Abstract
Mutations in the MYH9 gene encoding nonmuscle myosin IIA lead to macrothrombocytopenia as observed in MYH9-related disorders. We used mice with megakaryocyte-restricted MYH9 inactivation to explore the role of myosin in thrombopoiesis. In situ, bone marrow MYH9Δ megakaryocytes were irregularly shaped, appearing leaky with poorly defined limits. The demarcation membranes were abnormally organized and poorly developed, pointing to an insufficient reservoir for the future formation of platelets. The cytoskeletal-rich peripheral zone was lacking due to the absence of the myosin filament network that normally surrounds the granular zone in wild-type cells. In vitro studies of cultured cells showed that MYH9Δ megakaryocytes were unable to form stress fibers upon adhesion to collagen, suggesting that the leaky shape results from defects in internal tension and anchorage to the extracellular environment. Surprisingly, the proportion of cells extending proplatelets was increased in MYH9Δ megakaryocytes and the proplatelet buds were larger. Overall, this study provides evidence for a role of myosin in different steps of megakaryocyte development through its participation in the maintenance of cell shape, formation and organization of the demarcation membranes and the peripheral zone, anchorage to the extracellular matrix, and proplatelet formation.
Introduction
Nonmuscle myosin IIA plays a fundamental role in basic cellular functions such as cell division, adhesion to extracellular matrices, and migration.1 The myosin IIA heavy chain is encoded by the MYH9 gene and is the only isoform of nonmuscle myosin II present in blood platelets, unlike most cells that also express myosin IIB.2 Mutations in the MYH9 gene lead to dominant hereditary hemorrhagic diseases known as MYH9-related disorders (MYH9-RD, previously classified as the May-Hegglin anomaly and the syndromes of Sebastian, Fechtner, and Epstein) characterized by congenital thrombocytopenia with large platelets (macrothrombocytopenia) and leukocyte inclusions, sometimes associated with loss of hearing, cataracts, or nephritis during childhood and/or adulthood.3,4 The origin of the thrombocytopenia is unknown but most probably results from defective thrombopoiesis since the platelet half-life appears to be either normal or only slightly decreased in these patients.5,6
Blood platelets are produced by megakaryocytes following differentiation and maturation of hematopoietic stem cells into giant polyploid cells.7,8 The ultrastructure of these mature megakaryocytes reveals a large and highly organized cytoplasm.9,10 The granular zone contains an extensive membrane network called the demarcation membrane system (DMS) that constitutes a membrane reservoir for the future platelets,11 whereas the actin-rich peripheral zone is devoid of DMS and organelles.9,10,12 It is now thought that mature megakaryocytes release platelets through extension of cytoplasmic projections, the so-called proplatelets, into the vessel lumen.13,14
The role of myosin IIA in platelet biogenesis is still poorly understood and only a few reports are available concerning the impact of myosin IIA mutations on the bone marrow megakaryocytes of MYH9-RD patients. These individuals have either a normal or an increased number of megakaryocytes6,15-17 and normal megakaryocyte DNA ploidy.16,18 Recently, 2 separate in vitro studies using megakaryocytes differentiated from mouse embryonic stem cells or human CD34+ cells described the involvement of the Rho-Rho kinase-MLC-myosin IIA pathway in proplatelet formation.19,20 Inhibition of this pathway at either level resulted in enhanced proplatelet formation, suggesting myosin to be a negative regulator of platelet production. This may seem somewhat paradoxical in view of the macrothrombocytopenia in MYH9-RD patients and provides no explanation as to how the increased proplatelet formation translates into thrombocytopenia.
To gain insight into the mechanisms controlling the involvement of myosin IIA in platelet formation, we used the previously described megakaryocyte-restricted myosin-deficient mice (MYH9Δ mice).21 These animals exhibit severe myosin IIA deficiency and important hemostatic defects due to absence of the platelet contractile shape change and outside-in signaling. As in the human patients, MYH9Δ mice are thrombocytopenic and have large platelets.21 These platelets appear heterogeneous with a mixed population of normal discoid and more ovoid cells, a large proportion of them containing large amounts of rough endoplasmic reticulum.21 A normal number of microtubule coils was observed in both discoid and ovoid platelets, contrary to what was previously reported for giant platelets from patients with May-Hegglin anomaly and Epstein syndrome where the number of coils was increased 10- to 20-fold.22 Strikingly, at the ultrastructural level, some of the MYH9Δ platelets appear crowded with organelles, whereas others appear devoid of organelles, strongly suggesting a role for myosin IIA in the granule distribution.21 In the present study, the megakaryocytes of MYH9Δ bone marrow were examined in situ and the role of myosin IIA in matrix-induced cytoskeletal reorganization and in proplatelet formation was investigated in vitro using bone marrow–derived cultured megakaryocytes.
