Abstract
Eighteen relapsed patients with measurable indolent non-Hodgkin lymphoma (NHL) were vaccinated with dendritic cells (DCs) loaded with killed autologous tumor cells. Six patients had objective clinical responses including 3 continuous complete responses (CRs) and 3 partial responses (PRs), with a median follow up of 50.5 months. Eight patients had stable disease, whereas 4 had progressive disease. Clinical responses were significantly associated with a reduction in CD4+CD25+FOXP3+ regulatory T cells, an increase in CD3−CD56dimCD16+ natural killer (NK) cells, and maturation of lymphocytes to the effector memory stage in either postvaccination peripheral blood or tumor specimen samples. In partial responding patients, vaccination significantly boosted the IFN-γ–producing T-cell response to autologous tumor challenge. In one HLA-A*0201+ patient who achieved CR, IL-4 release by circulating T cells in response to tumor-specific IgH-encoded peptides was also documented. Immunohistochemical analysis of tumor biopsies using biotin-conjugated autologous serum samples revealed a tumor-restricted humoral response only in the postvaccination serum from responding patients. Collectively these results demonstrate that vaccination with tumor-loaded DCs may induce both T- and B-cell responses and produces clinical benefits in indolent NHL patients with measurable disease. This study is registered with the Istituto Superiore di Sanità: http://www.iss.it with protocol number 7578-PRE 21-801.
Introduction
Indolent non-Hodgkin lymphomas (NHLs) are chemosensitive, incurable, slow growing, and characterized by a continuous pattern of relapse.1-3 Follicular B-cell NHL (FL) has long been regarded as particularly immune responsive based on reports of spontaneous regressions4 and high response rates to monoclonal antibodies.5-7 Immunotherapy trials targeting the B-cell idiotype (Id) associated with disease have documented clinical responses in a conspicuous number of patients and the promotion of B cell– and T cell–mediated responses to autologous B-cell tumors in the majority of vaccinated patients.8-12 However, preliminary results of 2 phase 3 clinical trials of Id vaccination have indicated lack of clinical benefit in terms of progression-free survival (FavId phase-3 clinical trial; http://www.favrille.com/trials/phase-3clinical-trial-summary.htm).13 Novel lymphoma vaccine formulations based on autologous neoplastic cells have been recently studied. These approaches might potentially induce autoimmunity, but have the advantage of widening the spectrum of target tumor-associated antigens.14-16 With the latter intent, we used unfractionated whole tumor cell preparations as the source of antigens to load dendritic cells (DCs). DCs were loaded with heat-shocked apoptotic and necrotic tumor cells. In fact, apoptotic cell death of neoplastic cells promotes efficient transfer of tumor-associated antigens (TAAs) to the MHC class I processing pathway of professional antigen-presenting cells (APCs).17-19 In addition, stress stimuli such as heat shock have been shown to increase the immunogenicity of neoplastic cells.20,21
In this pilot study, 18 patients with measurable indolent NHL who had relapsed after at least one chemoradiotherapy regimen were injected subcutaneously with DCs loaded with autologous heat-shocked and UV-C–treated tumor cells. The vaccination was well tolerated without autoimmune reactions, achieved significant objective clinical responses, and was associated with significant immune modulation. Our results indicate that immunization of patients with tumor-loaded DCs represents a potentially effective and efficient strategy for the treatment of indolent NHL patients.
Methods
Patient characteristics
Between January 2003 and May 2005, 18 patients with FL (n = 12) or lymphoplasmocytoid lymphoma (n = 6), who were relapsed after their previous treatment, were accrued to this pilot study. The median prior number of regimens was 2 (range, 1-5). The study included 4 patients treated with high-dose sequential chemotherapy (HDS) supported by autologous hematopoietic stem cell transplantation. Eligibility criteria were as follows: confirmed diagnosis of indolent B-cell NHL (lymphoplasmocytoid or FL grades I-IIIa by REAL Classification22 ) relapsing after previous treatment; age of 18 years or older; Eastern Cooperative Oncology Group performance status of 2 or less. Patients were required to be at least 180 days from their last treatment, and to have measurable disease and adequate hematologic, renal, and hepatic functions. Patients were ineligible if they had more than 6 prior treatment regimens, had concurrent immunosuppressive therapy, had a history of central nervous system lymphoma, had serious coexistent active medical problems, had a history of a prior malignancy (excluding non-melanoma carcinomas of the skin and in situ cervical carcinomas) unless the patient was in remission for 2 or more years, or were pregnant or breastfeeding. Analysis of the disease response to DC vaccination was performed using Cheson criteria.23 Seven of eighteen patients were evaluated for clonal rearrangement of Bcl-2/IgH genes to evaluate minimal residual disease (MRD). All 18 patients were assessed for a response based on a central radiology and/or clinical evaluation. The study protocol was approved by the Italian Istituto Superiore di Sanità (ISS) and by the Institutional Scientific Review Committee. Written informed consent for vaccination procedure and for the investigational use of lymphocytes and serum samples and of tumor specimens was obtained from each patient before enrolling in the study in accordance with the Declaration of Helsinki.
Preparation and injection of vaccine
All procedures for preparation of the vaccine were carried out under Good Manufacturing Practice (GMP) conditions. NHL cells were isolated from lymph nodes (LNs) and/or from peripheral blood (PB). In 16 patients, LNs were processed by a mechanical dissociation device (BD Medimachine; BD Biosciences, Franklin Lakes, NJ) to yield single-cell suspensions. In the remaining 2 patients, circulating tumor cells were harvested by leukapheresis. CD19+ NHL cells were purified from PB and/or LNs using a high-gradient immunomagnetic technique according to the manufacturer's instructions (CD19 microbeads; Miltenyi Biotec, Gladbach, Germany). Tumor cells were heat shocked and then induced to undergo apoptosis by UV-C treatment as previously described.24 Briefly, 120 × 106 CD19+ cells were resuspended in 10% autologous human serum Iscove modified Dulbecco medium (Cambrex, Milan, Italy) and incubated for 60 minutes in a water bath at 46°C to induce the expression of heat shock proteins. The cells were irradiated (100 Gy) and finally exposed to a T-UV9 UV-C Germicidal Lamp (Philips, Eindhoven, The Netherlands) calibrated to provide 0.5 J/m2 per second, for 25 seconds, at a lamp to cell culture distance of 39 cm. Autologous PB monocytes, selected by a high-gradient immunomagnetic technique (CliniMacs CD14 microbeads; Miltenyi Biotec), were cultured for 5 days with rhGM-CSF (Leucomax; Novartis, Basel, Switzerland) and rhIL-4 (kindly provided by Schering-Plough, Kenilworth, NJ) as described.25 Then, immature DCs were cocultured for 48 hours with heat-shocked, irradiated, and UV-C–treated neoplastic B cells at a 1:2 ratio. Further DC maturation was finally promoted by adding TNF-α (Knoll, Ludwigshafen, Germany) for the last 12 hours of culture. Tumor-loaded DCs were finally resuspended in 1 mL 0.9% NaCl to be injected subcutaneously into patients. Four administrations of the vaccine in close vicinity to axillary and inguinal LNs were repeated at 2-week intervals.
