Abstract
NKG2D is an activating receptor expressed on CD8+αβ+ T cells, γδ+ T cells, natural killer (NK) cells, and some CD4+ T cells. For a long time, the interaction of NKG2D with its ligands (NKG2DLs) MICA, MICB, and ULBP1-3 has been considered a mechanism for recognition and elimination of tumor, infected, or otherwise “stressed” cells. However, a new role for NKG2D as an immunoregulatory receptor is emerging. Here, we show that NKG2D is strongly down-modulated on antigen-activated CD8+ T cells but only if CD4+ T cells are present. Down-modulation was caused by soluble factors produced by CD4+ T cells, and in particular soluble NKG2DLs were found in the supernatants of antigen-activated T-cell cultures. MICB was the ligand released at higher levels when CD4+ T cells were present in the cell cultures, suggesting that it could be the major player of NKG2D down-modulation. CD8+ T cells expressing low levels of NKG2D had impaired effector functions, as evaluated by proliferation, cytokine production, and cytotoxicity assays after combined triggering of NKG2D and TCR-CD3 complex. These findings show that activated CD4+ T cells expressing NKG2DLs can efficiently prevent NKG2D-mediated CD8+ T-cell functions, and suggest that the NKG2D/NKG2DL interaction can regulate immune responses.
Introduction
Regulation of T-cell responses involves diverse strategies of activation and inhibition to optimize recognition of infected or transformed cells, while preventing tissue damage as a result of autoreactivity and chronic inflammation. T-cell antigen receptor (TCR)–CD3-dependent responses are regulated by constitutive or inducible expression of costimulatory and inhibitory receptors, such as CD28 and its CTLA-4 counterpart. In recent years, however, it has become evident that the expression of natural killer (NK)–cell receptors of the NKG2 family (eg, NKG2D and CD94/NKG2 receptors) on CD8+αβ+ effector T cells may represent another mean to regulate cytolytic functions in the tissue microenvironment, effectively controlling antigen-specific killing.1-4 NKG2D is one of the most widely distributed “NK-cell receptors,” being expressed at the surface of all CD8+αβ+ T cells, γδ+ T cells, NK cells, as well as on certain activated CD4+ T cells.5-8 NKG2D is a potent costimulator of TCR-mediated effector functions and up-regulates antigen-specific T cell–mediated cytotoxicity directed against cells or tissues expressing stress-induced NKG2D ligands (NKG2DLs), particularly under conditions of suboptimal TCR engagement.3,4,9-13 In addition, NKG2D on T cells can also function independently of TCR,11,14 and in NK cells it can act as a primary activating receptor.5,15,16 Human NKG2D cell-surface expression and signaling require association with the adaptor molecule DAP10 that, upon tyrosine phosphorylation, recruits and activates phosphatidylinositol-3-kinase,6,17 thus resulting in the activation of cellular cytotoxicity, cytokine secretion, and proliferation.7
NKG2DLs belong to MIC and ULBP families.18 They are all membrane-bound glycoproteins, distantly related to MHC class I molecules, and under certain circumstances they can be shed.19-22 Generally, expression of NKG2DLs is low and restricted to few cell types, but it is inducible on most cells by different cellular “stress,” such as tumor transformation, infection, and DNA damage.23-25 Since NKG2D is expressed on all human CD8+ T cells, acquisition of ligands at the cell surface may render any cell a potential target, leading to the breakdown of tolerance and elimination of cells that should instead be spared. Thus, if NKG2DLs are thought to be “danger signals,” NKG2D is the “danger receptor” able to ensure full activation of CD8+ T cells. It must be then kept under strict control by regulation of its expression or expression of its ligands to avoid aberrant killing and tissue damage. In fact, aberrant NKG2D signaling and inappropriate activation of NK and/or CD8+ T cells by NKG2D has been implicated in various autoimmune diseases, including celiac disease, rheumatoid arthritis, and diabetes.26-28 Thus, modulation of NKG2D receptor expression or blockade of NKG2D signaling may have therapeutic implications for a variety of pathologies. This is particularly important in those circumstances where NKG2DLs are expressed on noninfected and nontransformed cells. In this context, we have recently demonstrated that antigen-activated CD4+ and CD8+ T cells express MICA and ULBP1-3 molecules,29 and this observation raised the question of what happens to the receptor expressed on CD8+ T cells. In human CD8+ T lymphocytes, NKG2D is generally up-regulated upon cell activation and expansion, induced by anti-CD3 monoclonal antibody (mAb) used alone or in combination with IL-2 or IL-15,3,14,30 or during active celiac disease.14 However, many reports have demonstrated a systemic NKG2D down-modulation on NK and T cells induced by soluble forms of its ligands released from some types of tumors in the serum of cancer patients.19-22,31-33 Elevated levels of soluble MIC molecules are present also in sera of pregnant women and are able to down-modulate NKG2D surface expression on maternal peripheral blood mononuclear cells (PBMCs).34 In nonobese diabetic (NOD) mice, NKG2D down-modulation has been observed on activated NK cells, but only upon expression of ligands on the same cells.26 In any case, systemic NKG2D down-regulation has the common outcome of an impaired NK- and/or T-cell cytotoxicity and IFN-γ production.19,26,31,33-36 Thus, NKG2DLs are involved not only in boosting cytotoxicity, but also in its suppression.
Based on these findings, we examined whether NKG2D on CD8+ T cells was modulated by the presence of its ligands on activated lymphocytes, and whether the expression of both NKG2D and NKG2DLs on lymphocytes could represent an inhibitory mechanism for the regulation of CD8+ T-cell functions.
Methods
Antibodies and reagents
Anti-MICA (MAB159227), anti-MICB (MAB236511), anti-ULBP1 (MAB170818), anti-ULBP2 (MAB165903), anti-ULBP3 (MAB166510), anti-NKG2D (MAB149810), anti–TGF-β1 (clone 9016), recombinant NKG2DL (rNKG2DL-Fc), and IL-21 receptor (IL-21R-Fc) were from R&D Systems (Minneapolis, MN). Control IgG1, anti-CD3/APC (HIT3a), anti-CD4/PerCP (SK3), anti-CD8/PerCP (SK1), anti-CD25/APC (2A3), anti-CD107a/FITC (H4A3), anti-CD3 (OKT3), anti-NKG2D (1D11), anti-CD28 (CD28.2), anti–DNAM-1 (DX11), and anti-CD11a (HI111) were from Becton Dickinson (San Jose, CA). Other antibodies (Abs) were anti-NKG2D (M585; Amgen, Thousand Oaks, CA); anti-MICB (BMO2; BAMOMAB, Munich, Germany); and anti-MICA/B (E16; Santa Cruz Biotechnology, Santa Cruz, CA). Control goat IgG and F(ab′)2 fragments of PE-conjugated goat-anti–mouse (GAM-PE) IgG were from Jackson Immunoresearch Laboratories (West Grove, PA), and the F(ab′)2 fragments of GAM IgG were from Cappel (Milan, Italy). Other reagents used were as follows: 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE), enterotoxin B (SEB) and enterotoxin A (SEA) from Staphylococcus aureus, sucrose, saponin, and bovine serum albumin (BSA; all from Sigma-Aldrich, St Louis, MO); human rIL-2 (Peprotech, Rocky Hill, NJ); and PHA (Biochrom AG, Berlin, Germany).
PBMCs and T lymphocytes: purification and stimulation
PBMCs, obtained by Ficoll separation of peripheral blood samples from healthy donors, were cultured with SEB (100 ng/mL) at 1.5 × 106 cells/mL; alternatively, before SEB stimulation, PBMCs were depleted of CD4+ cells using magnetic beads (Dynal; Invitrogen, Frederick, MD). CD3+ T cells, purified by immunomagnetic negative selection, were used to isolate subpopulations of CD8+ and CD4+ T cells by magnetic beads. CD8+ and CD4+ T cells were stimulated with SEB in the presence of autologous irradiated PBMCs. In some experiments, PBMCs were stimulated with SEA (1 or 100 ng/mL), PHA (1 μg/mL), PHA plus IL-2 (10 IU/mL), or plate-bound anti-CD3 mAb (1 μg/mL; data not shown). Mixed-lymphocyte reactions were performed as described29 (data not shown). Cells were CFSE labeled at day 0 as described.29
Immunofluorescence and fluorescence-activated cell-sorting analysis
CFSE-labeled PBMCs were stained with the specific mAbs, followed by GAM-PE. Cells were then incubated with normal mouse IgG, followed by anti-CD3/APC and anti-CD8/PerCP mAbs, and finally analyzed by flow cytometry on a FACSCalibur (Becton Dickinson). Proliferating CD8+ T cells were identified by gating on the CFSElowCD3+CD8+ population, whereas proliferating CD4+ T cells were the CFSElowCD3+CD8− population. The median of fluorescence intensity (MFI) value of the isotype cIgG was subtracted from the MFI relative to each molecule. For intracellular staining, PBMCs were stained with anti-CD3/APC and anti-CD8/PerCP mAbs, washed, fixed in 1% paraformaldehyde, and permeabilized with 0.5% saponin in PBS. After washing, cells were incubated with 0.5 μg anti-NKG2D/PE.
