural cleavage site. Nonetheless, hCXCL6 was not cleaved in the ELR sequence by MMP-12. However, cleavage occurred 3 residues downstream at Cys14-Leu15, which removes the ELR-containing peptide and so should also eliminate receptor activation. Cleavage at a site with a disulfide crosslinked Cys at P1 is extremely rare, making the absence of cleavage in the ELR even more unusual for MMP-12.
Nolan et al8 identified the novel murine ELR+ CXC chemokine termed dendritic cell inflammatory protein-1 (mCXCL3) and, using human neutrophils and murine CXCR2 transfected rat basophilic leukemia (RBL-1) cells, demonstrated that it had PMN chemotactic properties similar to those of hCXCL8. Murine CXCL3 has received limited attention so far, but our data confirm the weak chemotactic properties of the chemokine for murine and human PMNs. Previous groups have demonstrated that the ELR motif in human CXCL8 (IL-8) is crucial in cognate receptor binding and activation,5 as well as PMN chemotaxis.6 MMP-12–proteolyzed mCXCL3 or a synthetic analog of MMP-12–truncated mCXCL3(7-73) did not chemoattract human or murine PMNs and so MMP-12 proteolysis at Glu-Leu in the ELR motif is an inactivating cleavage. The general cleavage in the ELR sequence, and or degradation, in all but one of both murine and human ELR+ CXC chemokine subfamilies mechanistically suggests how MMP-12 plays a role in inhibiting PMN recruitment, a hypothesis supported by our in vivo results using Mmp12−/− mice.
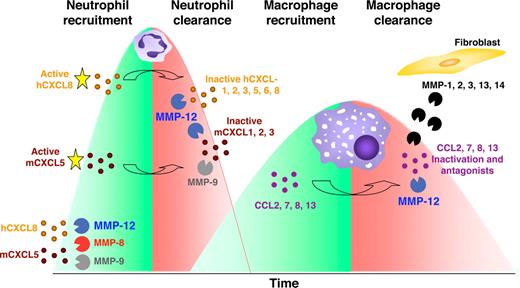
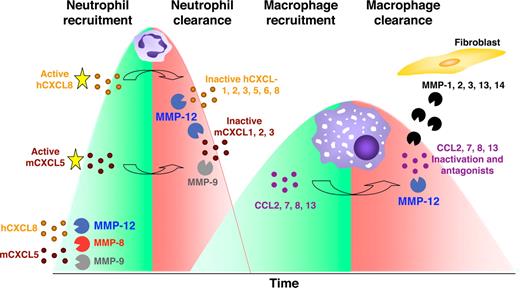
Using an LPS-induced acute lung injury model in Mmp12−/− and wild-type mice, we observed no difference in PMN or macrophage BALF numbers at 24 hours, similar to what was reported by Leclerc et al.47 A maximal response for PMNs was observed at 48 hours in both mouse strains. However, at 72 hours, the BALF PMN cell numbers decreased only in the wild-type mice and not the Mmp12−/− mice, revealing the absence of a MMP-12–dependent suppression of PMN infiltration. This is consistent with the cleavage and inactivation of most ELR+ CXC chemokines by MMP-12 (Figure 7).
Model of MMPs orchestrating the pro- and anti-inflammatory response. In response to host challenge, MMPs are released from epithelial cells, resident macrophages, and neutrophils cleaving and activating specific chemokines that recruit PMNs (mCXCL5, hCXCL5, and hCXCL8). After 24-48 hours, macrophages accumulate at the site of injury and secrete MMP-12 that inactivates ELR+ CXC chemokines. Hence, in addition to apoptosis reducing the numbers of cells from the PMN influx, by blocking the continued recruitment of PMNs, macrophage MMP-12 contributes to the reduction in the number of PMNs at the site of stimulus. In addition, MMP-12 and other MMPs released from surrounding stromal fibroblasts or epithelial cells cleave and inactivate MCPs (CCL2, -7, -8, and -13) converting these to receptor antagonists disrupting the recruitment of further macrophages and contributing to inflammatory resolution. Hence, macrophages terminate the PMN and macrophage influxes.
Model of MMPs orchestrating the pro- and anti-inflammatory response. In response to host challenge, MMPs are released from epithelial cells, resident macrophages, and neutrophils cleaving and activating specific chemokines that recruit PMNs (mCXCL5, hCXCL5, and hCXCL8). After 24-48 hours, macrophages accumulate at the site of injury and secrete MMP-12 that inactivates ELR+ CXC chemokines. Hence, in addition to apoptosis reducing the numbers of cells from the PMN influx, by blocking the continued recruitment of PMNs, macrophage MMP-12 contributes to the reduction in the number of PMNs at the site of stimulus. In addition, MMP-12 and other MMPs released from surrounding stromal fibroblasts or epithelial cells cleave and inactivate MCPs (CCL2, -7, -8, and -13) converting these to receptor antagonists disrupting the recruitment of further macrophages and contributing to inflammatory resolution. Hence, macrophages terminate the PMN and macrophage influxes.
It is noteworthy that the LPS-induced acute lung injury model also showed a specific increase in BALF macrophages at 72 hours in Mmp12−/− mice that was not apparent in the wild-type mice. This suggests that MMP-12 might also be involved in a negative feedback mechanism that attenuates macrophage recruitment. Indeed, we found that MMP-12 cleaves monocyte chemotactic proteins CCL2, -7, -8, and -13 at position 4-5, a known MMP truncation for the inactivation of the monocyte chemotactic ability of these chemokines in vitro and in vivo.11,23 Moreover, these N-terminal truncated CC chemokines are receptor antagonists that serve to actively suppress the macrophage infiltrate. Hence, when infiltrating macrophages arrive at the site of injury and phagocytose microorganisms, foreign material, or apoptotic PMNs, macrophage-secreted MMP-12 has the potential to abrogate further neutrophil and macrophage recruitment, thus contributing to inflammatory resolution and healing.
