Abstract
Ex vivo–expanded tumor-infiltrating lymphocytes infused into lymphodepleted recipients has clear antitumor efficacy. More practical sources of such antitumor lymphocytes would broaden the application of this approach. Previously, we described an in situ vaccination combining chemotherapy with intratumoral injection of CpG-enriched oligonucleotides, which induced T-cell immunity against established lymphoma. An ongoing clinical trial of this maneuver has demonstrated clinical responses in lymphoma patients. Here, we use this vaccine maneuver to generate immune cells for transfer into irradiated, syngeneic recipients. Transferred tumor-specific T-effector (Teff) cells preferentially expanded, increasing the Teff/T-regulatory (Treg) ratio in these “immunotransplantation” recipients and curing large and metastatic tumors. Donor T cells were necessary for tumor protection, and CD8 T-cell immune responses were enhanced by posttransplantation booster vaccination. Hematopoietic stem cell transplantation is a standard therapy for lymphoma. Therefore, in situ tumor vaccination followed by immunotransplantation of harvested tumor-specific T cells could be directly tested in clinical trials to treat otherwise resistant malignancies.
Introduction
Previously, we have described a practical way of achieving therapeutic antitumor immunity by administering cytotoxic therapy to release tumor antigens combined with intratumoral injection of an immunostimulatory CG-enriched oligodeoxynucleotide (CpG). This vaccination maneuver induces tumor-specific CD8 T-cell immunity and causes tumor remission both in a preclinical model of murine B-cell lymphoma1 and in patients with B-cell lymphomas.
To further improve this therapeutic maneuver, we have considered possible obstacles to the development of antitumor immunity, such as regulatory cells and myeloid suppressor cells. Myeloablative therapy with hematopoietic stem cell transplantation, a standard therapy for hematologic malignancies, could address these problems by eliminating regulatory immune cells2,3 and making “space” for the expansion of specific antitumor T cells. Dudley et al have demonstrated a remarkable antitumor effect of transferring cultured tumor infiltrating T cells (TILs) into lymphodepleted melanoma patients.4 However, it is unknown whether transferred peripheral blood mononuclear cells (PBMCs) from a vaccinated host could be as powerful. One concern is that transferred PBMCs would contain T-regulatory (Treg) cells, which may have persistent, suppressive effects on transferred tumor-specific T effectors (Teff).
The cytokines chiefly responsible for homeostatic T-cell proliferation (interleukin-7 [IL-7], IL-15, and IL-21) have been shown to preferentially affect Teff over Treg.5,6 We hypothesized that adoptive transfer of vaccine-primed, anticancer T cells into syngeneic hematopoietic stem cell transplantation recipients (“immunotransplantation”) would cause relatively greater proliferation of tumor-specific Teff and skewing against transferred Treg, resulting in a potent antitumor state.
We have devised such an immunotransplantation maneuver and demonstrated its preferential proliferative effect on transferred, tumor-specific Teff as well as its impressive power to treat large tumors. We anticipate that these results could be directly translated from the preclinical model to a clinical trial in patients with lymphoma.
Methods
Reagents
CpG 1826 5′-TCCATGACGTTCCTGACGTT was provided by Coley Pharmaceutical Group (Ottawa, ON). Bold sequences highlight hypomethylated cytosine–guanosine pairs. Cyclophosphamide (CTX) and neomycin were purchased from Sigma-Aldrich (St Louis, MO). Luciferin was purchased from Xenogen (Alameda, CA).
Cell lines and animal models
A20, a BALB/c B-cell lymphoma line, expressing major histocompatibility complex (MHC) class I and class II H-2d molecules, was obtained from ATCC (Manassas, VA). Tumor cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone Laboratories, Logan, UT), 100 U/mL penicillin and 100 μg/mL streptomycin (both from Invitrogen), and 50 μM 2-mercaptoethanol (Sigma-Aldrich). Cells were grown in suspension culture at 37°C in 5% CO2. Eight- to 10-week-old female BALB/c mice were purchased from Harlan (Indianapolis, IN). All experiments were conducted in accord with National Institutes of Health guidelines and with approval of the Stanford University Institutional Animal Care and Use Committee.
Flow cytometry
The following monoclonal antibodies were used in this study: phycoerythrin (PE) antimouse interferon-γ (IFN-γ), fluorescein isothiocyanate antimouse CD8, PE antimouse CD8β, peridinin chlorophyll protein (PerCP) antimouse CD3, PerCP antimouse CD4, PerCP antimouse CD8, and allophycocyanin (APC) antimouse CD44 (BD PharMingen, San Diego, CA). Cells were stained in phosphate-buffered saline (PBS), 1% bovine serum albumin, and 0.01% sodium azide and analyzed by flow cytometry on a BD FACSCalibur System. Donor splenocytes were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) in a 5-μM solution in PBS.
CpG/CTX vaccination
A20 lymphoma cells (107 in 100 μL PBS) were implanted subcutaneously on the lower back in 8- to 10-week-old BALB/c mice. Treatments began when tumors reached a size of approximately 100 mm2, typically at day 14 after tumor inoculation. The chemotherapy agent CTX was then administrated intraperitoneally at a dose of 100 mg/kg on each of 2 consecutive days. CpG was then injected intratumorally at a 100-μg/dose in 100 μL PBS on each of the 5 days after chemotherapy. Using this maneuver, a CD8 T-cell response caused most mice to be cured.1 Such mice were used as splenocyte donors 7 days after the completion of vaccination.
Immunotransplantation
Eight- to 10-week old, naive, recipient mice were irradiated with 900 cGy of total body irradiation (TBI) in a Philips x-ray unit (250 kV, 15 mA). Irradiated recipients were injected intravenously with 5 × 106 bone marrow (BM) cells in 0.5 mL RPMI 1640 medium in the tail vein admixed with splenocytes. Unless otherwise specified, splenocyte dose is one spleen per one recipient. In all experiments, BM was taken from the same source as donor spleens to more closely parallel the clinical application of this approach. Beginning 2 days before ablative therapy and thereafter, drinking water was supplemented with 1 mg/mL neomycin for gut decontamination. In the tumor-protective setting, recipient mice were implanted subcutaneously on day 3 after transplantation with 107 A20 cells. In the tumor-therapeutic setting, mice were challenged with 107 A20 cells 14 days before transplantation/transfer and selected at the time of treatment such that all cohorts harbored tumors of approximately 100 mm2. The growth of tumor was monitored by calipers 3 times per week. In some experiments, donor splenocytes were labeled with CFSE.