Flow cytometric analysis
Monocyte-derived DCs and tumor-loaded DCs were labeled with the following mouse antihuman monoclonal antibodies: FITC-labeled anti-hCD14, PerCP-labeled anti–hHLA-DR, FITC-labeled anti-hCD19 (BD Biosciences, San Jose, CA), PE-labeled anti-hCD80, PE-labeled anti-hCD83, PE-labeled anti-hCD86 (BD Pharmingen, San Jose, CA), and PE-labeled anti-hCD1a (Coulter, Krefeld, Germany). Tumor-loaded DCs were costained with propidium iodide (Bender Medsystem, Wien, Austria) and anti-hCD19 to verify that unloaded B cells present in the culture were nonviable. The maturation status of T cells from prevaccination and postvaccination LNs was assessed by labeling the LN cell suspension with the following mouse antihuman monoclonal antibodies: PE-labeled anti-hCD45RA (BD Pharmingen), PE-labeled anti-hCD4, PerCP-labeled anti-CD8, and APC-labeled anti-hCCR7 (BD Biosciences, San Jose, CA). Natural killer (NK) cells were characterized by staining PB samples with FITC-labeled anti-hCD56 (BD Biosciences, San Jose, CA), PE-labeled anti-hCD16 (Miltenyi Biotec), PerCP-labeled anti-hCD3 (BD Biosciences, San Jose, CA), and APC-labeled anti-hNKG2D or APC-labeled anti-hNKp46 (BD Pharmingen). The percentage of regulatory T cells (Tregs) was evaluated by intracellular hFOXP3 staining of PB and LN cell samples collected before and after vaccination using an FITC-labeled antihuman FOXP3 staining kit (eBioscience, San Diego, CA) following the manufacturer's instructions after staining cells with PE-labeled anti-hCD25 (Miltenyi Biotec), PerCP-labeled anti-hCD3, and APC-labeled anti-hCD4 (BD Biosciences, San Jose, CA) antibodies.
Amplification and sequencing of tumor-specific IgH and Bcl2/IgH rearrangements
Molecular monitoring of minimal residual disease (MRD; 7/18 patients) and sequencing of Ig genes expressed by neoplastic B cells (patient no. 14) were accomplished by use of either nested polymerase chain reaction (PCR) amplification of the Bcl-2/immunoglobulin heavy chain (IgH) as described by Corradini et al26 or seminested PCR amplification of clonal rearrangement of IgH genes, as reported,26 when Bcl-2 was not amplified. In the latter instances, DNA obtained from paraffin-embedded sections of diagnostic biopsies from each patient was PCR-amplified using VH and JH consensus primers for the framework region 2 of the IgH gene. To obtain a patient-specific primer, we subcloned the DNA into a suitable plasmid and sequenced the PCR products obtained. DNA extraction from BM and PB samples was performed using the QIAamp DNA Blood Extraction Kit (QIAGEN, Valencia, CA). A patient-specific positive control was included in all PCR experiments. Negative controls contained water instead of DNA. All PCRs were performed in duplicate for a minimum of 2 independent experiments.
Peptide prediction and HLA stabilization assay
Potential HLA-A*0201–binding peptides of 9 to 10 amino acids were identified by 3 different algorithms (http://bimas.dcrt.nih.gov/molbio/hlabind; http://www.syfpeithi.de/; and http://hlaligand.ouhsc.edu/SBT.htm [note: as of this paper's publication, this site is no longer operational]) within the tumor-specific IgH sequence from the HLA-A*0201+ patient (no. 14). Three peptides, showing high scores by all 3 algorithms, were selected: peptide 1 (sequence: TLLRLLFNWV) at the boundary between complementarity determining region 1 (CDR1) and framework region 2 (FR2); peptide 2 (QLVQSGAEVK) from FR1; and peptide 3 (YCARVPAGV) from CDR3. Synthetic peptides corresponding to these sequences (PRIMM s.r.l.; San Raffaele Biomedical Science Park, Milan, Italy) were then screened for binding to HLA-A2 by the HLA stabilization assay as described,27 using the HLA-A2–specific mAbs CR11.35128 and BB7.2 (ATCC, Manassas, VA). HLA-A2 stabilization was expressed as the fluorescence index (FI)29 based on the mean fluorescence intensity (MFI) for HLA-A2, as follows: (MFI[T2 loaded with peptide]/MFI[empty T2]) − 1.
ELISPOT assays
The IFN-γ and the IL-4 enzyme-linked immunosorbent spot (ELISPOT) assays were performed as described with minor modifications.30 Briefly, plates were coated at 4°C, overnight with 10 μg/mL 1-D1K anti–IFN-γ mAb or 10 μg/mL IL-4–I anti–IL-4 mAb (Mabtech, Stockholm, Sweden). PB-derived lymphocytes were stimulated with synthetic peptides (30 μg/mL). Treatment with 1% PHA (for IFN-γ ELISPOT), or to PMA plus ionomycin (for IL-4 ELISPOT) were used as positive controls. After incubation at 37°C for 48 to 72 hours, plates were labeled for 2 hours at room temperature with 1 μg/mL biotin-conjugated secondary anti–human IFN-γ 7B6-1 mAb or antihuman IL-4–II mAb (Mabtech). After the addition of streptavidin-ALP (Mabtech), the plates were developed with an alkaline phosphatase–conjugate substrate kit (Bio-Rad, Hercules, CA). The number of spots for each well was evaluated using the AID ELISPOT reader (Autoimmun Diagnostika, Strasburg, Germany). The number of spots produced by unstimulated PB lymphocytes was less than 20% of the number of spots obtained in response to peptides and was subtracted from the latter values. In some experiments, CD3+ T cells were purified by immunomagnetic sorting from involved LNs that were surgically removed before and after vaccination from 3 patients who achieved PR. These cells were then cultured for one week in the presence of autologous neoplastic B cells in medium supplemented with IL-2, and then evaluated by IFN-γ ELISPOT in response to autologous or allogeneic B-cell tumor challenge.
Purification of human Ig, biotinylation, and immunohistochemistry
Antibody purification from human sera was carried out using the MultiClear Kit (Cabru; Lesmo, Milan, Italy) following the manufacturer's suggested procedure. After purification, human IgG samples were biotinylated as previously described.31 The biotinylated antibodies were stored at 4°C until use for immunohistochemistry (IHC). IHC was carried out with formalin-fixed, paraffin-embedded tissue using heat-induced epitope retrieval with 10 mM citrate buffer (0.07 M pH 6.0; Bioptica, Milan, Italy), as described previously with minor modifications.32 Briefly, sections were incubated with biotynilated serum samples for 1 hour at room temperature and immune reaction was developed using peroxidase-conjugated streptavidin and finally visualized with the use of 3,3′-diaminobenzidine (Sigma Fast; Sigma-Aldrich, St Louis, MO) and counterstaining with hematoxylin.