We reported recently that PMN-specific MMP-8 N-terminally truncates the ELR+ CXC chemokines mCXCL5, hCXCL5, and hCXCL8 upstream from the ELR sequence, increasing their bioactivity and enhancing PMN recruitment in vitro and in vivo.16 It is noteworthy that some truncated mCXCL5 was recovered from the inflamed lungs of Mmp8−/− mice, suggesting processing by an additional protease.49 In the present study, we found that MMP-12 processing of mCXCL5 occurred at the same Ser4-Val5 bond as with MMP-8 activity and therefore also increases the PMN chemoattractant property of this chemokine. Hence, this is an interesting species difference between mouse and man—none of the human ELR+ CXC chemokines were activated by MMP-12. The relevance of this cleavage event in vivo was demonstrated in an acute inflammatory model that promotes subcutaneous PMN infiltration. We found that, similar to what was observed with the Mmp8−/− mice16 , Mmp12−/− mice 8 hours after LPS stimulation had a significant reduction in the influx of PMNs compared with wild-type control mice. This suggests that resident macrophages present in the tissue surrounding the air pouches50 might be activated by LPS and therefore secrete MMP-12 that in turn activates mCXCL5, thus enhancing early PMN recruitment in response to LPS.
This hypothesis is supported by other studies. Depletion of alveolar macrophages before bacterial instillation causes a significant decrease in early PMN recruitment48 ; addition of a natural MMP inhibitor, TIMP-2, with LPS reduces the PMN influx.40 Nénan et al34 reported that the instillation of recombinant MMP-12 catalytic domain into mouse airways resulted in increased PMN recruitment, into the BALF, until the 18-hour time point. These data correlate with those in a previous study by Warner et al35 in which they observed a 50% reduction in PMN influx into the alveolar space of Mmp12−/− mice compared with wild-type control mice in an immune complex-induced acute lung injury model. However, in both these studies, the detailed mechanistic aspects of the MMP-12 regulation on PMN influx were not elucidated. We suggest that the activation of mCXCL5 by MMP-12 accounts for these observations. Hence, in mice but not man, MMP-12 expression by resident macrophages has an early proinflammatory role that promotes initial LPS-induced acute inflammatory responses.
In view of the large number of ELR+ CXC chemokines cleaved and inactivated by MMP-12, we propose that a primary role of MMP-12 in innate immunity is to terminate the PMN recruitment signals generated by these chemokines. This proteolytic regulation of PMN chemoattractant signals provides a novel mechanistic explanation for reducing and controlling PMN numbers and is consistent with the phagocytic functions of the macrophage in removing apoptotic PMNs. Hence, these in vitro and in vivo data using Mmp12−/− mice have revealed a new biologically important role for the macrophage in innate immunity. We suggest that macrophages integrate the signaling information embedded in an inflamed tissue and dynamically down-regulate the PMN and macrophage infiltrates using macrophage-specific MMP-12. By this precise proteolysis of a variety of chemokines, MMP-12 activity is one of the mechanisms to dampen inflammation and promote resolution.
Our work on MMP-12 exemplifies how a protease can have multiple activities that affect different phases of the host response. The net balance of these activities and the strength of compensatory proteins or pathways in the cell or host tissue determine the overall role and net host benefit of a protease. Hence, one needs to be cautious in interpreting in vitro and simple in vivo experimental systems. Indeed, this reveals the necessity for using genetic knockouts and animal models of disease to comprehensively understand complex systems. The relative roles of leukocyte and resident cell MMPs in inflammation and their net protective effects through chemokine regulation, versus their degradative activities on the ECM are important issues to resolve—both understanding the mechanistic aspects in the control of chronic inflammatory influxes and the practical aspects of drug targeting. By elucidating MMP-driven regulation of the initiation and termination of innate immunity that allows for the switch to adaptive immunity, those activities driving pathology versus those beneficial to the host can be distinguished and thereby validate new drug targets and anti-targets for the treatment of chronic inflammatory diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
C.M.O. is supported by a Canada Research Chair in Metalloproteinase Proteomics and Systems Biology, with research grants from the Canadian Institutes of Health Research, the National Cancer Institute of Canada (with funds raised by the Canadian Cancer Society), and the Canadian Breast Cancer Research Alliance Special Program Grant on Metastasis, as well as with a Center Grant from the Michael Smith Research Foundation. J.H.C. is also supported by Michael Smith Foundation for Health Research. A.E.S. and J.H.C. are supported by Natural Sciences and Engineering Research Council of Canada, and CIHR Strategic Training Program STP-53877. A.D. is supported by the Fonds Québécois de la recherche sur la nature et les technologies.
Authorship
Contribution: R.A.D. and C.M.O. designed the research, analyzed the data and wrote the manuscript; and R.A.D., J.H.C., C.L.B., A.D., and A.E.S. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher M. Overall, University of British Columbia, Centre for Blood Research, UBC, 4.401 Life Sciences Centre, 2350 Health Sciences Mall, Vancouver, BC, Canada V6T 1Z3; e-mail: chris.overall@ubc.ca.