In some experiments, a vaccine “boost” was administered, consisting of 106 A20 cells cultured in the presence of CpG1826 at a final concentration of 3 μg/mL for 72 hours and irradiated to 50 Gy immediately before injection. These boosts were administered either intravenously or subcutaneously.
Immunotransplantations were performed using donor splenocyte cell subsets by positive isolation using Dynabeads FlowComp Mouse CD4 or CD8 Kits or depletion using Dynabeads Mouse Pan T (Thy 1.2) Kits (Invitrogen) as per protocols. Of note, FlowComp Mouse CD8 positive selection is based on anti-CD8α monoclonal antibody (mAb) binding such that selected cells can be phenotypically assessed using anti-CD8β mAb without risk of selecting/detecting mAb interference.
Detection of tumor-reactive T cells
Blood was collected from tail vein, anticoagulated with 2 mM ethylenediaminetetraacetic acid in PBS, then diluted 1:1 with Dextran T500 (Pharmacosmos, Holbaek, Denmark) 2% in PBS and incubated at 37°C for 45 minutes to precipitate red cells. Leukocyte-containing supernatant was removed and centrifuged, and remaining red cells were lysed with ammonium chloride potassium buffer (Quality Biological, Gaithersburg, MD). PBMCs were then cocultured with 5 × 105 irradiated A20 cells for 24 hours with 0.5 μg antimouse CD28mAb (BD PharMingen) and in the presence of monensin (Golgistop; BD Biosciences, San Jose, CA) for the last 5 hours at 37°C and 5% CO2. Tumor specificity of the response was assessed by parallel experiments coculturing PBMCs with 5 × 105 irradiated CT26 cells, a BALB/c colon cancer cell line (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Cells were then washed and stained with anti-CD8 fluorescein isothiocyanate and anti-CD4 allophycocyanin (BD Biosciences). Intracellular IFN-γ expression was assessed using BD Cytofix/Cytoperm Plus Kit per instructions and BD anti–IFN-γ PE-conjugated Ab. Cells were analyzed on a FACSCalibur flow cytometer.
Bioluminescence
The A20-luficerase cell line was provided by R. Negrin (Stanford University Medical Center, Stanford, CA).7 Mice were anesthetized with isofluorane, and an aqueous solution of luciferin (150 mg/kg intraperitoneally, Xenogen) was injected 5 minutes before imaging. Animals were placed into the light-tight chamber of the CCD Camera System (IVIS-200; Xenogen). Photons emitted from luciferase-expressing cells within the animal body were quantified using the software program Living Image (Xenogen) as an overlay on Igor (Wavemetrics, Seattle, WA).
Histology
Tissues were fixed in 10% buffered formalin and embedded in paraffin. Sections (5 μm) were stained with haematoxylin and eosin. Slides were viewed using a Nikon ECLIPSE E 800 microscope (Nikon, Melville, NY) using a 20× lens, and images were acquired using a SPOT RT-SLIDER camera and accompanying SPOT software version 4.6 (Diagnostic Instruments, Sterling Heights, MI).
Results
Treg increase in CpG/CTX-vaccinated mice, but immunotransplantation increases the Teff/Treg ratio
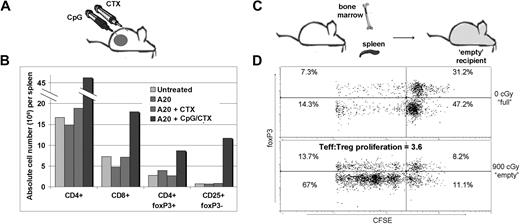
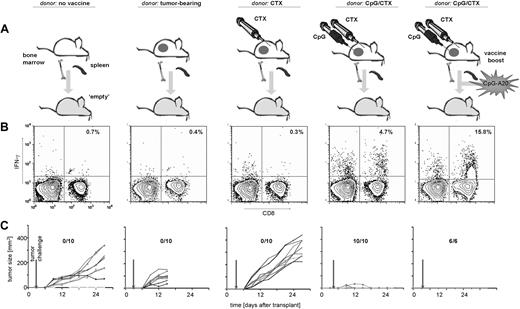
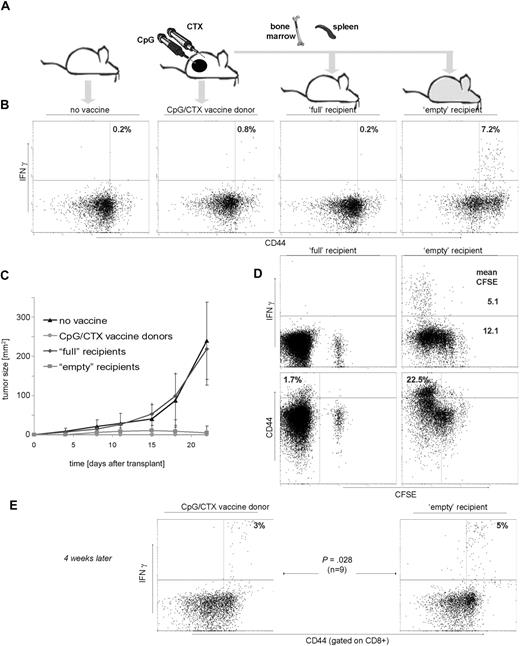
Our prior work described an intratumoral CpG plus systemic CTX vaccine maneuver (CpG/CTX). Briefly, mice with subcutaneous A20 tumors received systemic CTX followed by 5 daily intratumoral injections of CpG-1826, resulting in tumor regression mediated by CD8 T cells. As described in other tumor vaccine systems,8 there was an absolute increase in the number of Treg cells after vaccination despite the development of tumor-specific immunity (Figure 1B). Therefore, we asked whether the transfer of splenocytes into lympho-depleted recipients could skew the T-cell population against Treg. CFSE-labeled splenocytes were transferred into untreated (“full”) or lethally irradiated (“empty”) recipients. Recipient T cells were assessed at day 15 for CFSE dilution. “Empty” recipients showed marked Teff-cell proliferation: 3.6-fold more so than foxP3+ Treg (Figure 1C). Minimal proliferation occurred in “full” recipients.
Treg increase in CpG/CTX-vaccinated mice and preferential Teff proliferation during immunotransplantation. (A) Mice received CpG/CTX vaccination as described. (B) On day 15 after vaccination, donor mice splenocytes were assessed for T-cell subsets by flow cytometry. (C) Splenocytes were taken from wild-type donor mice and labeled with 5 μg/mL CFSE and injected along with unlabeled BM cells into recipients that received either no (0 cGy) or lethal (900 cGy) TBI. (D) On day 15 after transplantation, splenocytes were taken from 3 recipient mice and separately measured by flow cytometry for CFSE, surface CD4, and intracellular foxP3. Data shown are gated on live, CFSE+, CD4+ cells. Data shown are representative of the 3 individual recipients.