ELISA
Enzyme-linked immunosorbent assay (ELISA) for sMICA, sMICB, IFN-γ, and TGF-β was from R&D Systems and for IL-21 was from eBioscience (San Diego, CA). sULBP2 was detected as described,21 whereas for sULBP1 and sULBP3 we established specific ELISAs. For sULBP1, plates were coated with NKG2D-Fc (25 μg/mL), whereas for sULBP3, with mAb M550 (2 μg/mL) (Amgen), overnight at R/T. After blocking with 1% BSA, samples and standard recombinant proteins (rULBP1-Fc or rULBP3-Fc) were added and incubated at R/T for 2 hours. After washing, 2 μg/mL anti-ULBP1 (AUMO2) or anti-ULBP3 mAb (R&D Systems) were added for 2 hours at R/T. Plates were washed and anti–mouse IgG-HRP (1:750; Bio-Rad, Hercules, CA) or IgG2a-HRP (1:2000; Southern Biotech, Birmingham, AL) was added for 1 hour at R/T, for sULBP1 and sULBP3, respectively. Plates were washed and developed using a peroxidase substrate system (R&D Systems). Cell-culture supernatants were analyzed without prior dilution.
Acidic elution, Transwell, and cell-cell contact experiments
For acidic elution, total PBMCs were harvested after 5 days of SEB stimulation, incubated for 1 minute on ice with 0.133 M citric acid and 0.066 M Na2HPO4, pH 3.3,37 and then washed with PBS containing Tris-HCl 0.9 mM, pH 8.8. Cells were stained with anti-NKG2D or anti-CD94. To control the elution efficiency, staining with primary mAbs was followed by acidic treatment, and then by GAM-PE. Anti-CD3/APC and anti-CD8/PerCP mAbs were added afterward.
Diffusion chamber assays were performed using cell-culture inserts for 24-well plates with 0.4-μm polycarbonate membrane (Transwell; Corning, Corning, NY), in which CD4-depleted PBMCs were added to the top chamber and CD4-depleted PBMCs or total PBMCs were added to the bottom chamber (1.5 × 106 cells/mL).
To study the effect of CD4-associated membrane-bound molecules, CD4+ cells were purified at day 3 from SEB-activated total PBMCs, and fixed or not in 1% paraformaldehyde (5 × 106 cells/mL) for 7 minutes at R/T. Cells were washed with PBS, and plated with CD4-depleted PBMCs, previously CFSE labeled and stimulated with SEB for 3 days. Purified CD4+ cells were plated at different ratios, from 1:1 up to 1:5, with an excess of CD4+ cells. Two days later, cells were harvested and stained with anti-CD8, anti-NKG2D, or anti-CD48 mAbs.
Real-time PCR
Total RNA (1 μg), isolated by Trizol (Gibco, Carlsbad, CA), was used for cDNA first-strand synthesis in a 25-μL reaction volume, and 1 μL cDNA was used in a 25-μL polymerase chain reaction (PCR) in the presence of FastStart Taq DNA polymerase (Roche Diagnostics, Basel, Switzerland). Real-time PCR was carried out using the ABI Prism 7700 Sequence Detection system (Applied Biosystems, Foster City, CA). cDNA was amplified in triplicate with primers for NKG2D (Hs01095635_m1) and DAP10 (Hs00367159_m1), both conjugated with fluorochrome FAM and β-actin (4326315E) conjugated with fluorochrome VIC. The cycling conditions were 50°C for 10 minutes, followed by 40 cycles of 95°C for 30 seconds, and 60°C for 2 minutes. Data were analyzed using the Sequence Detector v1.7 analysis software (Applied Biosystems).
T-cell proliferation and IFN-γ production
Ninety-six–well plates were coated with GAM-IgG (5 μg/mL) overnight at 4°C, then washed and specific mAbs were added O/N at 4°C. Purified NKG2Dhigh and NKG2Dlow CD8+ T cells were stimulated with plate-bound mAbs for 48 hours. Wells were pulsed with 0.5 μCi (0.0185 MBq) 3H-thymidine for the final 18 hours of culture and incorporated activity was measured in a scintillation counter. Data are represented as the mean of cpm (triplicates). In some experiments, supernatants were collected after 24 hours for IFN-γ ELISA.
Cytotoxicity assays
Purified NKG2Dhigh and NKG2Dlow CD8+ T cells were used as effectors. P815 cells, used as targets, were loaded with anti-CD3 or anti-NKG2D mAbs, in redirected Ab-dependent lysis. In degranulation assays, effector and target cells were plated at different ratios, with 2 × 105 cells from each cell population for the E/T ratio of 1:1 (data shown for 1:2.5). Cells were incubated at 37°C for 2 hours, and anti-CD8/PE and CD107a/FITC staining (or cIgG/FITC) was performed for 45 minutes on ice.38 Cells were washed and analyzed by fluorescence-activated cell sorting (FACS). Cytotoxicity was also measured by standard 51Cr-release assays (data not shown).
Results
Modulation of NKG2D expression on activated CD8+ T cells
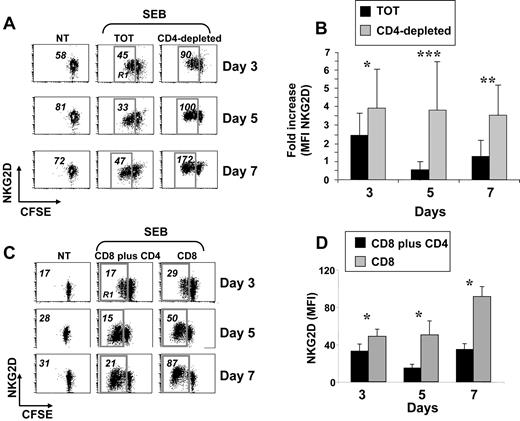
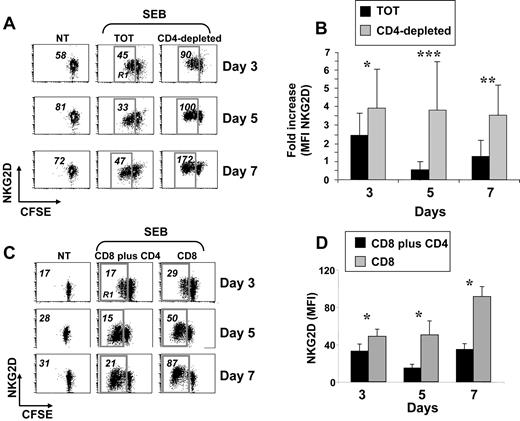
Expression of NKG2D was monitored on peripheral blood CD8+ T lymphocytes activated with the superantigen SEB, with PHA, or cocultured with irradiated allogeneic PBMCs. In all experimental settings, cells were also labeled with CFSE to follow their proliferative response. In all these conditions, and according to previous reports,3,14,30 NKG2D expression increased during the stimulation period, compared with untreated cells (data not shown). However, when CD8+ T cells were stimulated with SEB in the context of total PBMCs, a previously not described down-regulation of NKG2D was observed and it was particularly evident on proliferating cells after 5 days of stimulation (gate R1 in Figure 1A left and middle panels). Since one major difference between stimulation of purified CD8+ T cells and total PBMCs was the presence of CD4+ T cells, we investigated whether down-modulation of NKG2D was attributable to their presence. When PBMCs were depleted of CD4+ cells and activated with SEB, NKG2D down-modulation on CD8+ T cells was no longer observed (Figure 1A right panels). The most pronounced difference was at day 5 (Figure 1B). Notably, CD8+ T lymphocytes from the 2 cell cultures showed a similar proliferative capacity as measured by loss of CFSE fluorescence intensity (Figure 1A and data not shown).39 Similar results were obtained when CD8+ and CD4+ T cells were purified and then cultured together (Figure 1C-D), directly demonstrating that the capacity to down-regulate NKG2D was attributable mainly to CD4+ T lymphocytes and not to other CD4+ cells. In addition, NKG2D down-regulation did not occur on resting CD8+ T cells (data not shown), and it was specific as expression of other receptors did not show the same pattern (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Finally, NKG2D was down-regulated also when total PBMCs were stimulated with the superantigen SEA, with PHA with or without IL-2, or with the agonistic anti-CD3 mAb (data not shown).