Statistical analysis
Analysis of statistical significance was performed using the 2-sided Student t test. When the sample size was small, the Fisher exact test or the nonparametric Mann-Whitney test was used. The significance of the results was annotated as follows: * indicates P value less than .05; **, P value less than or equal to .01; and ***, P value less than or equal to .001.
Results
Quality control of vaccine preparations
The preparation of the vaccine was carried out when the percentage of κ or λ CD19+ cells was greater than or equal to 75%. The mean number of tumor cells harvested after CD19+ cell selection was 430 plus or minus 35 × 106 with viability greater than 90% as determined by trypan blue staining. PB-derived monocytes were skewed toward CD14−CD1a+CD83− immature DCs in the presence of rhGM-CSF and rhIL-4 as previously described.25 After 5 days of culture, the average number of generated DCs was 250 plus or minus 50 × 106 with a viability greater than 90% as determined by trypan blue staining. After coculturing the immature DCs with killed and heat-shocked tumor cells, more than 90% of the APCs had a mature DCs phenotype (CD80+, CD86+, CD83+, HLA-DR+, CD1a+; data not shown). All vaccine preparations were cleared from living CD19+ tumor cells, since B cells that eventually appeared in the cultures stained positive for propidium iodide. The mean number of injected DCs was 45 plus or minus 3 × 106. No microbiologic contaminations were observed in any stage of vaccine preparation.
Safety
All of the 18 enrolled patients received 4 doses of patient-specific vaccine. The only observed adverse events were injection site reactions that occurred in 66% of treated patients. Injections site reactions were transient (24-48 hours) and characterized by grade 1 (mild) or grade 2 (moderate) erythema (70%), pruritus (25%), tenderness (50%), and swelling (20%). None of the patients required medication. No cumulative toxicities were observed. No significant hepatic, renal, pulmonary, cardiac, hematologic, gastrointestinal, or neurologic toxicities attributable to the treatment were observed. In particular, no significant reduction of PB B cells or Ig levels was observed during or after the injections (data not shown). No signs of autoimmunity were detected, as measured by the absence of C-reactive protein and antinuclear antibodies (data not shown).
Efficacy
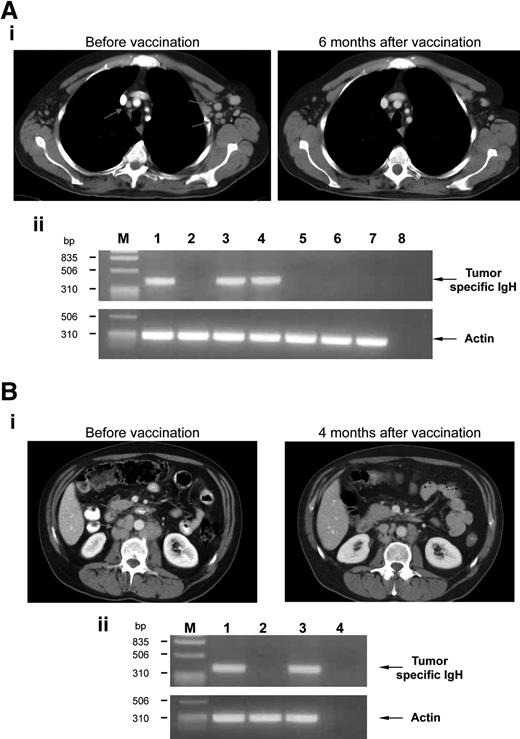
All patients were evaluated for vaccine activity with a median follow-up of 50.5 months (range, 7-63 months). Objective responses were identified in 6 (33.3%) of 18 patients (Table 1), including 3 complete responses (CRs) and 3 partial responses (PRs). The remaining 12 patients had stable disease (SD; n = 8) or progressive disease (PD; n = 4). The first patient experiencing a CR after vaccination (no. 12, Table 1) received the vaccine while in relapse after CVP regimen and with a tumor-specific IgH PCR signal in PB and BM. The CR achieved by this patient after CVP and before enrollment in the present study had lasted 36 months. A complete CT scan response of mediastinal and axillary LNs was achieved 6 months after the last injection (Figure 1Ai). In addition, the tumor-specific PCR signal disappeared from the PB and BM of this patient (Figure 1Aii). Presently, the patient remains in CR at 49 months without additional anticancer therapy. The second patient who experienced CR after vaccination (no. 13, Table 1) had relapsed after CVP followed by rituximab therapy. The CR achieved by this patient after rituximab therapy, and prior to enrollment in the present study, had lasted 24 months. After the initiation of the vaccination schedule, the overall tumor mass gradually reduced resulting in the attainment of CR 3 months after the last injection. Presently, the patient remains in CR at 46 months without additional anticancer therapy. The third patient (no. 14, Table 1) had relapsed after HDS and autologous hematopoietic stem cell transplantation. Of note, the CR achieved by this patient after autograft, and prior to enrollment in the present study, had lasted only 6 months. The patient attained CR 7 months after the last vaccine injection. The patient is still in CR with a follow-up of 45 months.
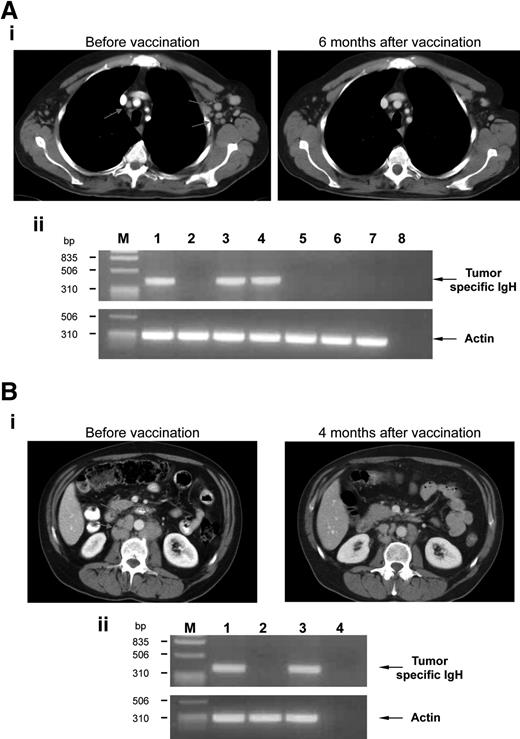
Therapeutic effects of vaccination. (A) Example of a clinical CR. (i) CT scan images of the chest of patient no. 12 revealed multiple small enlarged mediastinal and left axillary LNs (left, arrows) that normalized 6 months after vaccination (right). (ii) Involvement of BM (lanes 1 and 2: before vaccination and 6 months after vaccination, respectively) and PB (lanes 3, 4, 5, 6, and 7: before vaccination, and 3, 6, 12, and 18 months after vaccination, respectively) was evaluated by PCR amplification of the clonal tumor-specific IgH gene rearrangement using IgH-specific molecular probe. Actin amplification was performed as a control. Line 8 represents no DNA; M, marker. (B) Example of a clinical PR. (i) CT scan images of the retroperitoneum of patient no. 5 showed enlarged retroperitoneal LNs (left, arrows) that shrunk 4 months after vaccination (right). (ii) Involvement of BM (lanes 1, 2, and 3: before vaccination, and 4 and 12 months after vaccination, respectively) was evaluated by PCR amplification for panel A. Actin amplification was performed as a control. Line 4 represents no DNA; M, marker.