Treg increase in CpG/CTX-vaccinated mice and preferential Teff proliferation during immunotransplantation. (A) Mice received CpG/CTX vaccination as described. (B) On day 15 after vaccination, donor mice splenocytes were assessed for T-cell subsets by flow cytometry. (C) Splenocytes were taken from wild-type donor mice and labeled with 5 μg/mL CFSE and injected along with unlabeled BM cells into recipients that received either no (0 cGy) or lethal (900 cGy) TBI. (D) On day 15 after transplantation, splenocytes were taken from 3 recipient mice and separately measured by flow cytometry for CFSE, surface CD4, and intracellular foxP3. Data shown are gated on live, CFSE+, CD4+ cells. Data shown are representative of the 3 individual recipients.
An immunotransplantation maneuver enhances tumor-specific T-cell responses and protects against tumor challenge
The effect of immunotransplantation on tumor-specific T cells was assessed. CpG/CTX-vaccinated donors and immunotransplantation recipients were tumor-challenged 3 days after transplantation using 100-fold more than the A20 minimal lethal dose to provide a stringent test of the potency of the immune response.
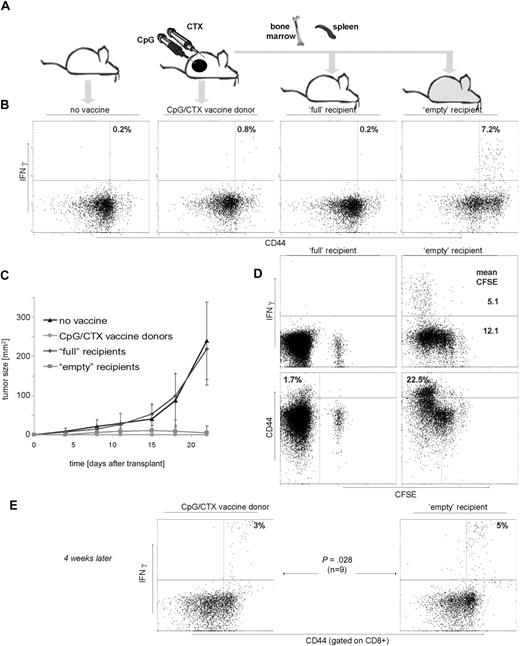
Fifteen days after transplantation, PBMCs were assayed for IFN-γ response to A20 tumor cells. Vaccinated donor mice demonstrated a significant tumor-specific T-cell response (Figure 2B). Transfer of splenocytes from these vaccinated donor mice into “full” recipients resulted in no detectable tumor-specific T cells (Figure 2B third panel), despite the measurable persistence of the transferred population over the same time course (Figure 2D left panels, CFSE+ population). In contrast, “empty” recipients demonstrated a robust, tumor-specific T-cell response, an order of magnitude greater than either “full” recipients or vaccinated donors.
Immunotransplantation enhances tumor-specific T-cell responses and protects against high-dose tumor challenge. (A) Mice received either no vaccine, vaccination with CpG/CTX, or syngeneic BM and CFSE-labeled splenocytes from vaccinated donors after either no irradiation (“full” recipients) or 900 cGy of TBI (“empty” recipients). On day 3 after transplantation, mice (10 per cohort) were challenged with 107 A20 cells subcutaneously. (B,D) On day 15 after transplantation, mice were bled and PBMCs tested for tumor-specific IFN-γ production. (B) Graphs are gated for CD3+ lymphocytes, and statistics are IFN-γ+ cells as a percentage of all CD44hi cells. (C) Tumor growth curves are composites for each cohort. Error bars represent plus or minus 1 SD. (D) (Top right panel) Mean fluorescent intensity (MFI) of IFN-γ–producing cells related to CFSE dilution. The MFI of IFN-γ+ cells (5.1) is lower than that of IFN-γ−, 12.1. (Bottom panels) “Empty” mice contain more (22.5%) CD3+CD44hi cells (defined as in panel A) than their “full” mice counterparts (1.7%). (E) On day 45 after transplantation, 9 mice were rebled and PBMCs were separately assayed as in panels B and D, except that gated are CD8+ cells and indicated are the proportion of IFN-γ+ cells as a percentage of CD44hi cells.
Immunotransplantation enhances tumor-specific T-cell responses and protects against high-dose tumor challenge. (A) Mice received either no vaccine, vaccination with CpG/CTX, or syngeneic BM and CFSE-labeled splenocytes from vaccinated donors after either no irradiation (“full” recipients) or 900 cGy of TBI (“empty” recipients). On day 3 after transplantation, mice (10 per cohort) were challenged with 107 A20 cells subcutaneously. (B,D) On day 15 after transplantation, mice were bled and PBMCs tested for tumor-specific IFN-γ production. (B) Graphs are gated for CD3+ lymphocytes, and statistics are IFN-γ+ cells as a percentage of all CD44hi cells. (C) Tumor growth curves are composites for each cohort. Error bars represent plus or minus 1 SD. (D) (Top right panel) Mean fluorescent intensity (MFI) of IFN-γ–producing cells related to CFSE dilution. The MFI of IFN-γ+ cells (5.1) is lower than that of IFN-γ−, 12.1. (Bottom panels) “Empty” mice contain more (22.5%) CD3+CD44hi cells (defined as in panel A) than their “full” mice counterparts (1.7%). (E) On day 45 after transplantation, 9 mice were rebled and PBMCs were separately assayed as in panels B and D, except that gated are CD8+ cells and indicated are the proportion of IFN-γ+ cells as a percentage of CD44hi cells.
Challenged tumors grew rapidly in untreated mice and in “full” recipients of splenocyte transfer (Figure 2C), whereas vaccinated donors and “empty” immunotransplantation recipients were completely protected. Interestingly, there was brief tumor growth in the “empty” recipients, peaking at day 14 followed by complete regression (Figure 2C), indicating antitumor immunity increasing over time.
Measurement of CFSE intensity of transferred T cells demonstrated their proliferation over time in “empty” but not “full” recipients (Figure 2D top panels). Tumor-specific T cells proliferated to an even greater degree than other T cells (mean CFSE = 5.1 vs 12.1).