Methods
Materials
Equine tendon collagen was from Nycomed (Munich, Germany). RNAse A, propidium iodide, BSA, and polyclonal anti–myosin IIA antibodies were from Sigma-Aldrich (Rueil-Malmaison, France). Anti–mouse β3 integrin (LucA5) was from Emfret (Wurzburg, Germany). Complete protease inhibitor cocktail, phalloidin-AF488, goat anti–rabbit AF488 or AF555, goat anti–rat AF555, IMDM medium, penicillin, streptomycin, and glutamine were from Invitrogen (Cergy-Pontoise, France). An FITC-labeled monoclonal anti-CD41 antibody was from Emfret and recombinant mTPO was from StemCell Technologies (Vancouver, BC).
Mice
The floxed MYH9 strain was crossed with PF4-Cre mice23 to obtain mice with deletion of the MYH9 exon 1 in the megakaryocytic lineage, as described previously.21 Homozygous wild-type (WT) and MYH9Δ littermates used in this study were from a mixed C57BL/6-129sv genetic background and were maintained in the animal facilities of the Etablissement Français du Sang-Alsace. For the measurement of circulating TPO level, blood was drawn from the abdominal aorta and anticoagulated with EDTA (6 mM). Plasma was obtained after centrifugation of whole blood at 9100g and the TPO level was measured using the mouse TPO Quantikine Elisa kit (R&D Systems, Lille, France). All experiments with mice conformed to French legislation for animal experimentation and followed the recommendations of the Guide for Care and Use of Laboratory at the Institute of Laboratory Animal Resources.
In situ megakaryocytes
Bone marrow histology.
Bone marrow was harvested from the diaphyses by flushing the femurs with Tyrode buffer, an iso-osmotic phosphate buffer at pH 7.35 containing glucose (0.1%, wt/vol), human serum albumin (HSA; 0.35%, wt/vol), calcium (2 mM), and magnesium (1 mM). Samples were immediately fixed for 24 hours in 4% paraformaldehyde (PFA) and embedded in paraffin and 5 μm sections were stained with hematoxylin and eosin (H&E).
DNA distribution.
Bone marrow cell suspensions were labeled with a FITC-conjugated anti-CD41 antibody. The cells were fixed in 70% ice-cold ethanol and stained with propidium iodide solution (50 μg/mL) in the presence of RNAse A (100 μg/mL). The ploidy distribution in the CD41+ population was determined by 2-color flow cytometry (FACSCalibur; BD Biosciences, Lyon, France).
Megakaryocyte ultrastructure.
Bone marrow samples were fixed in 2.5% glutaraldehyde and embedded in epon. Transversal thin sections of the entire bone marrow were cut, stained with uranyl acetate and lead citrate, and examined under a CM120 transmission electron microscope (TEM; FEI, Eindhoven, The Netherlands).21
Megakaryocyte quantification.
Megakaryocytes were counted under the TEM on whole transversal sections and the number of cells was expressed as the density per unit area (defined as one square of the grid, ie, 16 000 μm2). Megakaryocytes at stages I, II, and III were identified according to Zucker-Franklin24 using distinct ultrastuctural characteristics. Stage I corresponded to a cell 10 to 50 μm in diameter with a large nucleus; stage II, to a cell 20 to 80 μm in diameter containing platelet-specific granules; and stage III, to mature megakaryocytes having a well-developed DMS with clearly defined platelet territories and a peripheral zone. The DMS was quantified in the region of interest (ROI of 42 μm2) by measuring the perimeter of the electron lucent zones, where the perimeter corresponds to the demarcation membranes. The perimeter was determined in pixels using Metamorph software (Version 5; Universal Imaging, Downingtown, PA). A total of 23 megakaryocytes from 3 different mice were analyzed for both the WT and the MYH9Δ strain.
Myosin IIA immunolabeling of bone marrow megakaryocytes
Confocal imaging.
Bone marrow samples were fixed in 4% PFA, cryopreserved by sucrose immersion, and frozen. Cryosections (8 μm) were cut with a cryocut (Leica CM 3050 S; Leica Microsystems, Paris, France) and incubated with a polyclonal anti–myosin IIA antibody (10 μg/mL) followed by a secondary AF488-conjugated goat anti–rabbit antibody. In some cases, double labeling was performed with anti-β3 integrin antibody followed by secondary AF555-conjugated goat anti–rat antibody. The slides were mounted with Mowiol (Merck, Darmstadt, Germany) and examined under a confocal microscope (TCS SP5, objective 63×/1.4 oil; Leica Microsystems).