Therapeutic effects of vaccination. (A) Example of a clinical CR. (i) CT scan images of the chest of patient no. 12 revealed multiple small enlarged mediastinal and left axillary LNs (left, arrows) that normalized 6 months after vaccination (right). (ii) Involvement of BM (lanes 1 and 2: before vaccination and 6 months after vaccination, respectively) and PB (lanes 3, 4, 5, 6, and 7: before vaccination, and 3, 6, 12, and 18 months after vaccination, respectively) was evaluated by PCR amplification of the clonal tumor-specific IgH gene rearrangement using IgH-specific molecular probe. Actin amplification was performed as a control. Line 8 represents no DNA; M, marker. (B) Example of a clinical PR. (i) CT scan images of the retroperitoneum of patient no. 5 showed enlarged retroperitoneal LNs (left, arrows) that shrunk 4 months after vaccination (right). (ii) Involvement of BM (lanes 1, 2, and 3: before vaccination, and 4 and 12 months after vaccination, respectively) was evaluated by PCR amplification for panel A. Actin amplification was performed as a control. Line 4 represents no DNA; M, marker.
Among the 3 patients experiencing PR after vaccination, the first (no. 1, Table 1) had relapsed after CVP followed by rituximab therapy. The CR achieved by this patient after rituximab therapy, and prior to enrollment in the present study, had lasted 72 months. The patient achieved the PR 3 months after the last vaccine injection, and PR lasted 47 months. Between 47 and 60 months, the disease resumed slow progression and the patient was eventually admitted to second-line chemotherapy. The second patient (no. 5, Table 1) had relapsed after CVP followed by rituximab therapy. The CR achieved by this patient after rituximab therapy, and before enrollment in the present study, had lasted 15 months. The patient achieved a partial CT scan response of retroperitoneal LNs 6 months after the last injection (Figure 1Bi), which was associated with a negative BM tumor-specific IgH PCR signal (Figure 1Bii). The PR lasted 12 months until a BM PCR signal was documented (Figure 1Bii) that was associated with retroperitoneal LN progression. The patient was admitted to a HDS chemotherapy program and achieved continuous CR with a follow up of 42 months. The last patient (no. 6, Table 1) had relapsed after 3 chemotherapy (CHT) regimens (R-CEOP for 6 cycles, rituximab alone for 4 cycles, and HDS with autograft). The CR achieved by this patient after HDS, and prior to enrollment in the present study, had lasted 12 months. After vaccination, the patient experienced an initial PR that lasted 7 months followed by rapid tumor progression.
All nonresponding patients had conspicuous tumor burden or bulky disease at the time of enrollment. Disease stabilization was observed in 8 patients with a median duration of 43.5 months, whereas 4 patients progressed 1 month after the last vaccination. Three of 4 patients are alive after salvage HDS chemotherapy supported by autograft or allograft. One patient died following disease progression.
Immunologic monitoring of vaccinated patients
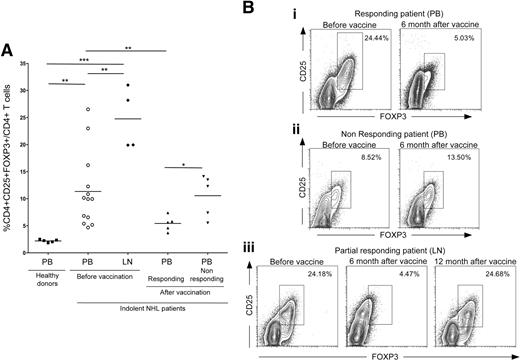
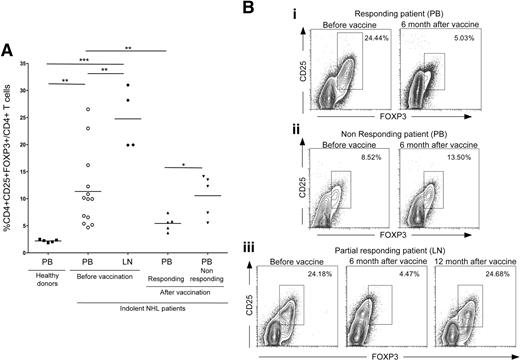
Recent findings demonstrated that the expansion and induction of suppressor cells with a “natural” CD25+CD4+FOXP3+ Treg phenotype in PB and in the tumor microenvironment of B-NHL patients are crucial factors contributing to the tumor immune evasion.33 To verify whether our DC-based vaccination could counteract this mechanism and restore the physiologic balance of Tregs in the PB and at the tumor site, the frequency of CD4+CD25+FOXP3+ T cells was evaluated for the enrolled patients before and after vaccination. At the time of the enrollment, NHL patients had a higher frequency of Tregs in the PB (P = .096) and LNs (P < .001) in comparison with healthy donors (Figure 2A). Interestingly, the PB Treg frequency decreased significantly within 6 months following vaccination, but only in the responder patients (P = .008; Figure 2A,Bi,ii). In addition, the frequency of CD4+CD25+FOXP3+ Tregs at tumor sites was considerably reduced at the time of response in partial responder patients (Figure 2Biii). Accordingly, when the disease progressed, the percentage of Tregs increased again to pretherapy values at the tumor site (Figure 2Biii). The absolute Treg count confirmed the reduction and the increase of Tregs in PB of responding and nonresponding patients after vaccination, respectively (data not shown).
Evaluation of Treg frequency in PB and LN of NHL patients before and after vaccination. (A) Schematic comparison of CD4+CD25+FOXP3+ T-cell frequencies in healthy donor PB, patient PB and LNs before vaccination, and responding and nonresponding patient PB after vaccination. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Representative examples of Treg frequencies in prevaccination versus postvaccination PB of the complete responding patient no. 14 (i), the nonresponding patient no. 7 (ii), and LN specimens collected before vaccination, 6 months after vaccination at the time of partial remission, and 12 months after vaccination at disease progression from the partial responding patient no. 5 (iii). All plots were gated on CD3+CD4+ cells. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur (Becton Dickinson, Lincoln Park, NJ). Analyses were performed using FlowJo 8.7.1 software version for Macintosh (TreeStar, Eugene, OR).
