In this issue of Blood, Cerchietti and colleagues describe the development of a highly active peptide inhibitor for the BCL6 oncoprotein that shows great promise for the clinical treatment of BCL6-dependent diffuse large cell lymphoma.
Diffuse large B-cell lymphoma (DLBCL) is a common and aggressive subtype of non-Hodgkin lymphoma that is frequently associated with deregulation of the BCL6 gene. Chromosomal translocations and mutations alter the BCL6 promoter without affecting the BCL6 coding region. Deregulated BCL6 transcription due to genetic alteration of the promoter appears to keep B cells in a rapidly proliferating germinal center–like state and promote the process of B-cell transformation. In mouse model systems, constitutive expression of BCL6 can immortalize primary B cells and promote the development of lymphomas, showing that BCL6 can act as an oncogene.
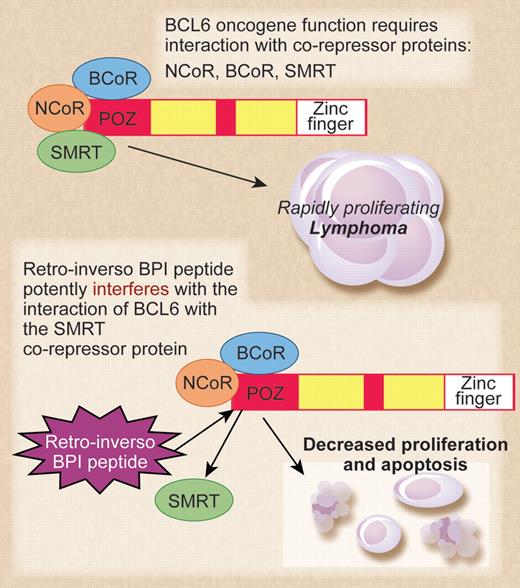
The top panel shows BCL6 and its corepressors acting as oncogenes in diffuse large B-cell lymphoma (DLBCL). The bottom panel shows mechanism of action of the retro-inverso BCL6 inhibitory peptide (BPI) in inhibiting lymphoma cell growth. Professional illustration by Debra T. Dartez.
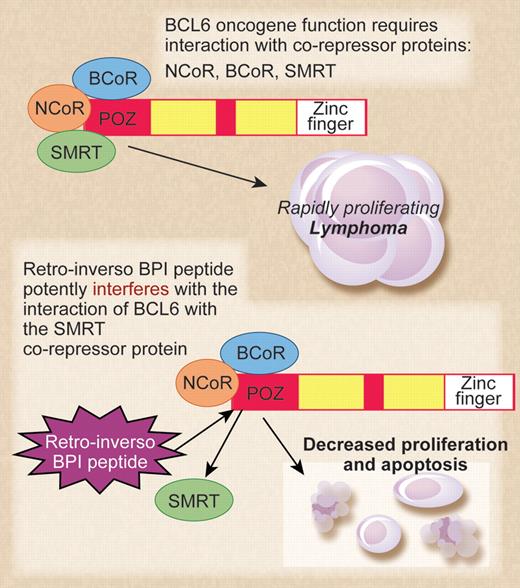
The top panel shows BCL6 and its corepressors acting as oncogenes in diffuse large B-cell lymphoma (DLBCL). The bottom panel shows mechanism of action of the retro-inverso BCL6 inhibitory peptide (BPI) in inhibiting lymphoma cell growth. Professional illustration by Debra T. Dartez.
The high levels of BCL6 expression in DLBCL coupled with the low expression of BCL6 in the vast majority of normal cells have made BCL6 an attractive candidate for anticancer drug development. However, BCL6 is a transcriptional repressor protein, and functional interference with BCL6 protein in the nucleus of tumor cells using pharmaceutical agents presents a challenge. In 2004, the Melnick group described the initial development of a peptide inhibitor for BCL6.1 This early version of BCL6 inhibitory peptide (BPI) was a relatively large 120 amino acid peptide that worked by interfering with the ability of BCL6 to bind its critical corepressor proteins, N-CoR and SMRT. BPI was able to enter cells via the addition of an HIV-TAT sequence to the peptide, facilitating the crossing of the cell membrane after macropinocytosis. While growth inhibition by BPI was highly specific and effective at low-peptide concentrations, the major drawback of BPI was its instability—cells required a fresh dose every few hours for a sustained inhibitory effect.
At this point, the Melnick group decided to retain the idea of a peptide inhibitor that interrupted the interaction of BCL6 with its corepressor proteins and focus their efforts on refining the inhibitory peptide to make it more stable. The result of that work is the current study from Cerchietti et al,2 which represents a technical tour-de-force in inhibitory peptide design and testing. The authors went through multiple iterations of peptide refinement by adding an additional fusogenic motif besides the TAT sequence, shortening the inhibitory sequence to 9 amino acids, modifying peptide configuration by mutating a proline residue, and lastly, making the peptide in retro-inverso (D isomer) configuration. The eventual result was the “S6.2 RI-BPI” peptide, which was highly stable in cells, required only 1 dose every 48 hours, and was also inhibitory at micromolar concentration. The S6.2 RI-BPI peptide worked to inhibit lymphoma growth in vivo, was remarkably specific for lymphoma subtypes dependent upon BCL6, and showed no overt signs of toxicity when injected into mice over a 1-year period. Normal germinal center B cells are dependent upon BCL6 function, and treatment of mice with the S6.2 RI-BPI peptide inhibited germinal center formation. This again showed that S6.2 RI-BPI peptide could inhibit BCL6 function in vivo. Importantly, the S6.2 RI-BPI peptide did not induce inflammatory disease in mice, which was a concern as Bcl6-deficient mice develop lethal inflammatory disease at a high frequency.3 Clearly more careful studies on toxicity of the S6.2 RI-BPI peptide in vivo are required, but these initial results are very encouraging.
Overall, this work by Cerchietti et al represents an excellent case study of rational drug design for cancer therapy. More importantly, this new study from the Melnick lab shows that therapeutic use of BCL6 peptide inhibitors to treat lymphoma should be a reality in the very near future.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■