TEM.
Bone marrow cells were fixed in 2% PFA and embedded in Lowicryl (EMS, Hatfield, United Kingdom).25 Sections were first incubated with antimyosin or irrelevant antibodies (10 μg/mL) followed by 10 nm gold-conjugated protein A and then stained with 1.8% uranyl acetate/0.2% methylcellulose. In some experiments, to visualize intracellular cytoskeletal filaments, bone marrow cells were permeabilized with 0.02% saponin for 5 minutes, fixed in 2% PFA, and labeled in suspension.26 The cells were incubated overnight with antimyosin or irrelevant antibodies (10 μg/mL) followed by 10 nm gold-conjugated protein A and the samples were embedded in epon.
In vitro megakaryocytes
Culture.
Mouse bone marrow was flushed from femurs and tibias with Tyrode buffer. The cells were then dissociated and cultured in IMDM medium supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 50 ng/mL TPO. After 3-day culture, mature megakaryocytes were recovered by passing the suspension through a discontinuous density BSA gradient.
Western blotting.
Megakaryocytes were suspended in lysis buffer (1% NP40, 50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA) in the presence of complete protease inhibitor cocktail. Proteins were separated on a 4% to 15% polyacrylamide gel and blotted onto a PVDF membrane and visualized with anti–myosin IIA (1 μg/mL) and anti-actin (1 μg/mL) antibodies. Quantification was performed using the ImageQuant TL software v2003.03 (Amersham Biosciences, Freiburg, Germany). The amount of myosin was normalized against total actin.
Megakaryocyte adhesion.
Megakaryocytes recovered by density gradient sedimentation were seeded onto collagen (100 μg/mL)–coated coverslips, allowed to adhere for 3 hours at 37°C, and fixed in 2% PFA for 15 minutes. The cells were incubated with blocking buffer (0.2% BSA, 0.02% saponin in PBS) followed by anti–myosin IIA (5 μg/mL) for 1 hour in the same buffer and then a secondary antibody (anti–rabbit AF555 1:400). Stress fibers were labeled by incubating the cells with phalloidin-AF488 (1/40) concomitantly with the primary antibody.
Proplatelet formation.
Day-3 megakaryocytes recovered by density gradient filtration were cultured for 1 additional day in the same medium. The percentage of megakaryocytes extending proplatelets was determined by differential interference contrast (DIC) microscopy using a DMIL inverted microscope (objective 40×/0.55; Leica Microsystems), about 300 cells being analyzed for each culture. The size of the proplatelet buds was measured with Metamorph software. Several photographs of the same cell were taken on different focal planes so as to permit the analysis of 1 to 4 isolated buds per megakaryocyte. A total of 58 and 65 megakaryocytes were examined for WT and MYH9Δ mice, respectively, from 3 independent cultures. To observe megakaryocytes forming proplatelets by scanning electron microscopy, the cells were recovered after fixation in 2% PFA and cytospun onto poly-l-lysine–coated slides.21
Results
The thrombocytopenia of MYH9Δ mice does not result from a defect in megakaryocyte number or ploidy
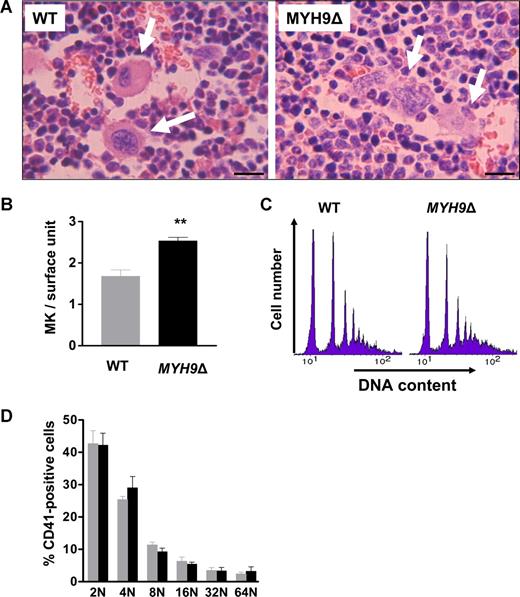
As previously described,21 MYH9Δ mice exhibit a 70% decrease in the circulating platelet count with a normal platelet half-life. To determine which steps in the platelet production process are affected by MYH9 inactivation, we first examined bone marrow by conventional histology. Whereas WT megakaryocytes appeared as giant round cells (Figure 1A left), MYH9Δ megakaryocytes seemed to be diffuse and it was difficult to precisely delimit their contours (Figure 1A right). To identify all megakaryocytes, the cells were further examined by immunofluorescence histology after labeling with antibodies against integrin β3 and VWF. In this case, the infiltrating morphology of MYH9Δ megakaryocytes was even more evident, compared with that of WT megakaryocytes, which appeared as well-delineated isolated cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As a result, MYH9Δ megakaryocytes were hard to count under the optical microscope and we counted them by TEM observation of bone marrow sections. The number of megakaryocytes from stages I to III was significantly increased (1.5-fold) in MYH9Δ compared with WT mice (Figure 1B). This increase is probably due to reactive thrombocytopoiesis. Indeed, the level of circulating TPO is increased in MYH9Δ mouse plasma (mean ± sem, 209.9 ± 10.7 and 301.8 ± 33.6 pg/mL for WT and MYH9Δ plasma, respectively; n = 9; P = .019 with unpaired Student t test).