Because homeostatic proliferation can induce a memory phenotype in transferred T cells,9,10 we compared CD44 expression in T cells repopulating “full” versus “empty” recipients (CD44hi = 1.7% vs 22.5%). Donor and “empty” recipient mice were also retested for tumor-specific immunity 4 weeks from the time of immunotransplantation. Intact tumor-specific memory was observed in donor mice and to an even higher degree in “empty” recipients (3% vs 5% of memory CD8 cells; Figure 2E).
Immunotransplantation protects against systemic tumor burden
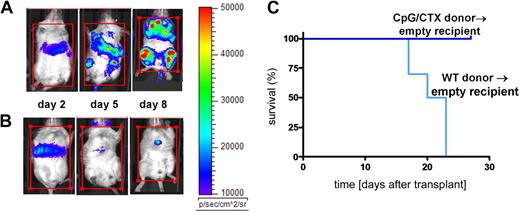
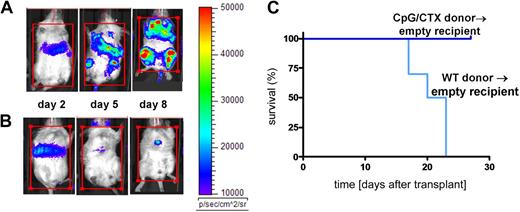
We next tested the efficacy of immunotransplantation on systemic tumor. Recipient mice were lethally irradiated and rescued with BM cells and splenocytes from donors that received either no vaccine (Figure 3A) or CpG/CTX vaccination (Figure 3B). One day after transplantation, mice were challenged intravenously with high-dose (106) A20-LUC (50 times the minimal lethal dose7 ) and followed by bioluminescence. All mice had initial tumor growth. Mice receiving unvaccinated-donor splenocytes rapidly progressed, whereas mice receiving vaccinated-donor splenocytes had rapid remission of disease. Similar results were demonstrated using intraperitoneal tumor challenge (data not shown).
Immunotransplantation protects against systemic tumor burden. Donor mice received (A) no treatment or (B) CpG/CTX vaccination as described. On day 0 (7 days after the completion of the CpG/CTX vaccine), recipient mice received 9 Gy of TBI followed by transplantation of 5 × 106 BM cells and splenocytes from donors. Mice were challenged on day 1 after transplantation with 106 A20-LUC cells intravenously and followed clinically and per their bioluminescence. The same representative mice are shown over time. (C) Cohorts of recipient mice (n = 10) receiving the same treatments and tumor challenge with 106 wild-type A20 cells intravenously were followed for clinical signs of illness and survival.
Immunotransplantation protects against systemic tumor burden. Donor mice received (A) no treatment or (B) CpG/CTX vaccination as described. On day 0 (7 days after the completion of the CpG/CTX vaccine), recipient mice received 9 Gy of TBI followed by transplantation of 5 × 106 BM cells and splenocytes from donors. Mice were challenged on day 1 after transplantation with 106 A20-LUC cells intravenously and followed clinically and per their bioluminescence. The same representative mice are shown over time. (C) Cohorts of recipient mice (n = 10) receiving the same treatments and tumor challenge with 106 wild-type A20 cells intravenously were followed for clinical signs of illness and survival.
Immunotransplanted mice were separately challenged with wild-type A20 cells and followed for survival (Figure 3C). Recipient mice receiving BM and splenocytes from unvaccinated donors died by day 25, whereas recipients of vaccinated-donor splenocytes all survived.
Immunotransplantation induces T cell–mediated tumor protection
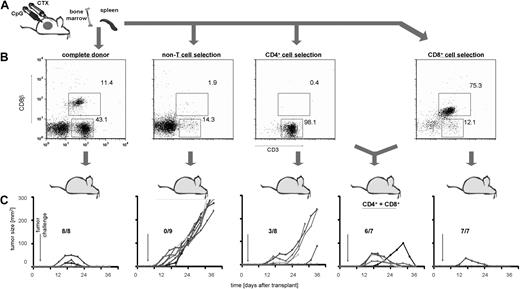
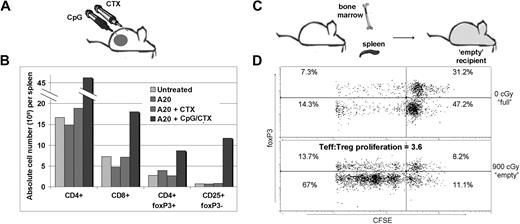
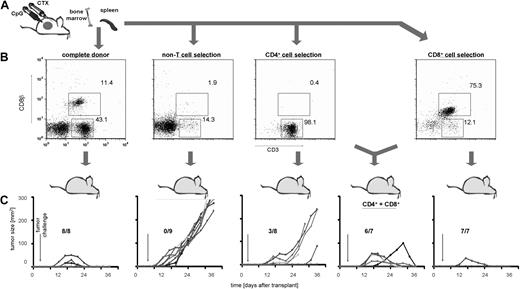
Because immunotransplantation of splenocytes from CpG/CTX-vaccinated mice resulted in an approximately 10-fold increase in CD8 T cell tumor-specific immune response (Figure 2B) but no apparent increase in tumor-specific immune response of other cell types (data not shown), we tested the relative importance of different cell subsets in mediating tumor protection. On day 8 after CpG/CTX vaccination, donor splenocyte populations are composed of approximately 11% CD8 T cells, 43% CD4 T cells, and 46% non-T cells (Figure 4B). Using mAb-conjugated ferromagnetic beads, purified splenocyte subsets were obtained and transferred into irradiated recipient mice at total cell numbers normalized to that population's proportion in donor splenocytes (ie, ∼105 × 106 complete splenocytes, 49 × 106 non-T cells, 45 × 106 CD4 T cells, or 12 × 106 CD8 T cells were transferred). Recipients were challenged with subcutaneous tumor on after transplantation day 3 and followed for tumor growth and survival. These data demonstrated that T cells were necessary to transfer complete tumor protection and suggested that CD8 cells were more potent in this regard (Figure 4C).
Immunotransplantation of CD8 T cells is both necessary and sufficient for tumor protection. (A) Donor mice received CpG/CTX vaccination, and splenocyte cell subsets were purified from CpG/CTX vaccinated donors using mAb-conjugated ferromagnetic beads to either positively select or deplete specific populations. (B) Resulting populations were gated on live lymphocytes, and purity was assessed by flow cytometry. (C) CpG/CTX donor splenocyte subsets were used in immunotransplantation as described. Recipient mice received high-dose tumor challenge on day 3 after transplantation and were followed for bidimensional tumor size. Proportions of tumor-free mice are indicated. One mouse in the “CD4+CD8” group died within a week after transplantation but still had palpable tumor.