Evaluation of Treg frequency in PB and LN of NHL patients before and after vaccination. (A) Schematic comparison of CD4+CD25+FOXP3+ T-cell frequencies in healthy donor PB, patient PB and LNs before vaccination, and responding and nonresponding patient PB after vaccination. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Representative examples of Treg frequencies in prevaccination versus postvaccination PB of the complete responding patient no. 14 (i), the nonresponding patient no. 7 (ii), and LN specimens collected before vaccination, 6 months after vaccination at the time of partial remission, and 12 months after vaccination at disease progression from the partial responding patient no. 5 (iii). All plots were gated on CD3+CD4+ cells. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur (Becton Dickinson, Lincoln Park, NJ). Analyses were performed using FlowJo 8.7.1 software version for Macintosh (TreeStar, Eugene, OR).
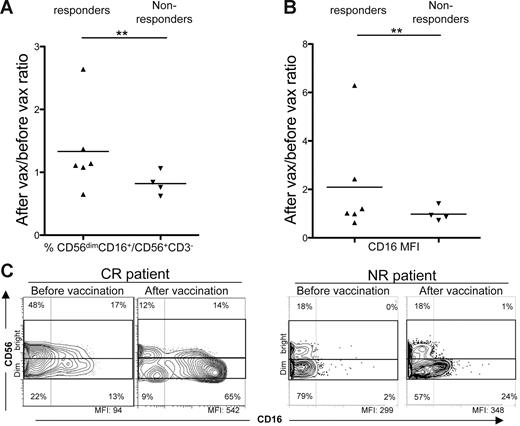
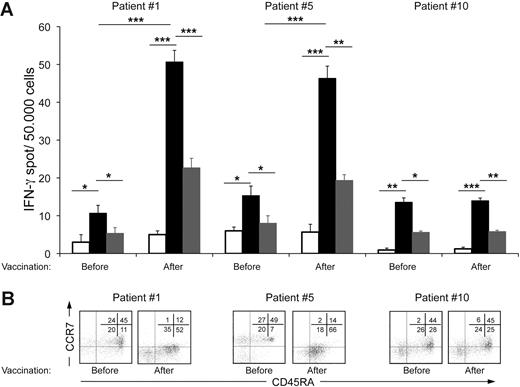
We then evaluated the frequency and phenotype of NK cells before and after vaccination in enrolled patients, because upon activation they would be expected to prevent Treg differentiation, and promote T-cell proliferation and effector functions.34,35 By evaluating the ratio of frequencies of postvaccination versus prevaccination NK cells with a cytotoxic immunophenotype (CD3−CD56dimCD16+) in PB samples, we found a statistically significant increase of positive cells (P = .009; Figure 3A) or CD16 MFI ratios (P = .008; Figure 3B) for NK cells in responder compared with nonresponder patients (Figure 3C). Moreover, 4 of 6 responder patients also showed increased expression of the NKp46 activating receptor after vaccination in comparison with 1 of 4 nonresponding patients with a similar change (P = .044; Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). To investigate the activity of our vaccination in boosting T cell–mediated responses to autologous tumors, we evaluated the function and maturation of lymphocytes from vaccinated patients. T cells producing IFN-γ in response to autologous tumor challenge were found in prevaccination LNs of these patients and with higher frequency in comparison with responses to allogeneic HLA-mismatched B-cell tumor challenge, included as positive control (Figure 4A). Moreover, the frequency of IFN-γ–producing T cells stimulated by the autologous B-cell tumor was significantly increased in LN samples harvested 6 months after the last vaccine administration (Figure 4A). A similar assay carried out in patients with SD or PD did not provide evidence of an increase of IFN-γ releasing T cells specific for autologous tumor antigens after vaccination (Figure 4A). Comparison of the maturation phenotype of prevaccination versus postvaccination CD4+ and CD8+ T cells from involved LNs of 2 of 3 patients with PR showed a shift toward the CCR7−CD45RA− T Teffector memory (TEM) and/or CCR7−CD45RA+ Tterminally differentiated (TTD) stages after vaccination (Figure 4B, patient nos. 1 and 5). Again, the maturation of tumor-infiltrating lymphocytes (TILs) in LNs of patients with SD or PD was not modified by the vaccination procedure (Figure 4B).
Evaluation of CD56dimCD16+ NK frequency in NHL patient PB before and after vaccination. (A) Ratios of CD56dimCD16+ NK frequencies in postvaccination versus prevaccination PB samples of responders and nonresponders.(B) Ratios of CD16 MFI in NK cells of postvaccination versus prevaccination PB samples of responders and nonresponders. Statistically significant differences were calculated using the Fisher exact test (**P ≤ .01). (C) Representative examples of CD56dimCD16+ NK frequencies in prevaccination versus postvaccination PB of a complete responder (left panels, patient no. 14), and of a nonresponder (right panels, patient no. 7). All plots were gated on CD3−CD56+ cells. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
Evaluation of CD56dimCD16+ NK frequency in NHL patient PB before and after vaccination. (A) Ratios of CD56dimCD16+ NK frequencies in postvaccination versus prevaccination PB samples of responders and nonresponders.(B) Ratios of CD16 MFI in NK cells of postvaccination versus prevaccination PB samples of responders and nonresponders. Statistically significant differences were calculated using the Fisher exact test (**P ≤ .01). (C) Representative examples of CD56dimCD16+ NK frequencies in prevaccination versus postvaccination PB of a complete responder (left panels, patient no. 14), and of a nonresponder (right panels, patient no. 7). All plots were gated on CD3−CD56+ cells. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
Functional and phenotypic analysis of T cells at tumor site from partial responding or nonresponding patients. (A) T cells, isolated from involved LNs before and after vaccination, were evaluated by IFN-γ ELISPOT assay when cultured alone (□), with the autologous tumor (■), or with an allogeneic tumor ( ). Results obtained for 2 of 3 partial responders and for a nonresponder patient are reported. Error bars indicate standard deviation of the mean. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Maturation profile of T cells at tumor site before and after vaccination was assessed by flow cytometry analysis of CCR7 and CD45RA expression in the CD3+CD8+ gated population. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
). Results obtained for 2 of 3 partial responders and for a nonresponder patient are reported. Error bars indicate standard deviation of the mean. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Maturation profile of T cells at tumor site before and after vaccination was assessed by flow cytometry analysis of CCR7 and CD45RA expression in the CD3+CD8+ gated population. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