Megakaryocyte numbers are increased in MYH9Δ bone marrow. (A) Sections of WT and MYH9Δ bone marrow samples were stained with hematoxylin-eosin. Arrows indicate megakaryocytes; bars: 30 μm. (B) In situ quantification of megakaryocytes in the bone marrow by TEM. Values are the mean plus or minus sem for 3 mice, with 3 transversal sections examined per mouse. **P < .01 using the Student unpaired t test. (C) Ploidy distribution of CD41+ bone marrow cells in MYH9Δ and WT mice. The histograms show the distribution of DNA labeling and are representative of 4 mice. (D) Proportion of megakaryocytes in each ploidy class. Results are the mean plus or minus sem for 4 mice.
Megakaryocyte numbers are increased in MYH9Δ bone marrow. (A) Sections of WT and MYH9Δ bone marrow samples were stained with hematoxylin-eosin. Arrows indicate megakaryocytes; bars: 30 μm. (B) In situ quantification of megakaryocytes in the bone marrow by TEM. Values are the mean plus or minus sem for 3 mice, with 3 transversal sections examined per mouse. **P < .01 using the Student unpaired t test. (C) Ploidy distribution of CD41+ bone marrow cells in MYH9Δ and WT mice. The histograms show the distribution of DNA labeling and are representative of 4 mice. (D) Proportion of megakaryocytes in each ploidy class. Results are the mean plus or minus sem for 4 mice.
DNA ploidy was determined as a marker of megakaryocyte differentiation. In the CD41+ population, a ploidy of up to 64N was observed in both WT and MYH9Δ cells (Figure 1C), without any difference in ploidy distribution (Figure 1D). Overall, these data indicate that the thrombocytopenia of MYH9Δ mice is unlikely to result from a reduced number of megakaryocytes or defects in endoreplication.
MYH9Δ mice display an abnormal megakaryocyte morphology with a decreased DMS
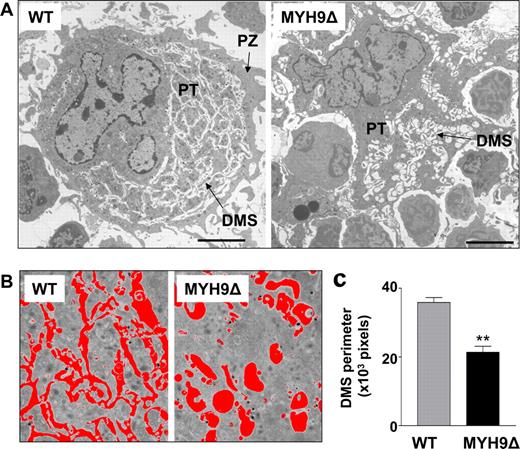
At the ultrastructural level, the abnormal shape of mature MYH9Δ megakaryocytes was even more striking (Figure 2A right). These cells appear to infiltrate without restraint between the other bone marrow cells. In addition, the cytoplasm of MYH9Δ megakaryocytes is totally disorganized compared with that of the WT. Whereas mature WT megakaryocytes display a distinct granular zone containing a well-developed DMS and a peripheral zone (PZ) devoid of DMS and organelles (Figure 2A left), no peripheral zone can be distinguished in MYH9Δ megakaryocytes (Figure 2A right). Moreover, the DMS of the latter is highly disorganized being dilated with a vacuole-like appearance and containing very few well-delimited platelet territories (PTs) (Figure 2A right). Image analysis was used to measure the perimeter of the DMS area and determine the amount of internal membrane (“Methods, In situ megakaryocytes, Megakaryocyte quantification”) (Figure 2B). The internal membranes were found to be 1.7 times less developed in MYH9Δ megakaryocytes than in WT cells (Figure 2C). These in situ data indicate that myosin is involved in maintenance of the cell shape and the intracytoplasmic organization of the DMS and peripheral zone in megakaryocytes.