Reduced expression of NKG2D on SEB-activated CD8+ T cells cultured in the presence of CD4+ cells. (A) PBMCs were CFSE-labeled, depleted or not (TOT) of CD4+ cells, and then stimulated with SEB. After 3, 5, and 7 days, cells were harvested and stained with anti-CD8, anti-CD3, and anti-NKG2D mAbs, in a 4-color FACS analysis. Data shown are from CD3+CD8+ cells. Numbers represent the NKG2D median of fluorescence intensity (MFI) values derived from unstimulated CD3+CD8+ T cells, or from the same cells that had divided at least once, as measured by loss of CFSE intensity (gate R1). Dot plots are from a representative donor, of 18 tested. Percentage of proliferating cells (gate R1) was as follows: 50 plus or minus 15 (TOT) and 37 plus or minus 19 (CD4−) at day 3; 73 plus or minus 17 (TOT) and 69 plus or minus 14 (CD4−) at day 5; and 78 plus or minus 17 (TOT) and 78 plus or minus 13 (CD4−) at day 7. (B) Summary of all donors. The NKG2D MFI values obtained on dividing cells from total or CD4-depleted PBMCs were divided per NKG2D MFI of unstimulated cells. IgG isotype control MFI values were always subtracted. *P less than .0002; **P less than .00001; and ***P less than .000001, with paired Student t test. (C) CD3+ T cells were isolated from PBMCs by negative selection and further purified to obtain subpopulations of CD4+CD3+ and CD8+CD3+ T cells. CD8+ T cells were cultured alone (CD8) or with CD4+ T cells (CD8 + CD4) and stimulated with SEB in the presence of irradiated autologous PBMCs. After 3, 5, and 7 days, cells were harvested and stained as described in panel A. Numbers represent NKG2D MFI values derived from unstimulated CD3+CD8+ T cells (NT), or from the same cells that had divided at least once, as measured by loss of CFSE intensity (gate R1). IgG isotype control MFI values were always subtracted. Dot plots are from a representative donor of 5 tested. Percentage of proliferating cells (gate R1) was as follows: 30 plus or minus 21 (CD8 + CD4) and 25 plus or minus 16 (CD8) at day 3; 47 plus or minus 20 (CD8 + CD4) and 49 plus or minus 19 (CD8) at day 5; 53 plus or minus 20 (CD8 + CD4) and 54 plus or minus 20 (CD8) at day 7. (D) Summary of 5 different donors. Mean plus or minus SE of NKG2D MFI values obtained on dividing cells from CD4 plus CD8 or CD8 T lymphocytes. *P value less than .04 with paired Student t test.
Reduced expression of NKG2D on SEB-activated CD8+ T cells cultured in the presence of CD4+ cells. (A) PBMCs were CFSE-labeled, depleted or not (TOT) of CD4+ cells, and then stimulated with SEB. After 3, 5, and 7 days, cells were harvested and stained with anti-CD8, anti-CD3, and anti-NKG2D mAbs, in a 4-color FACS analysis. Data shown are from CD3+CD8+ cells. Numbers represent the NKG2D median of fluorescence intensity (MFI) values derived from unstimulated CD3+CD8+ T cells, or from the same cells that had divided at least once, as measured by loss of CFSE intensity (gate R1). Dot plots are from a representative donor, of 18 tested. Percentage of proliferating cells (gate R1) was as follows: 50 plus or minus 15 (TOT) and 37 plus or minus 19 (CD4−) at day 3; 73 plus or minus 17 (TOT) and 69 plus or minus 14 (CD4−) at day 5; and 78 plus or minus 17 (TOT) and 78 plus or minus 13 (CD4−) at day 7. (B) Summary of all donors. The NKG2D MFI values obtained on dividing cells from total or CD4-depleted PBMCs were divided per NKG2D MFI of unstimulated cells. IgG isotype control MFI values were always subtracted. *P less than .0002; **P less than .00001; and ***P less than .000001, with paired Student t test. (C) CD3+ T cells were isolated from PBMCs by negative selection and further purified to obtain subpopulations of CD4+CD3+ and CD8+CD3+ T cells. CD8+ T cells were cultured alone (CD8) or with CD4+ T cells (CD8 + CD4) and stimulated with SEB in the presence of irradiated autologous PBMCs. After 3, 5, and 7 days, cells were harvested and stained as described in panel A. Numbers represent NKG2D MFI values derived from unstimulated CD3+CD8+ T cells (NT), or from the same cells that had divided at least once, as measured by loss of CFSE intensity (gate R1). IgG isotype control MFI values were always subtracted. Dot plots are from a representative donor of 5 tested. Percentage of proliferating cells (gate R1) was as follows: 30 plus or minus 21 (CD8 + CD4) and 25 plus or minus 16 (CD8) at day 3; 47 plus or minus 20 (CD8 + CD4) and 49 plus or minus 19 (CD8) at day 5; 53 plus or minus 20 (CD8 + CD4) and 54 plus or minus 20 (CD8) at day 7. (D) Summary of 5 different donors. Mean plus or minus SE of NKG2D MFI values obtained on dividing cells from CD4 plus CD8 or CD8 T lymphocytes. *P value less than .04 with paired Student t test.
Altogether, these results indicate that NKG2D down-modulation occurs on activated CD8+ T cells independently of the type of stimulation, but it is dependent on the presence of CD4+ T cells. For sake of simplicity, we will refer to CD8+ T lymphocytes from total PBMC cultures as NKG2Dlow cells, and to those from CD4-depleted PBMCs as NKG2Dhigh cells.
MICB is expressed on the surface of SEB-activated CD4+ and CD8+ T lymphocytes
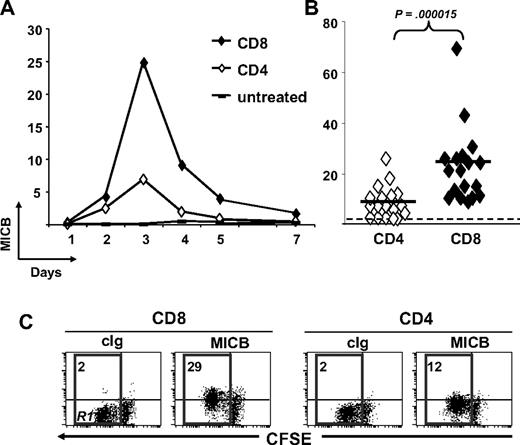
In our previous work,29 we observed that MICB mRNA was up-regulated on activated T lymphocytes, but we did not examine its surface expression because of the unavailability of a specific anti-MICB Ab. We now could extend our analysis using a newly commercially available anti-MICB mAb. CFSE-labeled PBMCs were stimulated with SEB, and MICB was monitored on both CD4+ and CD8+ T lymphocytes at different days. MICB started to be expressed at day 2, peaked at day 3, and declined afterward, disappearing at day 7 (Figure 2A). Similarly to the other NKG2DLs, MICB was preferentially expressed on proliferating T cells (Figure 2C). Interestingly, though MICA and ULBP expression levels were similar between CD4+ and CD8+ T cells,29 MICB was always more highly expressed on CD8+ T lymphocytes (Figure 2B).