Functional and phenotypic analysis of T cells at tumor site from partial responding or nonresponding patients. (A) T cells, isolated from involved LNs before and after vaccination, were evaluated by IFN-γ ELISPOT assay when cultured alone (□), with the autologous tumor (■), or with an allogeneic tumor ( ). Results obtained for 2 of 3 partial responders and for a nonresponder patient are reported. Error bars indicate standard deviation of the mean. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Maturation profile of T cells at tumor site before and after vaccination was assessed by flow cytometry analysis of CCR7 and CD45RA expression in the CD3+CD8+ gated population. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
). Results obtained for 2 of 3 partial responders and for a nonresponder patient are reported. Error bars indicate standard deviation of the mean. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Maturation profile of T cells at tumor site before and after vaccination was assessed by flow cytometry analysis of CCR7 and CD45RA expression in the CD3+CD8+ gated population. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
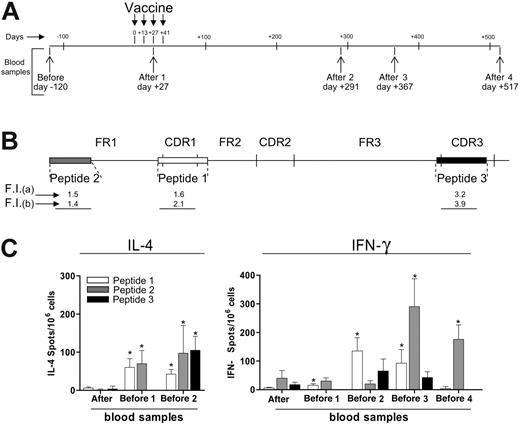
In contrast to the results obtained with T cells from the tumor site, IFN-γ ELISPOT for PB lymphocytes from both responding and nonresponding patients failed to show evidence of an increase in T cells reacting to autologous tumor antigens after vaccination (data not shown). Therefore, we attempted to document a T cell–mediated response to peptides encoded by the tumor-specific IgH sequence. One of the patients in CR after vaccination expressed HLA-A*0201 and this gave us the opportunity to evaluate PB samples, taken before and after vaccination (Figure 5A), for the presence and frequency of peptide-specific T cells directed to HLA-A2–binding peptides (Figure 5B) corresponding to different regions of the tumor-specific IgH sequence. Using the HLA stabilization assay, all 3 selected peptides were found to bind to HLA-A2 molecules as indicated by fluorescence index (FI) values after staining of T2 cells with 2 different anti–HLA-A2 mAbs (Figure 5B). By ELISPOT assay, an increased frequency of T cells releasing IL-4 or IFN-γ (Figure 5C) in response to all the 3 peptides was found in postvaccination PB samples of this patient in comparison with prevaccination samples (P < .05). Among the 3 tested peptides, the CDR1- and FR1-encoded peptides gave rise to a stronger and longer-lasting response, detected up to 517 days (for IFN-γ) or 291 days (IL-4) after the first vaccine administration. However, a significant increase in the frequency of IL-4–releasing T cells was found even against the CDR3-encoded peptide in a PB sample taken 291 days after vaccination (Figure 5C).
Circulating peptide-specific T cells directed to tumor-specific IgH-encoded epitopes in a vaccinated patient. (A) PB samples were obtained from patient no. 14 at the indicated time points before (pre) and after (post) vaccination. (B) Synthetic peptides (indicated as peptides nos. 1, 2, and 3) corresponding to CDR1, FR1, and CDR3 sequences of the tumor-specific IgH sequence from patient no. 14 were used to assess cytokine release by circulating T cells by ELISPOT. Fluorescence index (FI) as evaluated by the HLA-A2 stabilization assay after staining of T2 cells with mAb CR11.351 (a) or BB7.2 (b). (C) Frequencies of IL-4– or IFN-γ–producing T cells against peptides nos. 1, 2, and 3, as evaluated by ELISPOT in blood samples taken before and after vaccination from patient no. 14. The HLA-A*0201–binding HIV peptide ILKEPVHGV was used as negative control in both the IL-4 and IFN-γ assays. Response to this peptide was fewer than 10 spots/106 cells in all blood samples analyzed. * indicates frequency of cytokine-releasing T cells, in the indicated blood samples, was significantly higher compared with prevaccination values (Mann-Whitney test, P < .05). Error bars indicate standard deviation of the mean.
Circulating peptide-specific T cells directed to tumor-specific IgH-encoded epitopes in a vaccinated patient. (A) PB samples were obtained from patient no. 14 at the indicated time points before (pre) and after (post) vaccination. (B) Synthetic peptides (indicated as peptides nos. 1, 2, and 3) corresponding to CDR1, FR1, and CDR3 sequences of the tumor-specific IgH sequence from patient no. 14 were used to assess cytokine release by circulating T cells by ELISPOT. Fluorescence index (FI) as evaluated by the HLA-A2 stabilization assay after staining of T2 cells with mAb CR11.351 (a) or BB7.2 (b). (C) Frequencies of IL-4– or IFN-γ–producing T cells against peptides nos. 1, 2, and 3, as evaluated by ELISPOT in blood samples taken before and after vaccination from patient no. 14. The HLA-A*0201–binding HIV peptide ILKEPVHGV was used as negative control in both the IL-4 and IFN-γ assays. Response to this peptide was fewer than 10 spots/106 cells in all blood samples analyzed. * indicates frequency of cytokine-releasing T cells, in the indicated blood samples, was significantly higher compared with prevaccination values (Mann-Whitney test, P < .05). Error bars indicate standard deviation of the mean.
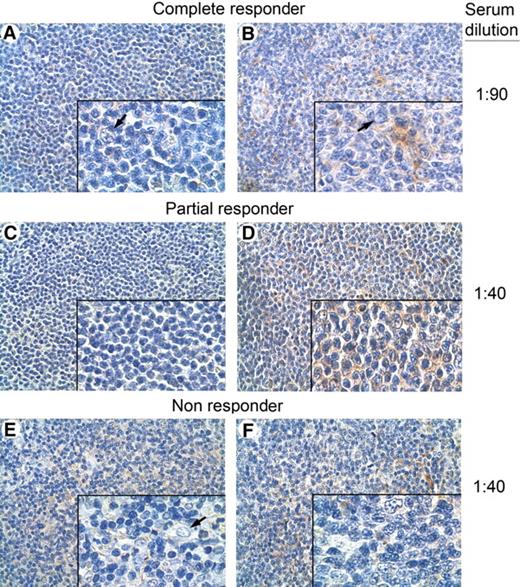
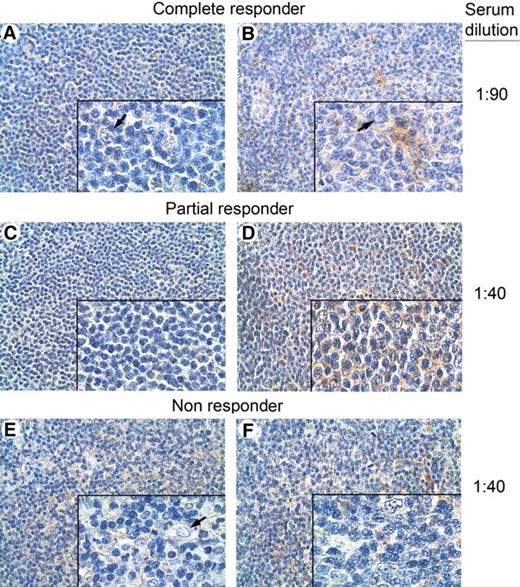
Finally, to determine whether responding patients also developed a tumor-specific antibody response, IgG antibodies were purified from prevaccination and postvaccination serum samples from 2 responding and 2 nonresponding patients, and used to immunostain autologous tumor biopsies (Figure 6). Only the antibodies isolated from the postvaccination sera of one patient in CR and one in PR showed tumor cell–restricted reactivity in comparison with the matched prevaccination serum samples (Figure 6A,B and 6C,D, respectively). Normal cells in the tumor biopsies from these patients showed no immunostaining (Figure 6A,B,E). No tumor-restricted immunostaining was detected using biotinylated IgG isolated from prevaccination and postvaccination serum samples from the 2 nonresponder patients (Figure 6E,F). These results may suggest that the promotion of a tumor-specific B-cell immune response occurs in responder patients.