Abnormal morphology of mature MYH9Δ megakaryocytes. (A) TEM images illustrating a typical mature WT megakaryocyte (left) and an irregularly shaped MYH9Δ megakaryocyte (right) containing a dilated DMS that results in rare and enlarged platelet territories (PTs). Bars represent 5 μm. (B) Representative fields and threshold areas (red) of the DMS of WT and MYH9Δ megakaryocytes. (C) Quantification of the DMS by measurement of the perimeter per field of the threshold area. Data are expressed in pixels and are the mean plus or minus sem for 3 mice, with a total of 23 megakaryocytes analyzed. **P < .01 using Student unpaired t test.
Abnormal morphology of mature MYH9Δ megakaryocytes. (A) TEM images illustrating a typical mature WT megakaryocyte (left) and an irregularly shaped MYH9Δ megakaryocyte (right) containing a dilated DMS that results in rare and enlarged platelet territories (PTs). Bars represent 5 μm. (B) Representative fields and threshold areas (red) of the DMS of WT and MYH9Δ megakaryocytes. (C) Quantification of the DMS by measurement of the perimeter per field of the threshold area. Data are expressed in pixels and are the mean plus or minus sem for 3 mice, with a total of 23 megakaryocytes analyzed. **P < .01 using Student unpaired t test.
Myosin IIA is concentrated at the periphery of WT megakaryocytes and is in contact with the DMS
To identify the site where myosin could act, we next examined the distribution of myosin in WT megakaryocytes using immunolabeling and confocal and transmission electron microscopy. As shown in Figure 3A, myosin is distributed throughout the cytoplasm with a particularly high concentration at the periphery of the cell. This corresponds to the presence of thick myosin filaments as revealed by TEM using gold-labeled antibodies (Figure 3B arrowheads). These filaments form a continuous network that entirely surrounds the granular zone and borders the peripheral zone (Figure 3B). Myosin is also detected within the peripheral zone (PZ) and appears to be in contact with actin filaments (Figure 3B arrows). The peripheral zone is absent from MYH9Δ megakaryocytes, strongly suggesting that the network of myosin is necessary to separate the cytoplasm into a peripheral and a granular zone. In addition, myosin is present near and in contact with the DMS (Figure 3C arrows), suggesting it is directly involved in the development and organization of these membranes.
Concentration of myosin IIA at the cell periphery and in contact with the DMS in WT megakaryocytes. (A) Sections of WT mouse femora stained with anti–myosin IIA followed by goat anti–rabbit AF488 antibodies were examined by confocal microscopy. Myosin is present throughout the cytoplasm with a high concentration at the cell periphery. Bar represents 5 μm. (B) Saponin-permeabilized megakaryocytes were incubated with an anti–myosin IIA antibody followed by 10 nm gold-conjugated protein A. After epon embedding, sections were cut and examined by TEM. Myosin IIA is organized in thick filaments at the base of the peripheral zone (PZ) (▲). m indicates mitochondria; α, alpha granule. Bar represents 200 nm. (C) Immunogold labeling of sections of Lowicryl-embedded megakaryocytes. Myosin IIA is in contact with the DMS ( ). Bar represents 200 nm.
). Bar represents 200 nm.
Concentration of myosin IIA at the cell periphery and in contact with the DMS in WT megakaryocytes. (A) Sections of WT mouse femora stained with anti–myosin IIA followed by goat anti–rabbit AF488 antibodies were examined by confocal microscopy. Myosin is present throughout the cytoplasm with a high concentration at the cell periphery. Bar represents 5 μm. (B) Saponin-permeabilized megakaryocytes were incubated with an anti–myosin IIA antibody followed by 10 nm gold-conjugated protein A. After epon embedding, sections were cut and examined by TEM. Myosin IIA is organized in thick filaments at the base of the peripheral zone (PZ) (▲). m indicates mitochondria; α, alpha granule. Bar represents 200 nm. (C) Immunogold labeling of sections of Lowicryl-embedded megakaryocytes. Myosin IIA is in contact with the DMS ( ). Bar represents 200 nm.