Immunotransplantation of CD8 T cells is both necessary and sufficient for tumor protection. (A) Donor mice received CpG/CTX vaccination, and splenocyte cell subsets were purified from CpG/CTX vaccinated donors using mAb-conjugated ferromagnetic beads to either positively select or deplete specific populations. (B) Resulting populations were gated on live lymphocytes, and purity was assessed by flow cytometry. (C) CpG/CTX donor splenocyte subsets were used in immunotransplantation as described. Recipient mice received high-dose tumor challenge on day 3 after transplantation and were followed for bidimensional tumor size. Proportions of tumor-free mice are indicated. One mouse in the “CD4+CD8” group died within a week after transplantation but still had palpable tumor.
Complete donor vaccination is required to transfer antitumor immune responses and tumor protection and can be enhanced by booster vaccination
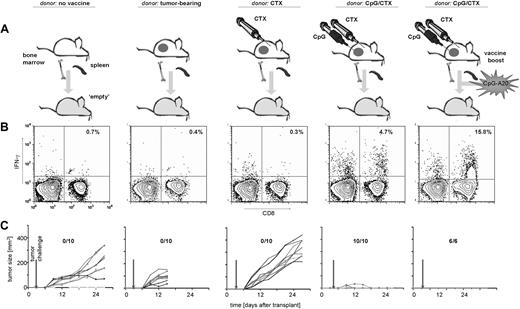
We next tested the requirements for vaccination of immune donors. Donor mice received no treatment, tumor implantation alone, tumor implantation followed by systemic CTX, or the complete CpG/CTX vaccination maneuver (Figure 5A). At the time of splenocyte harvest, tumor-bearing and CTX-treated donors both had measurable tumors. CpG/CTX-vaccinated donors had little or no measurable disease.
Donor requirements to transfer antitumor immunity. (A) Donor mice received no treatment, A20 tumor challenge, A20 tumor challenge followed by CTX, or A20 tumor challenge followed by CTX and intratumoral CpG. On day 0 (7 days after the completion of the CpG/CTX vaccine), recipient mice received 9 Gy of TBI followed by 5 × 106 BM cells and splenocytes from donors. One group received a simultaneous boost of 106 A20 cells, which were cultured with CpG-1826 at 3 μg/mL for 72 hours, then irradiated (50 Gy). On day 3 after transplantation, all cohorts of mice received high-dose tumor challenge. (B) On day 15, posttransplantation/transfer mice were bled and assayed by flow cytometry for tumor-specific CD8 T-cell responses as described. Indicated are the percentage of IFN-γ–producing CD8+ live lymphocytes (n = 3 per cohort). (C) Cohorts of mice were followed for bidimensional tumor size, and proportions of tumor-free mice are indicated. One mouse in the “no treatment donor” cohort (first column) showed minimal subcutaneous growth but manifested systemic disease (with hind-limb paralysis) and was killed on day 30.
Donor requirements to transfer antitumor immunity. (A) Donor mice received no treatment, A20 tumor challenge, A20 tumor challenge followed by CTX, or A20 tumor challenge followed by CTX and intratumoral CpG. On day 0 (7 days after the completion of the CpG/CTX vaccine), recipient mice received 9 Gy of TBI followed by 5 × 106 BM cells and splenocytes from donors. One group received a simultaneous boost of 106 A20 cells, which were cultured with CpG-1826 at 3 μg/mL for 72 hours, then irradiated (50 Gy). On day 3 after transplantation, all cohorts of mice received high-dose tumor challenge. (B) On day 15, posttransplantation/transfer mice were bled and assayed by flow cytometry for tumor-specific CD8 T-cell responses as described. Indicated are the percentage of IFN-γ–producing CD8+ live lymphocytes (n = 3 per cohort). (C) Cohorts of mice were followed for bidimensional tumor size, and proportions of tumor-free mice are indicated. One mouse in the “no treatment donor” cohort (first column) showed minimal subcutaneous growth but manifested systemic disease (with hind-limb paralysis) and was killed on day 30.
Recipient mice that received splenocyte transfer from unvaccinated, A20-bearing, or CTX-treated donors did not demonstrate significant tumor-specific CD8 T-cell responses, nor were they protected from tumor challenge (Figure 5B,C). Mice receiving splenocytes from A20-bearing donors were also not protected from the smaller intravenous tumor challenge of A20 cells contaminating the splenocyte population. In contrast, recipients receiving splenocytes from fully vaccinated donors demonstrated significant tumor-specific CD8 T-cell responses and 100% tumor protection (Figure 5B,C fourth panel).
We hypothesized that, if transferred CD8 T cells mediate immunotransplantation antitumor immunity, then delivering signals 1 and 2 (cognate antigen in the context of costimulatory molecules) along with the homeostatic proliferative signal would enhance this immune response. A20 tumor cells were cultured in vitro with CpG (to increase costimulatory molecule expression1 ), irradiated, and given with immunotransplantation either as an intravenous (Figure 5A) or subcutaneous (data not shown) boost. There was a marked, 3- to 4-fold increase in the proportion of antitumor CD8 T cells with both routes of immunization that was highly tumor-specific (Figures 5B, S1). We also observed a suggestion of more potent tumor protection, as the initial tumor growth usually observed in challenged immunotransplantation recipients no longer occurred (Figure 5C). In addition, late recurrences (beyond day 75) observed in a minority of challenged immunotransplanted mice did not occur in “boosted” cohorts. (All cohorts were followed for 90 days, and late recurrences were seen in 0% to 40% of recipients in repeated experiments.)