Immunohistochemical analysis of tumor biopsies with autologous serum. Immunohistochemical analysis of NHL specimens, with a homogeneous population of small lymphoid cells from a complete (A,B), a partial (C,D), and a nonresponding patient (A-F), immunostained with biotin-conjugated autologous prevaccination (A,C,E) and postvaccination (B,D,F) serum samples at the indicated serum dilutions. Lymphoid neoplastic cell-restricted autoreactivity was evidenced only when the postvaccination sera of the complete (B) and the partial (D) responders were used. No tumor-restricted immunostaining was detected either in prevaccination (E) or postvaccination (F) serum samples of a nonresponding patient. Nonneoplastic follicular DCs and endothelial cells present in the inset of panels A, B, and E showed no immunostaining ( ). Panel insets are shown at ×40 original magnification; panels, at ×10 original magnification. Images were acquired using Leica DMD108 (Heidelberg, Germany) equipped with HI Plan 10×/0.25 and 40×/0.65 objectives and processed in Adobe Photoshop CS3 software (San Jose, CA).
). Panel insets are shown at ×40 original magnification; panels, at ×10 original magnification. Images were acquired using Leica DMD108 (Heidelberg, Germany) equipped with HI Plan 10×/0.25 and 40×/0.65 objectives and processed in Adobe Photoshop CS3 software (San Jose, CA).
Immunohistochemical analysis of tumor biopsies with autologous serum. Immunohistochemical analysis of NHL specimens, with a homogeneous population of small lymphoid cells from a complete (A,B), a partial (C,D), and a nonresponding patient (A-F), immunostained with biotin-conjugated autologous prevaccination (A,C,E) and postvaccination (B,D,F) serum samples at the indicated serum dilutions. Lymphoid neoplastic cell-restricted autoreactivity was evidenced only when the postvaccination sera of the complete (B) and the partial (D) responders were used. No tumor-restricted immunostaining was detected either in prevaccination (E) or postvaccination (F) serum samples of a nonresponding patient. Nonneoplastic follicular DCs and endothelial cells present in the inset of panels A, B, and E showed no immunostaining ( ). Panel insets are shown at ×40 original magnification; panels, at ×10 original magnification. Images were acquired using Leica DMD108 (Heidelberg, Germany) equipped with HI Plan 10×/0.25 and 40×/0.65 objectives and processed in Adobe Photoshop CS3 software (San Jose, CA).
). Panel insets are shown at ×40 original magnification; panels, at ×10 original magnification. Images were acquired using Leica DMD108 (Heidelberg, Germany) equipped with HI Plan 10×/0.25 and 40×/0.65 objectives and processed in Adobe Photoshop CS3 software (San Jose, CA).
Discussion
In this trial, we tested the efficacy and the activity of a vaccination program based on autologous tumor-loaded DCs for the treatment of indolent NHL patients with measurable disease. Our vaccination resulted in enhanced antitumor activity in 6 of 18 patients, including 3 objective radiographic CR and 3 PRs. In all 3 patients with CR, the duration of the clinical response after vaccination was longer compared with the duration of the first and second complete remissions achieved with the previous treatments. In agreement with a recent study by Inogès et al,36 which used patient-specific Id vaccination, second longer remissions indicate a therapeutic effect. Differently from the study of Bendandi et al,10,36 our clinical results were observed in patients who were not in chemotherapy-induced remission at the time of vaccination.
In several published trials in indolent NHL patients, immunologic and clinical responses were observed after a vaccination against the tumor-specific Id used alone or with DCs as a natural adjuvant.8-11,36 However, recently 2 of 3 randomized phase 3 studies failed to demonstrate an improvement in the progression-free survival of FL patients after specific Id vaccination.13 As pointed out by Bendandi,37 the failure of randomized phase 3 studies with Id vaccine may be due to the clinical features of FL disease and to the heterogeneous characteristics of patient-tailored Id-based immunotherapy. Nevertheless, in the Levy study13 the patients mounting Id-specific immune responses after the vaccination showed a significant clinical benefit in terms of progression free survival. These results paved the way for identifying novel vaccination methods to increase the immunologic response rate. For this purpose, we used the whole tumor as a source of antigen with the intent to promote activation of immune responses not only to tumor-specific IgH-encoded epitopes, but also to the wide array of recently identified shared lymphoma-associated antigens recognized by T cells in NHL patients.38-40 In agreement with this hypothesis, Neelapu et al14 recently reported the clinical and immunologic results of a pilot clinical trial with a novel autologous whole tumor–derived proteoliposome vaccine formulation. To further improve this approach, we exposed the neoplastic B cells to γ-irradiation and UV-C after being heat shocked to increase the immunogenicity of tumor cells through an immunogenic death.21
The vast majority of studies of active immunotherapy targeting the B-cell Id have been designed to verify the role of vaccination in avoiding disease recurrences after chemotherapy with a debulking intent.10,11 The rationale of this approach was that the antitumor immune response promoted by the vaccine could be clinically effective only in presence of a limited tumor load. In the present study, we treated patients with clinically measurable disease, although all CRs and PRs were observed in patients with the smallest tumor burden. The responses were independent of the histologic type, prevaccination chemotherapy (comprising HDS therapy), the source of neoplastic cells for vaccine preparation (LN versus PB), DC maturation/activation profile, and other clinical characteristics. The evidence of lack of responses in the majority of patients might suggest that this immune intervention could not generate sufficient numbers of effector T cells with the appropriate specificity, functional status, homing properties, and survival characteristics to overcome existing tumor escape mechanisms and to promote effective antitumor responses in the presence of a significant tumor load.41,42
Immunologic monitoring of the enrolled patients documented significant changes in Treg, NK, and antitumor T-cell frequencies, and humoral responses in responding patients compared with nonresponding ones. This indicates that the clinical responses observed after DC-based vaccination may be the result of an active modulatory effect on different components of the immune system. The dramatic reduction of Treg frequency after vaccination in both PB and LN of responder patients suggests that our DC-based vaccine might induce antitumor responses even by downmodulating Treg-mediated surveillance. Accordingly, tumor progression was found to be associated with an increase in the Treg frequency in the PB and involved LNs. The mechanisms underlying the observed Treg reduction after vaccination in responding patients remain to be elucidated. Possibilities include recruitment and/or expansion from the circulating Treg population. Recent findings reported that NHL cells can recruit Tregs by production of chemokine CCL22.43 In addition, conventional T cells can be converted to functionally competent Tregs within the tumor microenvironment.33,44 In support of this hypothesis, it has been shown that B-NHL cells may produce factors such as TGF-β,45 which in turn could promote the generation/expansion of Tregs. Therefore, a reduced tumor load in responding patients may be responsible for the lowering of Treg frequency.45-47 Alternatively, Treg reduction may be directly induced by a successful immunization. In agreement with this mechanism, Hueman et al48 recently described a reduction in circulating Tregs upon immunization of breast cancer patients with a HER-2 neu peptide vaccine that was paralleled by a CD8+ vaccine-specific response. Another possibility is that activated NK cells may act as a functional bridge between the injected DCs and the observed reduction in FOXP3+ Tregs. In fact, as shown by Martín-Fontecha et al,49 the injection of mature DCs promotes the activation of NK cells that, in turn, provides a source of IFN-γ that is needed for Th1 polarization. In addition, a recent study has indicated that activated NK cells may suppress conversion to FOXP3+ regulatory function in CD4+ T lymphocytes.35 Interestingly, immune monitoring of vaccinated patients showed an increased fraction of NK cell subsets with a cytotoxic phenotype in the majority of the responding patients. Therefore, it is possible that effective DC-based vaccination may lead to the reduction of FOXP3+ CD25+ CD4+ Tregs through activation of NK cells.