). Bar represents 200 nm.
Careful examination of MYH9Δ megakaryocytes revealed the presence of residual myosin forming clusters in the cytoplasm (Figure S2A). Western blot experiments performed on cultured cells showed that MYH9Δ megakaryocytes express less than 1% of residual normal-sized myosin (Figure S2B arrowhead) together with a lower molecular weight myosin, probably the truncated protein lacking exon 1, which amounted to 17% of control platelets (Figure S2B arrow). These results confirm previous observations in circulating platelets,21 and, as in platelets, the residual amounts of myosin are not able to support a contractile function as in the next paragraph.
MYH9Δ megakaryocytes are unable to form stress fibers upon adhesion to fibrillar collagen
If the peripheral zone is involved in maintaining a round shape, its absence in MYH9Δ megakaryocytes could contribute to their tendency to leak between other cells. Another nonexclusive explanation for the MYH9Δ morphology could be a defect in the interaction of these cells with the stroma. To investigate this point, we performed in vitro adhesion assays on collagen, one of the principal matrix proteins found in bone marrow. Bone marrow–derived megakaryocytes cultured for 3 days were allowed to adhere to collagen-coated coverslips for 3 hours. Adhesion led to cell spreading and reorganization of the actin cytoskeleton. In WT cells, the actin filaments were organized in bundles anchored at the cell periphery and were present throughout the cell body, forming stress fibers interacting with myosin that was found along the actin fibers (Figure 4A-C). In contrast, stress fibers were absent from MYH9Δ megakaryocytes (Figure 4D-F) and although actin filaments were present, they were randomly distributed throughout the whole cell. Hence the loose, poorly delimited aspect of MYH9Δ megakaryocytes could result at least in part from defective signaling between the cells and their extracellular environment.
Defective actin reorganization in MYH9Δ megakaryocytes following adhesion to collagen. WT (A-C) and MYH9Δ (D-F) megakaryocytes were allowed to adhere to collagen for 3 hours. (A,D) Actin filaments were visualized by phalloidin-AF488 labeling (green). (B,E) Myosin was stained with an anti–myosin IIA antibody followed by goat anti–rabbit AF555 antibodies (red). (C,F) Merged images. No stress fibers are seen in MYH9Δ cells despite the presence of actin filaments. Images are representative of 3 experiments. Bars equal 25 μm.
Defective actin reorganization in MYH9Δ megakaryocytes following adhesion to collagen. WT (A-C) and MYH9Δ (D-F) megakaryocytes were allowed to adhere to collagen for 3 hours. (A,D) Actin filaments were visualized by phalloidin-AF488 labeling (green). (B,E) Myosin was stained with an anti–myosin IIA antibody followed by goat anti–rabbit AF555 antibodies (red). (C,F) Merged images. No stress fibers are seen in MYH9Δ cells despite the presence of actin filaments. Images are representative of 3 experiments. Bars equal 25 μm.
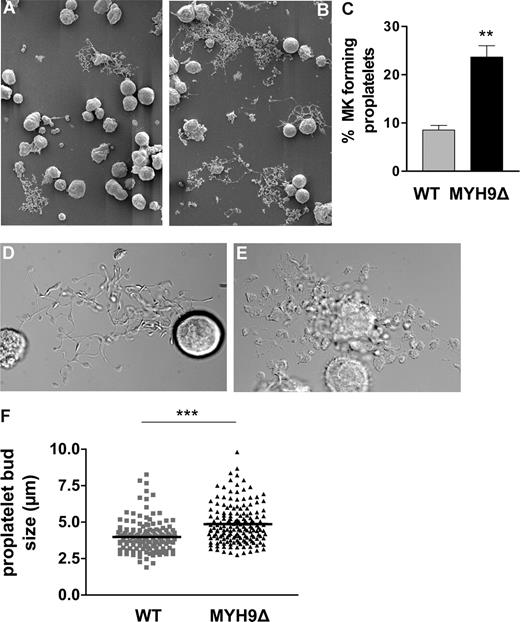
Proplatelet formation is abnormal and enhanced in MYH9Δ megakaryocytes
The efficacy of the intrinsic thrombopoiesis of megakaryocytes was further examined in vitro using bone marrow–derived cells cultured in a liquid medium for 4 days in the presence of thrombopoietin. Under these conditions, 9% of WT megakaryocytes formed proplatelets as counted on DIC microscopy images of live cells (Figure 5A,D). In MYH9Δ megakaryocyte cultures, the proportion of cells extending proplatelets was considerably higher, with levels of up to 25% (Figure 5B,C), suggesting that under normal conditions in WT cells, myosin may somehow control the extension of proplatelets. The morphology of WT proplatelets displayed the typical features usually observed in culture, with long proplatelet shafts, intermediate swellings, and platelet buds (Figure 5D).13 Conversely, the proplatelets formed by MYH9Δ megakaryocytes had a heterogeneous morphology, sometimes resembling a bunch of grapes (Figure 5E). Another feature of these proplatelets was the increased size of the proplatelet buds and intermediate swellings, which is reminiscent of the greater size of the circulating platelets (Figure 5F).