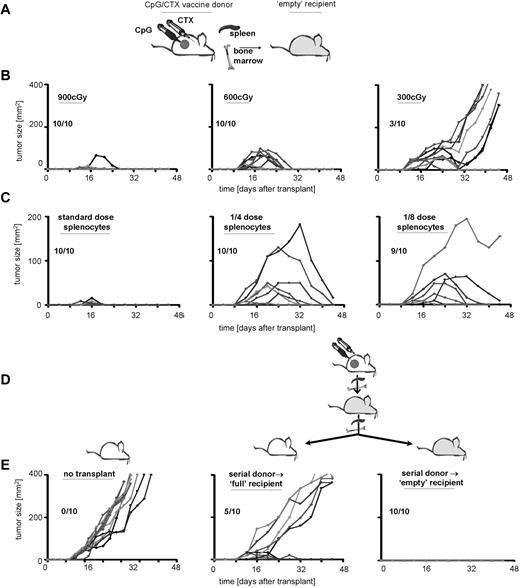
Immunotransplantation-induced tumor immunity increases with time and with serial transplantations
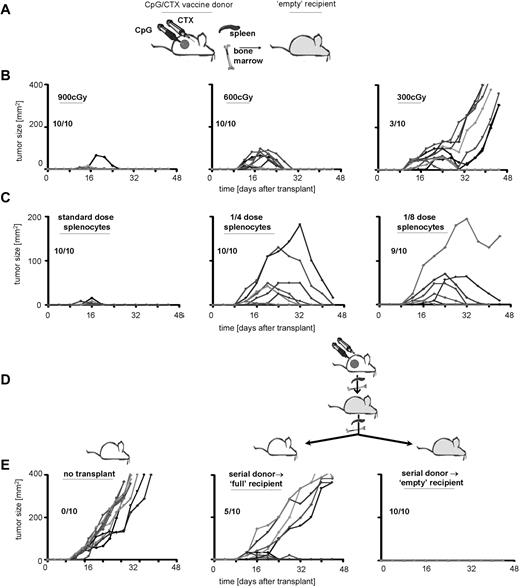
To investigate the mechanism of tumor protection in immunotransplantation and to determine the requirement for “emptiness,” we varied the conditioning-radiation dose. Recipients received 900, 600, or 300 cGy of TBI followed by immunotransplantation and then received high-dose A20 tumor challenge (Figure 6B). As shown previously, mice receiving full-dose TBI demonstrated transient tumor growth, peaking by day 20 but regressing by day 25. Mice receiving lower TBI doses showed variable protection, although there appeared to be a threshold dose. A 2-fold reduction (600-300 cGy) reduced tumor protection from 100% to 30%. In addition, tumor immunity in these recipients appeared to decrease with time, such that several mice demonstrated remission followed by rapid tumor recurrence (Figure 6C third column).
Immunotransplantation-induced tumor immunity increases with time and with serial transplantations. (A) Donor mice received CpG/CTX vaccination, and recipient mice received TBI. (B) Donor splenocytes and BM were transferred to recipients that received 900, 600, or 300 cGy as depicted. (C) Recipient mice received 900 cGy of TBI followed by 5 × 106 BM cells and splenocytes from donors at donor/recipient ratios of 1:1, 1:4, or 1:8, as depicted. (B,C) Recipient mice received high-dose tumor challenge on day 3 after transplantation and were followed for bidimensional tumor size. Proportions of tumor-free mice are indicated. (D) Serial immunotransplantation. Cured immunotransplantation recipients from experiments conducted as in panel A were subsequently used as donors in a serial immunotransplantation. Secondary recipients received either no irradiation or 900 cGy of TBI as depicted, followed by 5 × 106 BM cells and splenocytes from cured immunotransplantation mice. On day 3 after (secondary) transplantation, recipients were challenged with 107 A20 cells subcutaneously and (E) followed for tumor growth.
Immunotransplantation-induced tumor immunity increases with time and with serial transplantations. (A) Donor mice received CpG/CTX vaccination, and recipient mice received TBI. (B) Donor splenocytes and BM were transferred to recipients that received 900, 600, or 300 cGy as depicted. (C) Recipient mice received 900 cGy of TBI followed by 5 × 106 BM cells and splenocytes from donors at donor/recipient ratios of 1:1, 1:4, or 1:8, as depicted. (B,C) Recipient mice received high-dose tumor challenge on day 3 after transplantation and were followed for bidimensional tumor size. Proportions of tumor-free mice are indicated. (D) Serial immunotransplantation. Cured immunotransplantation recipients from experiments conducted as in panel A were subsequently used as donors in a serial immunotransplantation. Secondary recipients received either no irradiation or 900 cGy of TBI as depicted, followed by 5 × 106 BM cells and splenocytes from cured immunotransplantation mice. On day 3 after (secondary) transplantation, recipients were challenged with 107 A20 cells subcutaneously and (E) followed for tumor growth.
To determine the potency of CpG/CTX donor splenocytes, we transferred them at various doses into lethally irradiated recipients: one donor spleen → one recipient versus one-fourth or one-eighth dose (Figure 6C). As shown previously, recipient mice receiving the complete dose of splenocytes were protected from tumor, with some recipients exhibiting transient tumor growth, peaking at day 10 and regressing by day 15. Recipients of lower splenocyte doses were variably protected. In addition, peak tumor immunity appeared to increase with time. In lower splenocyte-dose cohorts, initial immunity was insufficient to stop rapid tumor growth, but by day 20 tumor immunity was powerful enough to eradicate tumors as large as 200 mm2.
Although the “empty signal” increases the antitumor effect of transferred T cells, it is a transient effect of ablative therapy and diminishes with hematopoietic reconstitution. Because T-cell transfer into the “empty” mouse induces both proliferation as well as qualitative changes (eg, increased proportion of tumor-specific and memory T cells), we asked whether these changes were reversible (ie, dependent on continued “emptiness” of the recipient) or irreversible (ie, persistent after secondary transfer to a “full” recipient). To address this question, we performed serial immunotransplantations using cured recipients as donors into either “empty” or “full” secondary recipients. These secondary recipients received high-dose tumor challenge on day 3 after transplantation.
The secondary transfer of immunotransplantation-cured donor splenocytes into “full” recipients demonstrated significant protection. We observed delayed tumor growth compared with tumor-challenged control mice and cure in 50% of recipients (Figure 6E). This differed markedly from the transfer of CpG/CTX vaccine-primed splenocytes into “full” recipients, which yielded no tumor protection (Figure 2B). In addition, serial immunotransplantation into “empty” secondary recipients protected 100% of these recipients from subsequent tumor challenge without the transient tumor growth seen in standard immunotransplantation recipients (Figures 2B, 5C, 6B,C), indicating an increased antitumor effect.