MICB is expressed mostly on the surface of SEB-activated CD8+ T cells. PBMCs were CFSE labeled and then stimulated with SEB. Cells were harvested at the indicated times and stained with anti-CD8–, anti-CD3–, and anti-MICB–specific mAbs in a 4-color FACS analysis. Subpopulations of CD8+ and CD4+ T lymphocytes were identified by gating on CD8+CD3+ or CD8−CD3+ cells, respectively, and further analyzed for the expression of MICB. (A) Time-course analysis of MICB expression on CD4+ and CD8+ T cells. MFI values relative to MICB are reported. One representative donor of 14 is shown. (B) Expression levels of MICB analyzed on SEB-activated CD4+ or CD8+ T lymphocytes from different donors (n = 14) at day 3. Each donor is represented by a symbol. The dashed line delimits negative donors (MFI values < 2). Mean values of MFI are indicated by horizontal bars and were calculated on positive donors. They were 25 plus or minus 14 on CD8+ T cells (ranging from 12 to 69) and 9 plus or minus 6 on CD4+ T cells (ranging from 3 to 26). Statistical analysis on all donors with paired Student t test. (C) MICB is preferentially expressed on proliferating cells. Progressive loss of CFSE fluorescence intensity in CD4+ or CD8+ T lymphocytes is indicated by the R1 region. The MFI relative to MICB and calculated on cells gated in R1 is shown. A representative donor of 14 analyzed is shown at day 3 after SEB stimulation.
MICB is expressed mostly on the surface of SEB-activated CD8+ T cells. PBMCs were CFSE labeled and then stimulated with SEB. Cells were harvested at the indicated times and stained with anti-CD8–, anti-CD3–, and anti-MICB–specific mAbs in a 4-color FACS analysis. Subpopulations of CD8+ and CD4+ T lymphocytes were identified by gating on CD8+CD3+ or CD8−CD3+ cells, respectively, and further analyzed for the expression of MICB. (A) Time-course analysis of MICB expression on CD4+ and CD8+ T cells. MFI values relative to MICB are reported. One representative donor of 14 is shown. (B) Expression levels of MICB analyzed on SEB-activated CD4+ or CD8+ T lymphocytes from different donors (n = 14) at day 3. Each donor is represented by a symbol. The dashed line delimits negative donors (MFI values < 2). Mean values of MFI are indicated by horizontal bars and were calculated on positive donors. They were 25 plus or minus 14 on CD8+ T cells (ranging from 12 to 69) and 9 plus or minus 6 on CD4+ T cells (ranging from 3 to 26). Statistical analysis on all donors with paired Student t test. (C) MICB is preferentially expressed on proliferating cells. Progressive loss of CFSE fluorescence intensity in CD4+ or CD8+ T lymphocytes is indicated by the R1 region. The MFI relative to MICB and calculated on cells gated in R1 is shown. A representative donor of 14 analyzed is shown at day 3 after SEB stimulation.
NKG2D ligands are released by SEB-activated PBMCs
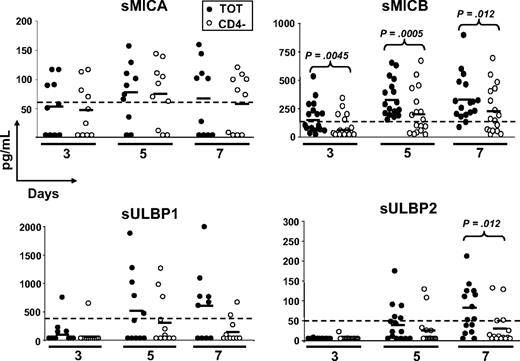
To study whether NKG2DLs were shed, possibly causing ligand-induced down-regulation of NKG2D on CD8+ T cells, we tested cell culture supernatants of total and CD4-depleted PBMCs stimulated with SEB for different days, in ELISA (Figure 3). Despite that MICA was expressed on the cell surface of activatedT cells in the great majority of donors (∼ 90%; Cerboni et al29 and data not shown), the soluble molecule was detected in only 40% to 70% of donors depending on the time point analyzed, with no major differences between total and CD4-depleted PBMCs. MICB was instead released by 100% of donors tested at day 5, and at all time points sMICB was significantly higher in the presence of CD4+ T cells. In fact, when CD8+ and CD4+ T lymphocytes were purified and stimulated with SEB, we observed that sMICB was released at significantly higher levels by CD4+ T cells, particularly at day 5 (Figure 4A). Of note, there was an inverse correlation between release of sMICB and its surface expression among CD4+ and CD8+ T lymphocytes (Figure 4B). sULBP1 was detected in no more than 50% of donors and only supernatants from total PBMCs had average sULBP1 levels above the detection limit, though they were never statistically different from the levels detected in CD4-depleted cultures. For sULBP2, maximal release was observed at day 7, when 60% of donors were positive and the levels were higher in the presence of CD4+ T cells. For sULBP3, we set up a specific ELISA (Figure S2). Cell culture supernatants from total or CD4-depleted PBMCs were negative for sULBP3, at all time points and in all donors tested (n = 9; data not shown).
Release of soluble NKG2D ligands by SEB-activated PBMCs. Total (●) or CD4-depleted (○) PBMCs were activated with SEB and cell-culture supernatants were harvested after 3, 5, and 7 days and tested in ELISA for the presence of the different ligands. Each circle represents the mean of triplicates of one donor tested on both cell populations. The numbers of donors analyzed were 10 (MICA), 17 (MICB), 11 (ULBP1), and 15 (ULBP2). All donors were monitored also for cell-surface expression of the different ligands by flow cytometry, and those negative in both types of assays were excluded from the analysis. For all soluble ligands, absorbance was measured by subtracting readings at 540 nm from readings at 450 nm. Dotted lines show the detection limit of each ELISA, and they were as follows: sMICA = 62.5 pg/mL, sMICB = 156 pg/mL, sULBP1 = 400 pg/mL, and sULBP2 = 50 pg/mL. Solid lines indicate the average of all measurements in the respective group. Statistical analysis on all donors with paired Student t test.
Release of soluble NKG2D ligands by SEB-activated PBMCs. Total (●) or CD4-depleted (○) PBMCs were activated with SEB and cell-culture supernatants were harvested after 3, 5, and 7 days and tested in ELISA for the presence of the different ligands. Each circle represents the mean of triplicates of one donor tested on both cell populations. The numbers of donors analyzed were 10 (MICA), 17 (MICB), 11 (ULBP1), and 15 (ULBP2). All donors were monitored also for cell-surface expression of the different ligands by flow cytometry, and those negative in both types of assays were excluded from the analysis. For all soluble ligands, absorbance was measured by subtracting readings at 540 nm from readings at 450 nm. Dotted lines show the detection limit of each ELISA, and they were as follows: sMICA = 62.5 pg/mL, sMICB = 156 pg/mL, sULBP1 = 400 pg/mL, and sULBP2 = 50 pg/mL. Solid lines indicate the average of all measurements in the respective group. Statistical analysis on all donors with paired Student t test.
MICB is released at higher levels by purified and activated CD4+ T cells. CD3+ T cells were isolated by negative selection and further purified to obtain subpopulations of CD4+CD3+ (CD4) and CD8+CD3+ (CD8) T cells. CD4+ and CD8+ T cells were stimulated with SEB in the presence of irradiated autologous PBMCs. (A) Cell culture supernatants were harvested after 3 and 5 days and tested in ELISA for the presence of sMICB. Each symbol represents one donor tested on both cell populations. Dotted line shows the detection limit of the assay. Solid lines indicate the average of all measurements in the respective group. Statistical analysis on all donors with paired Student t test. (B) The same donors were monitored also for cell-surface expression of MICB by anti-CD3 and anti-CD8 staining, followed by flow cytometry. IgG isotype control MFI values were always subtracted. Mean values of MFI are indicated by horizontal bars and were calculated on all donors. Statistical analysis with paired Student t test.
MICB is released at higher levels by purified and activated CD4+ T cells. CD3+ T cells were isolated by negative selection and further purified to obtain subpopulations of CD4+CD3+ (CD4) and CD8+CD3+ (CD8) T cells. CD4+ and CD8+ T cells were stimulated with SEB in the presence of irradiated autologous PBMCs. (A) Cell culture supernatants were harvested after 3 and 5 days and tested in ELISA for the presence of sMICB. Each symbol represents one donor tested on both cell populations. Dotted line shows the detection limit of the assay. Solid lines indicate the average of all measurements in the respective group. Statistical analysis on all donors with paired Student t test. (B) The same donors were monitored also for cell-surface expression of MICB by anti-CD3 and anti-CD8 staining, followed by flow cytometry. IgG isotype control MFI values were always subtracted. Mean values of MFI are indicated by horizontal bars and were calculated on all donors. Statistical analysis with paired Student t test.
Altogether, our results demonstrate for the first time that MICA, MICB, ULBP1, and ULBP2, but not ULBP3, are released from antigen-activated T cells. Of note, only MICB was released by all donors analyzed and always at significantly higher amounts by CD4+ T cells.