Abnormal proplatelet formation in cultured bone marrow–derived MYH9Δ megakaryocytes. WT (A,D) and MYH9Δ (B,E) megakaryocytes after 4 days of culture as observed by scanning electron microscopy (SEM) (A,B) and DIC microscopy (D,E). (C) Quantification by DIC microscopy of the number of megakaryocytes (MKs) extending proplatelets after 4 days of culture. The proportion of megakaryocytes forming proplatelets is increased in MYH9Δ cells. Data are expressed as the percentage of cells extending proplatelets and are the mean of 3 independent experiments. **P < .01 using the Student t test. (F) Measurement of the proplatelet buds in WT and MYH9Δ megakaryocytes forming proplatelets from 3 independent cultures. The size of the proplatelet buds is significantly increased in MYH9Δ cells. ***P < .001, unpaired Student t test.
Abnormal proplatelet formation in cultured bone marrow–derived MYH9Δ megakaryocytes. WT (A,D) and MYH9Δ (B,E) megakaryocytes after 4 days of culture as observed by scanning electron microscopy (SEM) (A,B) and DIC microscopy (D,E). (C) Quantification by DIC microscopy of the number of megakaryocytes (MKs) extending proplatelets after 4 days of culture. The proportion of megakaryocytes forming proplatelets is increased in MYH9Δ cells. Data are expressed as the percentage of cells extending proplatelets and are the mean of 3 independent experiments. **P < .01 using the Student t test. (F) Measurement of the proplatelet buds in WT and MYH9Δ megakaryocytes forming proplatelets from 3 independent cultures. The size of the proplatelet buds is significantly increased in MYH9Δ cells. ***P < .001, unpaired Student t test.
Discussion
In the present study, we explored the role of myosin in thrombocytopoiesis using megakaryocyte-restricted myosin IIA–deficient mice. We provide evidence for a role of myosin IIA in megakaryocyte development through its participation in the maintenance of cell shape, formation and organization of the DMS and the peripheral zone, anchorage to the extracellular matrix, and proplatelet formation.
The most striking defects observed in the bone marrow are the abnormal shape and irregular aspect of mature MYH9Δ megakaryocytes. In situ, cell morphology is controlled by intracellular cytoskeletal tensions and adhesive interactions with the extracellular environment. Stress fibers are absent from adherent MYH9Δ megakaryocytes, suggesting that both internal tensions and focal adhesions are strongly perturbed. Previous in vitro studies in nonmegakaryocytic cell types have demonstrated the importance of myosin IIA for normal cell shape through its involvement in the formation of stress fibers and focal adhesions, with ablation of myosin IIA leading to unusually large membrane protrusions.27-29 Thus, a lack of internal tension and proper interaction with the extracellular matrix is probably responsible for the leaky shape of MYH9Δ megakaryocytes in the bone marrow.
In addition to their irregular appearance, the overall cytoplasmic organization is perturbed in MYH9Δ megakaryocytes. First, the DMS is less abundant and abnormally dilated. This clearly indicates that myosin itself is directly required to achieve normal DMS development and organization, a conclusion that is further supported by our observation that myosin is associated with the DMS in WT megakaryocytes. During elaboration of the DMS, phosphatidylinositol 4,5-bisphosphate accumulates at the cytoplasmic face of these membranes and promotes the local assembly of actin filaments.11 Hence actomyosin could be necessary to apply a contractile force to the membranes and thereby organize and stabilize the DMS in maturing megakaryocytes.