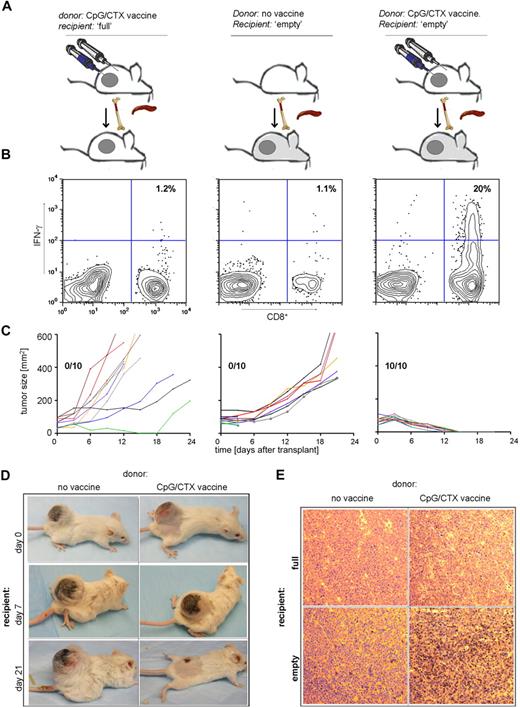
Immunotransplantation induces a tumor-specific immune response and cures mice with large tumors
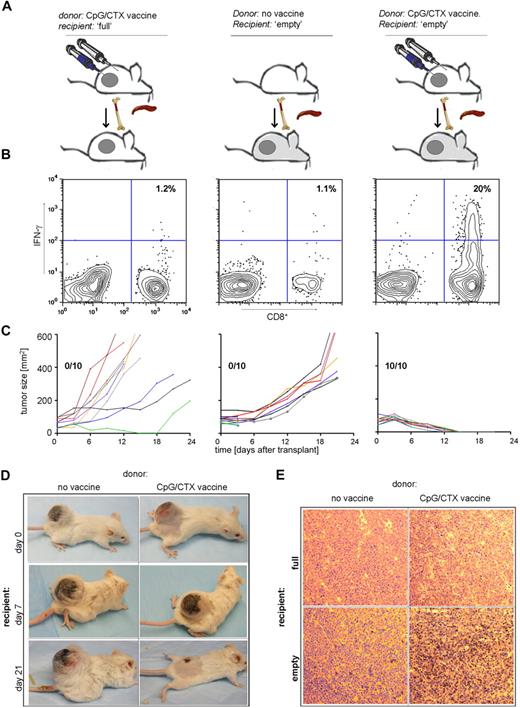
Because of the striking regression of large tumors in the prophylactic setting, we asked whether immunotransplantation could be effective in the therapeutic setting. On day 14 after subcutaneous implantation of 107 A20 cells, tumors are approximately 100 mm2, radiation resistant, and uniformly lethal in less than 20 days. These tumor-bearing mice were used as immunotransplantation recipients to test the therapeutic effect of this maneuver.
Mice bearing 100-mm2 tumors received either no irradiation or 900 cGy of TBI and received splenocytes and BM from CpG/CTX-vaccinated donors, as shown previously. The nonirradiated recipients also received a “boost” of irradiated, CpG-treated A20 cells to optimize their chance of tumor protection. On day 15 after transplantation, PBMCs were assayed for tumor-specific IFN-γ–producing cells.
In these large tumor-bearing recipients, immunotransplantation led to the induction of tumor-specific IFN-γ–producing CD8 T cells and to rapid cure (within 10 days) of 100% of mice (Figure 7B,C right panel). In contrast, splenocyte transfer from unvaccinated donors neither induced tumor-specific CD8 T cells nor caused any reduction in tumor size (Figure 7B,C middle panel). These results were identical to mice receiving BMT alone without splenocyte transfer (data not shown). Similarly, in nonirradiated recipient mice, transfer of BM plus vaccinated-donor splenocytes plus CpG-A20 “boost” was ineffective (Figure 7B,C left panel).
Immunotransplantation cures mice with large tumors. (A) Donor mice received no treatment or CpG/CTX vaccination. Recipient mice were challenged with 107 A20 cells subcutaneous 14 days earlier, then received either no irradiation or 900 cGy of TBI followed by 5 × 106 BM cells and splenocytes from donors. Nonirradiated mice also received an intravenous boost of A20 cells stimulated with CpG followed by irradiation (CpG-A20 cells) at the time of transplantation. (B) On day 15 after transplantation, mice were bled, and IFN-γ–producing tumor-specific CD8 T cells were assayed by flow cytometry as described (n = 3). (C) Cohorts of mice (n = 10) were followed for bidimensional tumor size, and proportions of tumor-free mice are indicated. Three mice from the “no vaccine donor” cohort died within 3 days after transplantation, still with palpable tumor. (D) Tumor (400-mm2)–bearing mice were used as immunotransplantation recipients as in panels A to C from donors receiving no treatment or CpG/CTX vaccination; shown are photographs of the same mice over time. (E) Tumor (100 mm2)–bearing mice were used as immunotransplantation recipients and were killed on day 8 after transplantation. Excised tumors were stained for CD3 and visualized per standard immunoperoxidase protocol (original magnification ×20, representative of 10 fields examined).
Immunotransplantation cures mice with large tumors. (A) Donor mice received no treatment or CpG/CTX vaccination. Recipient mice were challenged with 107 A20 cells subcutaneous 14 days earlier, then received either no irradiation or 900 cGy of TBI followed by 5 × 106 BM cells and splenocytes from donors. Nonirradiated mice also received an intravenous boost of A20 cells stimulated with CpG followed by irradiation (CpG-A20 cells) at the time of transplantation. (B) On day 15 after transplantation, mice were bled, and IFN-γ–producing tumor-specific CD8 T cells were assayed by flow cytometry as described (n = 3). (C) Cohorts of mice (n = 10) were followed for bidimensional tumor size, and proportions of tumor-free mice are indicated. Three mice from the “no vaccine donor” cohort died within 3 days after transplantation, still with palpable tumor. (D) Tumor (400-mm2)–bearing mice were used as immunotransplantation recipients as in panels A to C from donors receiving no treatment or CpG/CTX vaccination; shown are photographs of the same mice over time. (E) Tumor (100 mm2)–bearing mice were used as immunotransplantation recipients and were killed on day 8 after transplantation. Excised tumors were stained for CD3 and visualized per standard immunoperoxidase protocol (original magnification ×20, representative of 10 fields examined).
The 100% cure rate of mice bearing 100-mm2 tumors prompted us to treat mice with larger tumors. Mice with tumors as large as 400 mm2 received immunotransplantation from either untreated or CpG/CTX-vaccinated donors. In only the latter group, we observed multiple instances of remarkable tumor regressions (Figure 7D).
To determine whether there was an influx of effector cells to the tumor site, paralleling the development of tumor-specific T cells in the peripheral blood, we excised tumors on day 8 after transplantation from tumor-bearing recipients treated with 0 or 900 cGy of TBI and receiving immunotransplantation from either unvaccinated or CpG/CTX-vaccinated donors. A significant proportion of tumor-infiltrating CD3 cells were seen only in recipients receiving the complete immunotransplantation maneuver (Figure 7E).