The role of membrane-bound versus soluble factors in the down-modulation of NKG2D
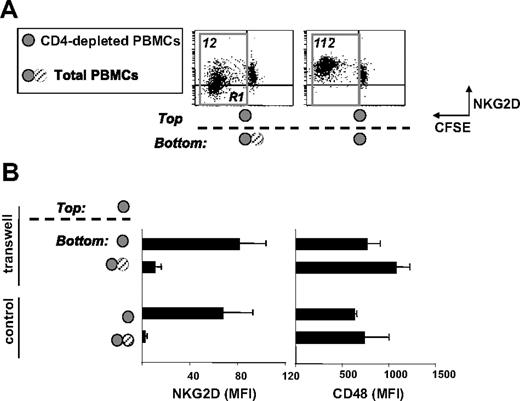
The observation that T lymphocytes express cell-surface NKG2DLs and release them as soluble molecules raised the question of the contribution of membrane-bound versus soluble factors in the modulation of NKG2D on CD8+ T cells. We thus assessed whether soluble factor(s) produced by CD4+ T cells were involved in CD4-induced NKG2D down-modulation. We performed Transwell experiments where CD4-depleted PBMCs were placed in the top chamber and total or CD4-depleted PBMCs, in the bottom chamber (Figure 5). After 5 days of SEB stimulation, NKG2D was analyzed on CD8+ T cells in the top chamber. Interestingly, NKG2D down-modulation was observed only when total PBMCs were present in the bottom chamber, suggesting the involvement of soluble factor(s) released by CD4+ T cells. The degree of receptor down-modulation was similar to that observed in standard cell culture conditions and it was specific since expression of CD48 was not affected (Figure 5B). These findings, together with the evidence that MICB was the ligand released by all donors and always at higher levels when CD4+ T cells were present in the cell cultures (Figures 3–4), prompted us to investigate whether MICB was involved in NKG2D down-modulation. Total PBMCs were activated with SEB for 3 days and neutralizing Abs against MICA, MICB, MICA/B,34 ULBP1, and ULBP2 were added to the wells for an additional 48 hours. NKG2D expression was partially restored only when MICB was blocked (by anti-MICA/B or anti-MICB Abs; Table 1). All other combinations of neutralizing Abs did not affect NKG2D, thus excluding a major role for MICA and ULBP1-2 in receptor down-regulation. However, we considered the possibility that among soluble factors down-modulating NKG2D also TGF-β and IL-21 could have a role, since they are able to reduce NKG2D expression.40-43 In general, both cytokines were detected at higher levels in the supernatants of total PBMC cultures, particularly at day 3 (IL-21) or at day 5 (TGF-β) (Figure S3). However, they did not substantially contribute in NKG2D down-modulation, since neutralizing experiments with a blocking anti–TGF-β1 mAb or with the recombinant IL-21R-Fc44 had no influence on NKG2D expression (Table 1).
Down-modulation of NKG2D is dependent mainly on soluble factor(s) released by CD4+ cells. (A,B) PBMCs were CFSE labeled and depleted of CD4+ cells (CD4-depleted PBMCs) or not depleted (total PBMCs) before SEB stimulation. Cells were grown in 24-well or in Transwell plates. In Transwell plates, CD4-depleted PBMCs were cultured in the top chamber, whereas total or CD4-depleted PBMCs were plated in the bottom chamber, as indicated. After 5 days, NKG2D or CD48 expression was evaluated on CD3+CD8+ T cells derived from the top chamber. In panel A, progressive loss of CFSE fluorescence intensity in CD8+ T cells is indicated by the R1 region. The MFI relative to NKG2D and calculated on cells gated in R1 is shown. Dot plots presented derive from a representative donor of 5 tested. In panel B, results obtained from 5 different donors are shown and are presented as the mean plus or minus SD of NKG2D or CD48 (used as control) MFI calculated as described for panel A. In parallel, NKG2D and CD48 expression was evaluated on CD3+CD8+ T cells grown in 24-well plates.
Down-modulation of NKG2D is dependent mainly on soluble factor(s) released by CD4+ cells. (A,B) PBMCs were CFSE labeled and depleted of CD4+ cells (CD4-depleted PBMCs) or not depleted (total PBMCs) before SEB stimulation. Cells were grown in 24-well or in Transwell plates. In Transwell plates, CD4-depleted PBMCs were cultured in the top chamber, whereas total or CD4-depleted PBMCs were plated in the bottom chamber, as indicated. After 5 days, NKG2D or CD48 expression was evaluated on CD3+CD8+ T cells derived from the top chamber. In panel A, progressive loss of CFSE fluorescence intensity in CD8+ T cells is indicated by the R1 region. The MFI relative to NKG2D and calculated on cells gated in R1 is shown. Dot plots presented derive from a representative donor of 5 tested. In panel B, results obtained from 5 different donors are shown and are presented as the mean plus or minus SD of NKG2D or CD48 (used as control) MFI calculated as described for panel A. In parallel, NKG2D and CD48 expression was evaluated on CD3+CD8+ T cells grown in 24-well plates.
Next, we investigated the contribution of cell-bound molecules in NKG2D down-regulation by comparing cell cultures with fixed or nonfixed cells. In particular, we cultured CFSE-labeled CD4-depleted (CD4−/CFSE+) or total (TOT/CFSE−) PBMCs for 3 days in the presence of SEB. Then, CD4+ T cells were purified from TOT/CFSE− cultures, fixed or not, and plated with CD4−/CFSE+ PBMCs at different ratios. NKG2D expression was higher in the presence of fixed CD4+ T cells, indicating the involvement of soluble factor(s) in maintaining a low NKG2D expression (Figure S4). Furthermore, we investigated whether NKG2D down-modulation was reversible in the absence of a SEB-conditioned medium. Cells were harvested after 5 days of stimulation, washed, and then cultured under different conditions for an additional 3 days (Figure S5). In the presence of fresh medium, expression of NKG2D increased, even though it did not reach the levels observed on CD4-depleted PBMCs (data not shown). Similar results were obtained by purifying CD8+ T cells at day 5 and replating them with fresh or SEB-conditioned medium for 3 more days (data not shown).
Taken together, these data strongly suggest that sMICB plays a major role in CD4-mediated NKG2D down-regulation. Other factors, including other soluble NKG2DLs, TGF-β, IL-21, as well as cell-bound molecules, might also contribute, though to a lesser extent, in the regulation of NKG2D expression on CD8+ T cells.
NKG2D down-modulation involves receptor internalization and not transcriptional regulation
Next, we investigated the mechanism(s) responsible of NKG2D down-modulation. To exclude the possibility that the NKG2D epitope was masked by the binding of soluble ligands, cells were briefly washed in acidic buffer (pH 3.3) before the staining with anti-NKG2D mAb. This treatment did not increase NKG2D MFI values (Figure 6A), thus excluding the possibility of epitope masking.37 We also excluded that decreased NKG2D expression was due to the failure of Ab binding as a consequence of changes in receptor conformation since we stained cells with other 2 anti-NKG2D mAbs and obtained similar results (data not shown).
Analysis of the mechanisms involved in NKG2D down-modulation. (A) Acidic treatment does not alter NKG2D expression on NKG2Dlow cells. Total PBMCs were stimulated with SEB for 5 days and FACS analysis was performed on CFSE+CD3+CD8+ cells. The C-type lectin receptor CD94 was used as control. ■ indicate normal staining;  , acidic elution; and □, control of the elution. The latter staining was performed by adding the anti-NKG2D or anti-CD94 mAb and, before adding the secondary GAM-PE mAb, cells were briefly washed with the acidic buffer. The lower staining demonstrates that the acidic treatment is able to break the antigen-Ab interaction. (B) Large amounts of intracellular NKG2D are found in NKG2Dlow cells. NKG2Dhigh and NKG2Dlow cells were harvested, fixed, and permeabilized to analyze the total amount of NKG2D (TOT = extracellular + intracellular) on CD3+CD8+ cells. Staining of extracellular (EC) NKG2D was performed as usual. In the right panel, results are presented as the mean plus or minus SD of the percentage of EC/TOT NKG2D obtained from 4 donors. (C) Clathrin-dependent endocytosis is involved in NKG2D down-modulation. Total or CD4-depleted PBMCs were stimulated with SEB for 4 days, sucrose (0.1 M) was added and left for an additional 18 hours. NKG2D and LFA-1 (used as control) expression was evaluated on CFSE+CD3+CD8+ cells. Treatment with sucrose did not reduce viability and CD3+CD8+ T-cell proliferation (data not shown). (D) NKG2D and DAP10 transcripts, quantitated by real-time PCR, in SEB-activated CD8+ T cells. Total or CD4-depleted PBMCs were stimulated with SEB and cells were harvested at different times as indicated. CD8+ T lymphocytes were further purified by immunomagnetic positive selection, total RNA was isolated, and real-time PCR was performed. Data were normalized by the amount of β-actin mRNA. Data are shown as the mean cycles of normalized NKG2D or DAP10 expression plus or minus SD (triplicates). All panels derive from 1 representative donor of 3 (D) or 4 (A-C) tested.