A second observation is the absence of a peripheral zone in MYH9Δ megakaryocytes due to the absence of the myosin filament network surrounding the granular zone. The role of the peripheral zone is still unknown. It has been suggested that a wide peripheral zone is characteristic of more mature megakaryocytes30 and could play a role in the association of the cells with the sinusoid subendothelium.31 Tablin et al reported that upon adhesion to matrigel, cultured megakaryocytes underwent cytoplasmic reorganization with breakdown of the actin-rich peripheral zone before extension of pseudopodia.32 One may speculate that the peripheral zone, or at least its myosin network component, forms a barrier that prevents the premature extension of proplatelets until the megakaryocyte is fully mature and located at the right place to release platelets. In favor of this hypothesis is the present observation that MYH9Δ megakaryocytes, which lack a peripheral zone, have an increased capacity to extend proplatelets in vitro. A potential role of myosin IIA in preventing proplatelet formation has been suggested from studies of megakaryocytes derived from human CD34+ cells20 and mouse embryonic stem cells.19 Our results support and extend this proposal, showing that the size of MYH9Δ proplatelet buds is greater than in the WT, which could explain the increased size of circulating MYH9Δ platelets.21
How then does a lack of myosin, which increases the amount of megakaryocytes extending proplatelets in vitro, result in thrombocytopenia in vivo? One hypothesis could be that a premature release of platelets before the megakaryocyte has reached full maturity would lead to a decrease in circulating platelets. Such a premature platelet release has indeed been observed in WASP-deficient mice where platelets were frequently found in the marrow interstitium.33 However, electron microscopy never revealed released platelets outside the vasculature in MYH9Δ mice, suggesting that the mechanism of thrombocytopenia may be different in this case. Another hypothesis is based on the observation that, in situ, the DMS is reduced in MYH9Δ megakaryocytes. Whether this lesser development of the DMS reflects an immature state of the MYH9Δ cells or a decreased capacity to generate these membranes is still unclear. Since the DMS is known to be the only source of proplatelet membranes,11 our observations could at least partly explain the thrombocytopenia found in MYH9Δ mice as being due to the lack of a sufficient membrane reservoir for platelet formation. The fact that proplatelet extensions are possible in MYH9Δ megakaryocytes does not preclude the possibility that the number of platelets produced per megakaryocyte is reduced due to the smaller amount of DMS. In this regard, a link between underdeveloped/disorganized DMS and thrombocytopenia has been reported in various genetically engineered mouse strains such as GPIbα-, GATA1-, and NF-E2–deficient mice34-36 and strains overexpressing transcription factor E2F-1.37
Overall, this study provides further evidence that myosin is involved in different steps of thrombocytopoiesis. Whether all or only some of these steps are affected in MYH9-RD patients remains to be determined. Although few data are available concerning the ultrastructure of MYH9-RD megakaryocytes in situ, some ultrastructural abnormalities have been reported in megakaryocytes from patients with Fechtner, Sebastian, and May-Hegglin syndromes.15,18,38 In all cases, the DMS was arranged in a disorderly fashion with poorly demarcated platelet fields, either being concentrated in areas devoid of organelles or radiating from the core of the cell to its periphery.15,18,38 The peripheral zone was also affected and in some cells reduced to a narrow rim with frequent openings.18 These findings suggest that, although myosin IIA–deficient mice are not the exact model of MYH9-RD, the defects in cytoplasmic organization seen in MYH9-RD and MYH9Δ megakaryocytes are a common consequence of the impairment of myosin activity.
In summary, we show that myosin is required to maintain megakaryocyte shape through internal tension and anchorage to the extracellular matrix. Our data also strongly suggest that myosin is directly involved in formation/stabilization of the DMS. Finally, myosin is seen to play a crucial role in the organization of the cytoplasm into granular and peripheral zones, and our results lead to the hypothesis that the presence of a peripheral zone in megakaryocytes could be a determining factor for the extension of proplatelets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jean-Yves Rinckel, Fabienne Proamer, Josiane Weber, and Amandine Moriot for expert technical assistance and J. N. Mulvihill for reviewing the English of the paper.
This study was supported by ARMESA (Association de Recherche et Développement en Médecine et Santé Publique, Paris, France) and ANR (Agence National pour la Recherche, Grant no. ANR-07-MRAR-016-01). C.L. is the recipient of a “contrat d'interface” between EFS and Inserm.
Authorship
Contribution: A.E. performed and analyzed electron microscopy imaging and wrote the paper; C.S. performed megakaryocyte culture; M.F. was responsible for the production of knockout mice; J.-P.C. and F.L. discussed results; C.G. designed research, analyzed the data, and wrote the paper; and C.L. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. Gachet, Inserm U949, Etablissement Français du Sang-Alsace (EFS-Alsace), 10, rue Spielmann, B.P. N°36, 67065 Strasbourg Cedex, France; e-mail: christian.gachet@efs-alsace.fr.








 ). Bar represents 200 nm.
). Bar represents 200 nm.