In contrast to nonresponding patients, the frequency of antitumor T cells in LNs of patients with PR was significantly increased compared with prevaccination samples, suggesting that immune modulation promoted by the vaccine contributed to the observed tumor responses. In addition, CD4+ and CD8+ TILs showed a shift toward the TEM and/or TTD maturation stage after vaccination, consistent with the promotion of T-cell effector functions at tumor sites. Nevertheless, IFN-γ ELISPOT in PB samples did not detect T cells reacting to autologous tumors after vaccination in patients who achieved PR or CR. It is likely that the frequency of tumor-specific T cells in the PB was below the assay detection limit, probably due to their selective homing to tumor sites. However, in the complete responding patient who expressed HLA-A*0201, an increased frequency of IFN-γ– or IL-4–releasing lymphocytes against CDR1-, FR1-, and even CDR3-derived peptides of the tumor-specific IgH sequence was found after vaccination. The responses against these epitopes were observed up to 10 (for IFN-γ) and 17 (for IL-4) months after vaccination, suggesting that immunization with tumor-loaded DCs has a long-lasting effect on the immune response to tumor-specific IgH-encoded peptides, as seen even with Id-specific vaccination.11 The identification of an increased frequency of IL-4–releasing T cells in response to IgH-encoded peptides in one CR patient also suggests that the vaccine can promote a TH2-type immune response. Such responses would be expected to provide helper functions for B-cell differentiation, a key process in the promotion of a humoral response to neoplastic cells. Accordingly, postvaccination sera from the responding patients, but not from nonresponders, contained antibodies that specifically stained autologous neoplastic cells. These results suggest that the promotion of antitumor humoral immunity by the vaccine contributed to the observed clinical responses. This hypothesis is in agreement with results in FL patients after idiotype vaccination, where a strong association of clinical outcome with antibody response has been documented.50 In conclusion, we demonstrated that active immunization with DCs loaded with patient-specific heat-shocked and irradiated tumor cells provided clinical and immunologic efficacy in some previously treated indolent NHL patients with measurable disease. The efficacy of the vaccine may also be enhanced in first-line therapy, when the immune competence of patients is not be impaired by previous chemotherapy regimens, or in a setting of MRD when active immunotherapy may offer the best chance of controlling disease relapse.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients for their generous participation in this study. We also thank Ilaria Bersani, Sara Napoli, Luca Romagnoli, Stefania Cresta, Paolo Longoni, Marco Milanesi, and Gianni Roncato for excellent technical assistance. We are grateful to Drs Maddalena Marchesi, Alfonso Marchianò, Carlo Spreafico, and Daniele Morelli (Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy) for their contribution to the clinical management of patients and Dr Umberto Vitolo (Molinette Hospital, Turin, Italy) for patient referral.
This study was supported in part by research funding by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan, Italy) and Fondazione Michelangelo (Milan, Italy) (M.D.N.).
Authorship
Contribution: M.D.N., A.A., and A.M.G. designed the research and wrote the paper; R.Z., C.C., R.M., M.M., and P.B. performed experiments; M.D.N. and R.Z. analyzed results and made the figures; A.C. and S.M.P. performed immunohistochemistry; and L.D., P.M., and F.R. were involved in patient care and follow-up.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Massimo Di Nicola, “Cristina Gandini” Bone Marrow Transplantation Unit, Istituto Nazionale per lo Studio e la Cura dei Tumori, Via Venezian 1, 20133 Milan; e-mail: massimo.dinicola@istitutotumori.mi.it.
References
Author notes
*A.A. and A.M.G. contributed equally to this study.




 ). Results obtained for 2 of 3 partial responders and for a nonresponder patient are reported. Error bars indicate standard deviation of the mean. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Maturation profile of T cells at tumor site before and after vaccination was assessed by flow cytometry analysis of CCR7 and CD45RA expression in the CD3+CD8+ gated population. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.
). Results obtained for 2 of 3 partial responders and for a nonresponder patient are reported. Error bars indicate standard deviation of the mean. Statistically significant differences, by 2-sided Student t test, are reported (*P ≤ .05; **P ≤ .01; ***P ≤ .001). (B) Maturation profile of T cells at tumor site before and after vaccination was assessed by flow cytometry analysis of CCR7 and CD45RA expression in the CD3+CD8+ gated population. Data were acquired using BD CellQuest software version 3.3 on the 4-color analysis flow cytometer BD FACSCalibur. Analyses were performed using FlowJo 8.7.1 software version for Macintosh.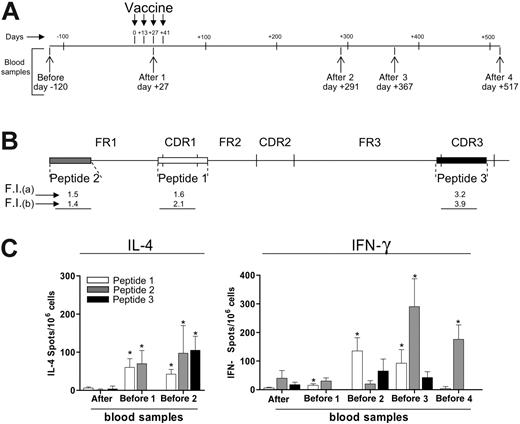
 ). Panel insets are shown at ×40 original magnification; panels, at ×10 original magnification. Images were acquired using Leica DMD108 (Heidelberg, Germany) equipped with HI Plan 10×/0.25 and 40×/0.65 objectives and processed in Adobe Photoshop CS3 software (San Jose, CA).
). Panel insets are shown at ×40 original magnification; panels, at ×10 original magnification. Images were acquired using Leica DMD108 (Heidelberg, Germany) equipped with HI Plan 10×/0.25 and 40×/0.65 objectives and processed in Adobe Photoshop CS3 software (San Jose, CA).