, acidic elution; and □, control of the elution. The latter staining was performed by adding the anti-NKG2D or anti-CD94 mAb and, before adding the secondary GAM-PE mAb, cells were briefly washed with the acidic buffer. The lower staining demonstrates that the acidic treatment is able to break the antigen-Ab interaction. (B) Large amounts of intracellular NKG2D are found in NKG2Dlow cells. NKG2Dhigh and NKG2Dlow cells were harvested, fixed, and permeabilized to analyze the total amount of NKG2D (TOT = extracellular + intracellular) on CD3+CD8+ cells. Staining of extracellular (EC) NKG2D was performed as usual. In the right panel, results are presented as the mean plus or minus SD of the percentage of EC/TOT NKG2D obtained from 4 donors. (C) Clathrin-dependent endocytosis is involved in NKG2D down-modulation. Total or CD4-depleted PBMCs were stimulated with SEB for 4 days, sucrose (0.1 M) was added and left for an additional 18 hours. NKG2D and LFA-1 (used as control) expression was evaluated on CFSE+CD3+CD8+ cells. Treatment with sucrose did not reduce viability and CD3+CD8+ T-cell proliferation (data not shown). (D) NKG2D and DAP10 transcripts, quantitated by real-time PCR, in SEB-activated CD8+ T cells. Total or CD4-depleted PBMCs were stimulated with SEB and cells were harvested at different times as indicated. CD8+ T lymphocytes were further purified by immunomagnetic positive selection, total RNA was isolated, and real-time PCR was performed. Data were normalized by the amount of β-actin mRNA. Data are shown as the mean cycles of normalized NKG2D or DAP10 expression plus or minus SD (triplicates). All panels derive from 1 representative donor of 3 (D) or 4 (A-C) tested.
Analysis of the mechanisms involved in NKG2D down-modulation. (A) Acidic treatment does not alter NKG2D expression on NKG2Dlow cells. Total PBMCs were stimulated with SEB for 5 days and FACS analysis was performed on CFSE+CD3+CD8+ cells. The C-type lectin receptor CD94 was used as control. ■ indicate normal staining;  , acidic elution; and □, control of the elution. The latter staining was performed by adding the anti-NKG2D or anti-CD94 mAb and, before adding the secondary GAM-PE mAb, cells were briefly washed with the acidic buffer. The lower staining demonstrates that the acidic treatment is able to break the antigen-Ab interaction. (B) Large amounts of intracellular NKG2D are found in NKG2Dlow cells. NKG2Dhigh and NKG2Dlow cells were harvested, fixed, and permeabilized to analyze the total amount of NKG2D (TOT = extracellular + intracellular) on CD3+CD8+ cells. Staining of extracellular (EC) NKG2D was performed as usual. In the right panel, results are presented as the mean plus or minus SD of the percentage of EC/TOT NKG2D obtained from 4 donors. (C) Clathrin-dependent endocytosis is involved in NKG2D down-modulation. Total or CD4-depleted PBMCs were stimulated with SEB for 4 days, sucrose (0.1 M) was added and left for an additional 18 hours. NKG2D and LFA-1 (used as control) expression was evaluated on CFSE+CD3+CD8+ cells. Treatment with sucrose did not reduce viability and CD3+CD8+ T-cell proliferation (data not shown). (D) NKG2D and DAP10 transcripts, quantitated by real-time PCR, in SEB-activated CD8+ T cells. Total or CD4-depleted PBMCs were stimulated with SEB and cells were harvested at different times as indicated. CD8+ T lymphocytes were further purified by immunomagnetic positive selection, total RNA was isolated, and real-time PCR was performed. Data were normalized by the amount of β-actin mRNA. Data are shown as the mean cycles of normalized NKG2D or DAP10 expression plus or minus SD (triplicates). All panels derive from 1 representative donor of 3 (D) or 4 (A-C) tested.
, acidic elution; and □, control of the elution. The latter staining was performed by adding the anti-NKG2D or anti-CD94 mAb and, before adding the secondary GAM-PE mAb, cells were briefly washed with the acidic buffer. The lower staining demonstrates that the acidic treatment is able to break the antigen-Ab interaction. (B) Large amounts of intracellular NKG2D are found in NKG2Dlow cells. NKG2Dhigh and NKG2Dlow cells were harvested, fixed, and permeabilized to analyze the total amount of NKG2D (TOT = extracellular + intracellular) on CD3+CD8+ cells. Staining of extracellular (EC) NKG2D was performed as usual. In the right panel, results are presented as the mean plus or minus SD of the percentage of EC/TOT NKG2D obtained from 4 donors. (C) Clathrin-dependent endocytosis is involved in NKG2D down-modulation. Total or CD4-depleted PBMCs were stimulated with SEB for 4 days, sucrose (0.1 M) was added and left for an additional 18 hours. NKG2D and LFA-1 (used as control) expression was evaluated on CFSE+CD3+CD8+ cells. Treatment with sucrose did not reduce viability and CD3+CD8+ T-cell proliferation (data not shown). (D) NKG2D and DAP10 transcripts, quantitated by real-time PCR, in SEB-activated CD8+ T cells. Total or CD4-depleted PBMCs were stimulated with SEB and cells were harvested at different times as indicated. CD8+ T lymphocytes were further purified by immunomagnetic positive selection, total RNA was isolated, and real-time PCR was performed. Data were normalized by the amount of β-actin mRNA. Data are shown as the mean cycles of normalized NKG2D or DAP10 expression plus or minus SD (triplicates). All panels derive from 1 representative donor of 3 (D) or 4 (A-C) tested.
We then investigated whether NKG2D was internalized upon interaction with its ligands induced on activated T cells. To address this issue, we first examined total NKG2D protein levels on fixed and permeabilized NKG2Dhigh and NKG2Dlow CD8+ T cells (Figure 6B). The total NKG2D content of permeabilized NKG2Dlow cells was significantly higher compared with its extracellular amount, indicating that a large proportion of the receptor was inside the cell. By contrast, the amount of NKG2D on the cell surface was similar to its total content both in NKG2Dhigh and in untreated cells, indicating that in these cells the greatest fraction of the receptor was expressed on the cell surface (Figure 6B and data not shown).
We then investigated whether the reduction of NKG2D was due to clathrin-dependent endocytosis, which is inhibited by hypertonic conditions.26 Total or CD4-depleted PBMCs were stimulated with SEB for 5 days, and sucrose was added for the last 18 hours. Decreased NKG2D expression on NKG2Dlow CD8+ T cells was partially reversed by the hypertonic medium, whereas LFA-1 expression was not modulated by this treatment (Figure 6C).
Next, we asked whether reduction of NKG2D expression could occur at the mRNA level, by examining the levels of both DAP10 and NKG2D transcripts in NKG2Dlow and NKG2Dhigh CD8+ T cells by real-time PCR. The 2 populations expressed equivalent NKG2D and DAP10 mRNA, thus indicating that the dramatic NKG2D down-modulation is independent from mRNA levels (Figure 6D).
Altogether, these findings show that NKG2D down-modulation results from clathrin–dependent receptor internalization triggered by the presence of CD4+ T cells.
