In this issue of Blood, Yokota and colleagues define ESAM as a novel marker that facilitates isolation of multipotential lympho-myeloid hematopoietic stem and progenitor cells in mice throughout ontogeny.
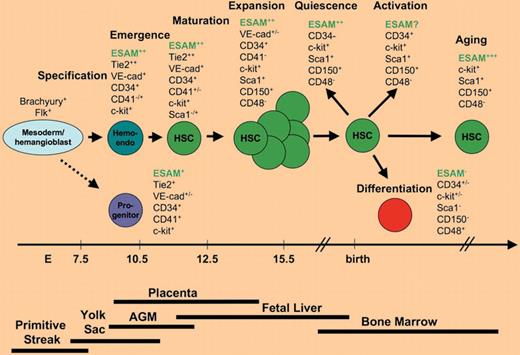
Distinction of hematopoietic stem cells (HSCs) from differentiated cells is essential for improving our understanding of HSC development and unique biological properties and for purification of HSCs for therapeutic applications. However, most HSC markers are developmental stage, context and species dependent, and multiple surface markers need to be combined to provide significant enrichment for HSCs (see figure; reviewed in Mikkola and Orkin1 ). Sca1, the classical mouse HSC marker, is expressed only in certain mouse strains, and its expression is not fully penetrant in newly emerged HSCs. Conversely, CD41, the first embryonic HSC/progenitor marker, is only expressed on nascent HSCs. Many traditional endothelial markers are expressed on HSCs as they emerge from the hemogenic endothelium. But they become down-regulated when HSCs colonize the liver (VE-cadherin) or in early postnatal life when the highly proliferative fetal HSCs switch to a quiescent adult phenotype (CD34). In addition to the phenotypic evolution that occurs throughout ontogeny, the surface markers on HSCs change on activation in culture or in response to mobilization or myeloablative treatment.2,3 Recently, SLAM markers CD150 and CD48 were shown to facilitate enrichment of HSCs from the fetal liver stage onwards, including mobilized and aging HSCs,4 whereas the analysis of their expression in nascent HSCs is still pending. Furthermore, despite the general conservation of hematopoietic sites, HSC/progenitor hierarchy, and transcription factors between mice and humans, the surface markers that facilitate identification of HSCs in the 2 species have turned out to be strikingly different. CD41, Sca1, and SLAM markers are relevant for HSC purification in mice but not humans, whereas CD34+CD38−, the classical human HSC profile, does not select comparable hematopoietic populations in mice. The complexity of HSC surface markers makes it difficult to study progressive evolution of HSCs from one developmental stage or environment to another, and across species.
ESAM was identified as a novel marker that distinguishes multipotential hematopoietic stem and progenitor cells from differentiated cells. In contrast to many other HSC surface markers that are expressed in developmental stage and context dependent fashion, high level of ESAM expression was observed already at the time when HSCs emerge from hemogenic endothelium (hemo-endo) and was sustained throughout development and adult life.
ESAM was identified as a novel marker that distinguishes multipotential hematopoietic stem and progenitor cells from differentiated cells. In contrast to many other HSC surface markers that are expressed in developmental stage and context dependent fashion, high level of ESAM expression was observed already at the time when HSCs emerge from hemogenic endothelium (hemo-endo) and was sustained throughout development and adult life.
This study by Yokota et al defines endothelial cell selective adhesion molecule (ESAM) as a novel marker that enriches for multipotential hematopoietic stem and progenitor cells in mice throughout ontogeny.5 ESAM belongs to the family of transmembrane proteins with immunoglobulin-like extracellular domains, and its expression has been documented in various species in endothelial cell junctions, platelets, and most recently in HSCs.6-8 The authors identified ESAM as a candidate HSC marker from a microarray screen that used Rag1-GFP reporter strain to separate the ckit+Sca1 Rag1− HSC subset from the ckit+Sca1+Rag1+ early lymphoid progenitors in midgestation fetal liver. Further studies showed that expression of ESAM was highly correlated with HSC phenotype, and high levels of ESAM expression could alone be used as means to isolate both multipotential progenitors as well as transplantable HSCs in the fetal liver.
In the AGM (aorta-gonad mesonephros region) where HSCs emerge, ESAM+ cells coexpressed c-kit and the endothelial markers Tie2, CD34, and PECAM that are known to be present in fetal HSCs. Interestingly, ESAM+ cells formed a distinct subpopulation that selected all hematopoietic progenitors with lymphoid potential, which is of importance as the establishment of lymphoid potential during the onset of fetal hematopoiesis distinguishes the true multipotential hematopoietic stem and progenitor cells from the earlier, yolk sac–derived progenitors that have limited lifespan and developmental potential. Comparison with the yolk sac further suggested that ESAMHickit+Tie2Hi cells represent emerging HSCs whereas the myeloerythroid progenitor cells are harbored in ESAMLockitHiTie2Lo fraction. In contrast to many endothelial markers that become down-regulated in HSCs later during development, ESAM expression was not only maintained in the Lin−ckit+Sca1Hi HSC fraction in the adult bone marrow, but its expression level even increased in aging mice.
The finding that ESAM is faithfully expressed in long-term repopulating HSCs and their immediate precursors throughout ontogeny may have many important applications. Purification of hematopoietic cells based on ESAM expression may facilitate investigation of the mechanisms that dictate cell fate toward multipotential HSCs rather than short-lived myeloerythroid progenitors during embryogenesis, and assessment of the development of these distinct hematopoietic programs in vivo as well as in vitro from embryonic stem cells or induced pluripotent cells. Furthermore, the high level and fairly specific expression of ESAM in multipotential hematopoietic cells could potentially be used for tracking HSCs during development and localizing them in distinct cellular niches by various imaging techniques. In addition, unraveling the molecular networks that regulate ESAM expression may give important clues about the cell intrinsic programs that govern the identity and functional properties of multipotential hematopoietic stem and progenitor cells. The importance of the findings in this study is further enhanced by recent findings that ESAM expression in HSCs appears to be conserved between different mouse strains and across species, as human HSCs were also shown to express this marker.8 Another important question is whether ESAM is functionally required for establishing and maintaining HSC properties, unlike most surface markers associated with HSC potential. Although ESAM−/− mice are viable and fertible and do not exhibit major hematopoietic failure, their hematopoietic lineage distribution is skewed and the number of their HSCs appears to be even slightly higher, implying that ESAM may be functionally involved in HSC-niche interactions in the bone marrow.8 Nevertheless, as a novel HSC marker with unique characteristics, ESAM is a highly appreciated addition to the “hematopoietic tool box” and will likely benefit both basic and translational studies on hematopoietic stem cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■