Discussion
The remarkable therapeutic effect of transferring ex vivo primed TIL into lymphodepleted melanoma patients11 compels the further development of this approach. It has been suggested that more aggressive lymphodepletion (ie, lethal myeloablation with stem cell rescue) would make this approach more powerful12 and that discovering a way to replace TILs with vaccine-primed T cells would facilitate broader clinical application. Although such an attempt by the same group was not encouraging,13 our findings suggest that some vaccine strategies are more effective in inducing transferable immunity. It is possible that more effective vaccinations, such as the CpG/CTX used here, will be the key to making immunotransplantation effective without the need for ex vivo expansion of T cells.
Here we demonstrate that an in situ vaccine maneuver, although generating tumor-specific T-cell response, also generates an increased number of Treg (Figure 1B).8 Using vaccine-primed donors as a source of T cells for immunotransplantation prompts the question of whether this increase in Treg will hinder the transferred immunity. Supporting recent results by Mirmonsef et al14 in a transgenic T-cell system, herein, we show that immunotransplantation increases the Teff/Treg in transferred splenocytes (Figure 1D) and causes preferential proliferation of tumor-specific CD8 T cells (Figure 2D).
The tumor-specific, IFN-γ–producing, CD8 T-cell response occurs primarily among memory CD44hi T cells, a subset also known to be proportionally increased by homeostatic proliferation.9,10 The preferential proliferation of tumor-specific T cells may be related to this memory phenotype or other qualities of these cells that make them more responsive to “homeostatic cytokines.” Alternately, the tumor-specific T-cell preferential expansion could be the additive or synergistic effect of the “empty” environment combined with encountering their cognate antigen (in tumor-bearing mice). The lack of tumor-specific T-cell proliferation in the presence of cognate antigen but in the absence of the homeostatic-proliferative signal (ie, in the “full” recipients; Figure 2D first panel) suggests synergy between the 2 signals.
Along with these changes in the transferred T cells, we have shown the consequent increased T-cell memory (Figure 2E) and the induction of tumor protection against even high-dose subcutaneous or systemic tumor challenge (Figures 2C, 3). This protection is entirely dependent on both the “emptiness” of recipients as well as the potent vaccination of donors. In contrast to studies showing an inherent immune response to murine and human tumors,15-17 we have shown that donors with lesser “vaccinations,” such as tumor alone or tumor treated with chemotherapy, do not transfer antitumor immunity against high-dose tumor challenge (Figure 4). Of note, Borrello et al used intravenous tumor-challenged donors and transferred tumor-purged splenocytes into lymphodepleted recipients,18 whereas our experiments use no in vitro tumor purge. Although their system differs from ours in the degree of tumor protection, both demonstrate the enhanced antitumor immunity of T cells transferred into the “empty” recipient.
The additional improvement of our immunotransplantation maneuver by a vaccine “boost” gives an indication of its mechanism and ultimate clinical application. The proliferative and qualitative changes that transferred T cells undergo in the “empty” recipient are primarily the result of increased availability of cytokines, such as IL-7 and IL-15, which function independently of T cell–receptor stimulation and costimulation. Culturing A20 cells with CpG in vitro increases the expression of CD40,19 CD80, and CD86.1 We therefore reasoned that a posttransplantation boost with CpG-stimulated A20 should optimally drive proliferation of tumor-specific T cells by simultaneously sending homeostatic proliferative signals along with signals 1 and 2. Our data show that both intravenous and subcutaneous routes of vaccine boost enhance tumor-specific immunity (Figure 4; and data not shown) and avoid late recurrences that sometimes occur in the absence of the boost. The clinical benefit of boosting argues for its inclusion in clinical translation of immunotransplantation. This is consistent with a clinical trial of immunotransplantation of pneumonia vaccine-primed T cells demonstrating that such a boosting approach was necessary for effective T-cell expansion.20
Tumor protection in our immunotransplantation maneuver demonstrates a strict dependence on the degree of irradiation (Figure 5B). This is consistent with our previous findings of the strict dependence on “emptiness” for homeostatic proliferation of transferred T cells (data not shown). We hypothesize that it is the “emptiness” of lethally irradiated recipients that induces the changes seen in transferred tumor-specific T cells as opposed to other effects of recipient irradiation, such as microbial translocation,12 radiation-induced “cytokine storm,”21,22 or other radiation-induced immune effects.23 We conjecture that those latter types of direct irradiation effects would be greatest shortly after treatment, whereas the homeostatic proliferation induced by emptiness is a time-dependent process, taking weeks to maximally increase T-cell numbers.24 Using lower-dose splenocyte transfer, we demonstrate that immunity does increase over time, allowing for the late elimination of tumors as large as 200 mm2, despite the 300-fold greater cell number compared with the time of tumor challenge (Figure 5C).
Several studies have shown preclinical efficacy of immunotherapies against established, but minimal, disease burden.18,25-27 Therefore, it is not surprising that translating such approaches into successful clinical trials has been difficult. Our initial results were encouraging enough to study the therapeutic setting of large, established tumors, which differ in several ways from standard-dose tumor challenge. First, in the A20 model, tumors of less than 0.25 mm2 are radio-sensitive and cured by lethal dose TBI (data not shown), whereas tumors of 100 mm2 are radio-resistant (Figure 6C middle panel). Perhaps the greatest obstacle is that mechanisms of immune evasion change with increasing tumor size. Specifically, Elpek et al demonstrated in the A20 tumor model that an immunotherapy effective against lower tumor burdens had no effect on larger tumors.28 Therefore, the cure of 100% of immunotransplantation recipients (Figure 6C) is in accord with our initial hypothesis: immunotransplantation eliminates multiple components of tumor immune-evasion strategies. These results allowed us to test even larger tumors that are more applicable to the clinical situation (Figure 6D).
The superior antitumor potency of immunotransplantation in curing large tumors could feasibly predict its successful translation into clinical trials. Because the in situ vaccine maneuver combining cytotoxic therapy and intratumoral CpG has already translated well from the preclinical model to objective clinical responses, we are optimistic that immunotransplantation will also translate to an improved clinical effect.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Shoshana Levy and Eric Berlin for helpful discussion in preparation of this manuscript.
This work was supported by grants from the National Institute of Health (CA-34233, CA-33399, CA-49605) and by a Career Development Award from the Lymphoma Research Foundation (J.D.B.). R.L. is an American Cancer Society Clinical Research Professor.
National Institutes of Health
Authorship
Contribution: J.D.B. and M.J.G. designed and performed research, analyzed data, and wrote the paper; D.K.C. designed and performed research and analyzed data; and R.L. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Division of Oncology, Department of Medicine, Stanford University Medical Center, Center for Clinical Sciences Research Room 1105, 269 Campus Drive, Stanford, CA 94305-5151; e-mail: levy@stanford.edu.