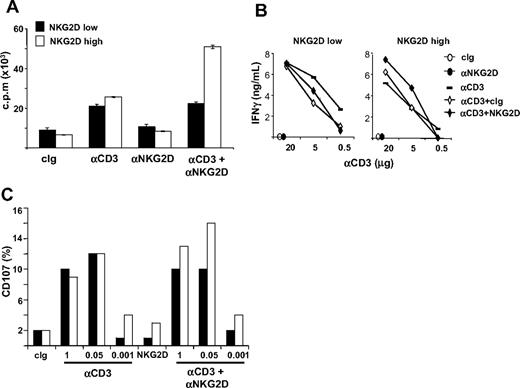
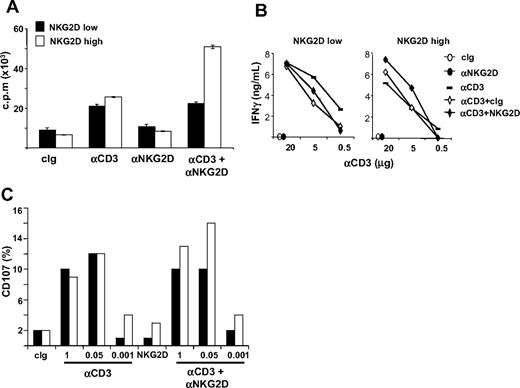
NKG2D costimulation of CD3-induced proliferation, IFN-γ production, and cytotoxicity is impaired in NKG2Dlow CD8+ T lymphocytes
We investigated whether NKG2D down-modulation on CD8+ T cells had functional consequences (Figure 7 and Figure S6). NKG2Dlow and NKG2Dhigh CD8+ T cells were purified and restimulated with plate-bound anti-CD3 and anti-NKG2D mAbs, alone or in combination, and T-cell proliferation was assessed 48 hours later. Interestingly, anti-NKG2D mAb costimulated CD3-induced proliferation only in NKG2Dhigh T cells, whereas if used alone it had no activity on the 2 populations (Figure 7A and Figure S6). Similarly to proliferation, NKG2D increased also CD3-induced IFN-γ production only in NKG2Dhigh T cells (Figure 7B).
NKG2D costimulation of CD3-induced functions is impaired in CD3+CD8+ NKG2Dlow T lymphocytes. (A) Analysis of the proliferative response. Total or CD4-depleted PBMCs were stimulated with SEB for 5 days and CD8+ T cells were then purified. NKG2Dlow and NKG2Dhigh CD8+ T cells were restimulated with the indicated combinations of plate-bound Abs (at 5 μg/mL) and T-cell proliferation was assessed 48 hours later. Wells were pulsed with 3H-thymidine for the final 18 hours of culture and incorporated activity was measured in a scintillation counter. Data are represented as the mean plus or minus SD (triplicates) and are representative of 1 of 4 different donors tested. (B) IFN-γ production. CD8+ T cells were obtained as described in panel A and stimulated with plate-bound mAbs for 24 hours. Supernatants were collected and tested for the presence of IFN-γ. (C) CD8+ T cells were obtained as described in panel A and they were mixed with P815 cells preincubated with anti-NKG2D (at 1 μg/106 cells) and different amounts of the anti-CD3 mAb. After 2 hours at 37°C, cells were stained with anti-CD8/PE and anti-CD107a/FITC mAbs. Cell-surface expression of CD107a was analyzed on CD8+ cells.
NKG2D costimulation of CD3-induced functions is impaired in CD3+CD8+ NKG2Dlow T lymphocytes. (A) Analysis of the proliferative response. Total or CD4-depleted PBMCs were stimulated with SEB for 5 days and CD8+ T cells were then purified. NKG2Dlow and NKG2Dhigh CD8+ T cells were restimulated with the indicated combinations of plate-bound Abs (at 5 μg/mL) and T-cell proliferation was assessed 48 hours later. Wells were pulsed with 3H-thymidine for the final 18 hours of culture and incorporated activity was measured in a scintillation counter. Data are represented as the mean plus or minus SD (triplicates) and are representative of 1 of 4 different donors tested. (B) IFN-γ production. CD8+ T cells were obtained as described in panel A and stimulated with plate-bound mAbs for 24 hours. Supernatants were collected and tested for the presence of IFN-γ. (C) CD8+ T cells were obtained as described in panel A and they were mixed with P815 cells preincubated with anti-NKG2D (at 1 μg/106 cells) and different amounts of the anti-CD3 mAb. After 2 hours at 37°C, cells were stained with anti-CD8/PE and anti-CD107a/FITC mAbs. Cell-surface expression of CD107a was analyzed on CD8+ cells.
We then assessed the lytic capacity of NKG2Dhigh and NKG2Dlow T cells against P815 cells loaded with anti-NKG2D mAb and with titrating concentrations of anti-CD3 mAb by analyzing the expression of CD107a on T cells (Figure 7C and Figure S6), or by 51Cr-release assays (data not shown). Interestingly, reduced anti-NKG2D–mediated costimulation was again observed in NKG2Dlow compared with NKG2Dhigh T cells. NKG2Dhigh and NKG2Dlow T cells showed similar killing capacity when anti-CD3 mAb was used alone, whereas triggering of the NKG2D receptor did not result in lysis, in accordance with previous studies.4,45
Thus, CD4-induced NKG2D down-modulation on SEB-activated CD8+ T cells resulted in an impaired ability of NKG2D to costimulate CD3-induced proliferation, IFNγ, production and cytotoxicity. Notably, the ability of NKG2D to costimulate T-cell proliferation and effector functions was consistently observed in approximately 50% of the donors analyzed.
Discussion
In this study, we show for the first time that NKG2D expression is down-regulated in response to antigen stimulation. Previous studies performed on CD8+ T cells generally demonstrated an up-regulation of NKG2D following cell activation/proliferation3,14,30 and we obtained the same results whether purified CD8+ T lymphocytes were activated with the superantigen SEB or with PHA, or were cocultured in mixed-lymphocyte reactions (data not shown). However, we found that if CD8+ T cells were cultured in the context of total PBMCs, expression of NKG2D was strongly down-regulated. Such down-modulation was dependent on the presence of CD4+ T cells, and, among different cell-surface receptors analyzed, it was observed only for NKG2D.
Our findings also suggest that release of soluble NKG2DLs is a major mechanism by which CD4+ T cells down-regulate NKG2D, as demonstrated by the combination of fixation and transwell experiments. Moreover, ELISA measurements showed higher levels of soluble NKG2DLs, and in particular of sMICB, in the presence of CD4+ T cells. This observation, together with the data showing a much lower cell-surface expression of MICB on antigen-activated CD4+ T cells, suggests that this could be the key molecule released by CD4+ T lymphocytes causing NKG2D down-modulation. These data also indicate that MICB is differentially regulated, expressed, and/or released among different lymphocyte subsets, and future experiments will be aimed at investigating the mechanism(s) responsible for the different expression of MICB on activated CD4+ and CD8+ T cells. On the other hand, it will be also interesting to understand why MICA cell-surface expression on T cells from the majority of donors29 was not accompanied by its shedding. Indeed, many reports on cancer patients and on tumor cell lines have shown that MICA is often released as a soluble molecule; thus shedding of MICA may be differentially regulated between lymphocytes and other cell types.19,20,33 ULBP1 and ULBP2 were also shed, but in 50% to 60% of donors and with a delayed kinetics compared with the decrease in NKG2D expression. Their kinetics, however, paralleled the expression of ULBP1 and ULBP2 on the cell surface (data not shown). However, since neutralizing experiments with anti-ULBP1 and -ULBP2 mAbs did not rescue NKG2D expression, we tend to believe that these ligands do not contribute in modulating NKG2D expression. As far as sULBP3 is concerned, we were able to set up a specific ELISA, but sULBP3 was absent from cell-culture supernatants thus excluding its involvement in NKG2D down-modulation.
In an attempt to better investigate the contribution of sMICB in NKG2D down-regulation, we used 2 neutralizing Abs: a cross-reactive anti-MICA/B polyclonal Ab and a specific anti-MICB mAb. These treatments partially restored NKG2D expression, which was instead not affected by a blocking anti-MICA mAb, thus indicating a role for MICB in receptor modulation. However, we could not exclude the involvement of other factors/cytokines in down-regulating NKG2D expression. IL-21 and TGF-β were other candidates as their ability to down-regulate NKG2D and to impair NK and CD8+ T-cell activity has been reported.40-43 In our experimental settings, a significant difference in the levels of IL-21 and TGF-β was observed only after 3 or 5 days of SEB stimulation, respectively, with lower amounts of both cytokines in CD4-depleted cell cultures. However, experiments performed with a blocking anti–TGF-β mAb or with the soluble recombinant IL-21 receptor (IL-21R-Fc) did not rescue NKG2D expression levels, at any time point and at any of the concentrations used, thus arguing against a role of these cytokines in NKG2D down-regulation. We also tend to exclude that in our experimental settings IL-21 and TGF-β are involved in the modulation of NKG2D, as their effect is associated with a marked reduction of DAP10 or NKG2D transcripts,41,43 which was not observed in this study. Our findings instead indicate that NKG2D is internalized upon ligand binding. Staining of permeabilized NKG2Dlow and NKG2Dhigh populations showed that significant, high amounts of NKG2D protein were found in the cytoplasm of NKG2Dlow cells. In addition, down-modulation of NKG2D was inhibited by hypertonic medium, indicating that NKG2D may be endocytosed via a clathrin-dependent pathway, as shown in NOD mice.26 We also observed that the NKG2Dlow phenotype was reversible, since NKG2D expression could be in part recovered when cells were further cultured in the presence of fresh medium. Thus, the higher total NKG2D content observed in permeabilized NKG2Dlow cells together with its partly reversible expression suggest that at least a proportion of NKG2D was not degraded but rather recycled. Altogether, these data provide evidence that the dramatic NKG2D down-modulation on SEB-activated CD8+ T cells cultured in the presence of CD4+ T cells is an event independent from transcription, but likely due to receptor internalization induced by NKG2DLs, and in particular by MICB.
Our new findings raise the question of why NKG2D should be kept under control by a mechanism relying on the presence of CD4+ T cells. A prolonged exposure to ligands expressed on T cells may desensitize CD8+ lymphocytes, rendering them functionally “anergic” to NKG2D costimulation, and this would protect CD4+ T cells (as well as other NKG2DL+ cells) from NKG2D-mediated recognition. Sparing CD4+ T cells would allow a continuation of their interplay with CD8+ lymphocytes and/or with other immune cells, especially in those sites where they are in close contact (eg, lymph nodes). On the other hand, desensitization of CD8+ T cells resulting from NKG2D down-modulation would affect their effector functions and it could represent a novel mechanism to switch them off. The control of CD8+ T-cell proliferation and effector functions could then result from both the up-regulation of inhibitory receptors (ie, CTLA-4 and PD-1)46 and down-regulation of activating ones (eg, TCR/CD3, CD8, NKG2D).47-49 Our functional data, indeed, demonstrate that CD8+ T cells expressing low levels of NKG2D are not responsive to its costimulation, either in terms of proliferation or of cytokine production and cytotoxic activity.
NKG2DL diversity may provide multiple modes of immunoregulation in vivo: different signaling, different expression patterns, and membrane association (GPI-link vs membrane-anchor, which may underlie different modes of shedding), great polymorphism that may permit donor-to-donor variation in the capacity of NKG2DLs to be shed and thereby to act distally as immunosuppressant. This, together with the individual variation in T-cell responses, and with the fact that NKG2D-mediated functions on effector CD8+ T cells could result from a critical balance between its cell-surface expression levels and the cytokine milieu present during the primary antigenic stimulation of naive CD8+ T cells, may explain why the costimulatory capacity of NKG2D was not observed in all donors we tested (see also Ehrlich et al50 ).
The acute expression of NKG2DLs can be considered a key component of the regulation of an immune response and exerts a pressure for a sort of immune evasion, as shown in tumors where the release of soluble NKG2DLs in the sera of cancer patients causes a systemic down-modulation of the receptor and a decreased functional activity, on both NK and CD8+ T cells.19,20,31,33 However, the observation of a decreased expression of NKG2D in mice with inducible Rae-1 expression,51 on PBMCs from pregnant women,34 and on antigen-activated T cells (this study) strongly indicates that a new scenario is emerging where the immune system is sensitive to changes in NKG2DL expression not only on transformed or infected cells but also on T lymphocytes. Since sustained NKG2DL expression seems to promote chronic immune activation (as shown for the atypically high Rae-1 or MICA expression in normal tissues that has been linked to the immunopathology of some autoimmune diseases14,26-28 ), detuning effector responsiveness might help to avoid overstimulation and/or potential damage to normal cells. A future goal is the identification of the “tipping point” that defines the transition of NKG2D-mediated immunoactivation into immunosuppression. This may clarify whether the transition requires a particular amount and/or duration of NKG2DL expression, or a particular form of NKG2DL (eg, soluble vs cell bound).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Oreste Segatto and Rocco Fraioli for expertise and assistance with recombinant protein purification, and Dr Lewis Lanier for the kind gift of BAF cells transfected with ULBP3.
This work was supported by grants from the Italian Association for Cancer Research (AIRC, Milan, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy), the Italian Ministry of Public Health, and the Center of Excellence (BEMM, Rome, Italy).
Authorship
Contribution: C.C. and A.Z. designed research, performed experiments, and wrote the paper; M.A. performed experiments and contributed to paper writing; and A.S. designed research and contributed to paper writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cristina Cerboni, Department of Experimental Medicine, University of Rome “La Sapienza,” Policlinico “Umberto I,” Viale Regina Elena 324, 00161 Rome, Italy; e-mail: cristina.cerboni@uniroma1.it.
References
Author notes
*A.S. and A.Z. contributed equally to this work.









 , acidic elution; and □, control of the elution. The latter staining was performed by adding the anti-NKG2D or anti-CD94 mAb and, before adding the secondary GAM-PE mAb, cells were briefly washed with the acidic buffer. The lower staining demonstrates that the acidic treatment is able to break the antigen-Ab interaction. (B) Large amounts of intracellular NKG2D are found in NKG2Dlow cells. NKG2Dhigh and NKG2Dlow cells were harvested, fixed, and permeabilized to analyze the total amount of NKG2D (TOT = extracellular + intracellular) on CD3+CD8+ cells. Staining of extracellular (EC) NKG2D was performed as usual. In the right panel, results are presented as the mean plus or minus SD of the percentage of EC/TOT NKG2D obtained from 4 donors. (C) Clathrin-dependent endocytosis is involved in NKG2D down-modulation. Total or CD4-depleted PBMCs were stimulated with SEB for 4 days, sucrose (0.1 M) was added and left for an additional 18 hours. NKG2D and LFA-1 (used as control) expression was evaluated on CFSE+CD3+CD8+ cells. Treatment with sucrose did not reduce viability and CD3+CD8+ T-cell proliferation (data not shown). (D) NKG2D and DAP10 transcripts, quantitated by real-time PCR, in SEB-activated CD8+ T cells. Total or CD4-depleted PBMCs were stimulated with SEB and cells were harvested at different times as indicated. CD8+ T lymphocytes were further purified by immunomagnetic positive selection, total RNA was isolated, and real-time PCR was performed. Data were normalized by the amount of β-actin mRNA. Data are shown as the mean cycles of normalized NKG2D or DAP10 expression plus or minus SD (triplicates). All panels derive from 1 representative donor of 3 (D) or 4 (A-C) tested.
, acidic elution; and □, control of the elution. The latter staining was performed by adding the anti-NKG2D or anti-CD94 mAb and, before adding the secondary GAM-PE mAb, cells were briefly washed with the acidic buffer. The lower staining demonstrates that the acidic treatment is able to break the antigen-Ab interaction. (B) Large amounts of intracellular NKG2D are found in NKG2Dlow cells. NKG2Dhigh and NKG2Dlow cells were harvested, fixed, and permeabilized to analyze the total amount of NKG2D (TOT = extracellular + intracellular) on CD3+CD8+ cells. Staining of extracellular (EC) NKG2D was performed as usual. In the right panel, results are presented as the mean plus or minus SD of the percentage of EC/TOT NKG2D obtained from 4 donors. (C) Clathrin-dependent endocytosis is involved in NKG2D down-modulation. Total or CD4-depleted PBMCs were stimulated with SEB for 4 days, sucrose (0.1 M) was added and left for an additional 18 hours. NKG2D and LFA-1 (used as control) expression was evaluated on CFSE+CD3+CD8+ cells. Treatment with sucrose did not reduce viability and CD3+CD8+ T-cell proliferation (data not shown). (D) NKG2D and DAP10 transcripts, quantitated by real-time PCR, in SEB-activated CD8+ T cells. Total or CD4-depleted PBMCs were stimulated with SEB and cells were harvested at different times as indicated. CD8+ T lymphocytes were further purified by immunomagnetic positive selection, total RNA was isolated, and real-time PCR was performed. Data were normalized by the amount of β-actin mRNA. Data are shown as the mean cycles of normalized NKG2D or DAP10 expression plus or minus SD (triplicates). All panels derive from 1 representative donor of 3 (D) or 4 (A-C) tested.