Abstract
We describe a novel localization of C7 as a membrane-bound molecule on endothelial cells (ECs). Data obtained by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), Western blot analysis, Northern blot analysis, and mass spectrometry revealed that membrane-associated C7 (mC7) was indistinguishable from soluble C7 and was associated with vimentin on the cell surface. mC7 interacted with the other late complement components to form membrane-bound TCC (mTCC). Unlike the soluble SC5b-9, mTCC failed to stimulate ECs to express adhesion molecules, to secrete IL-8, and to induce albumin leakage through a monolayer of ECs, and more importantly protected ECs from the proinflammatory effect of SC5b-9. Our data disclose the possibility of a novel role of mC7 that acts as a trap for the late complement components to control excessive inflammation induced by SC5b-9.
Introduction
The endothelium is organized into a monolayer of cells that cover the luminal side of the blood vessels along the vascular tree forming a physical barrier between circulating blood and the surrounding tissue. An important role of endothelial cells (ECs) of microvessels is to control the passage of soluble molecules and to regulate the migration of blood leukocytes into the extravascular sites in response to a variety of perturbing agents. Activation of ECs sets in motion a multistep process associated with sequential expression of adhesion molecules leading the leukocytes to adhere to ECs, to spread out on their surface, and eventually to egress into the surrounding tissue crawling through the interendothelial junctions.1,2
A large number of molecules including bacterial products, such as lipopolysaccharides (LPS), cytokines, and chemokines, have been shown to stimulate ECs and to contribute to the development of the inflammatory process.3 Among the proinflammatory molecules, a special role is played by the biologically active products of the complement (C) system that are released systemically or locally as a result of C activation through the classical, the alternative, or the lectin pathway. The terminal C complex (TCC) is one of these products that has received special attention for its ability to interact with ECs and to cause vascular changes in pathologic conditions associated with ischemia-reperfusion and vasculitis.4 TCC is formed by the assembly of C5b and the remaining late components on the cell surface after C activation by cell-bound antibodies or other non-antibody C-activating substances.5 This complex may insert into the cell membrane as a cytolytic membrane attack complex (MAC), but more often binds to EC surface as a sublytic complex.
A large proportion of the terminal complex is formed in various pathologic conditions including bacterial sepsis, immune complex-mediated diseases, traumata, ischemia-reperfusion, and others that are associated with massive C activation. Assembly of this complex on ECs in a lytic form may cause extensive cell damage, while insertion of the same complex in a sublytic form induces persistent stimulation of the endothelium and marked inflammatory reaction. Despite the inability to cause cytotoxic cell damage, the sublytic complex is still able to stimulate ECs to express P-selectin,6 to release interleukin-1 (IL-1) and to enhance the stimulating effect of tumor necrosis factor-α (TNF-α) on the expression of adhesion molecules.7 This complex has also been shown to trigger the coagulation process by promoting the assembly of prothrombinase complex and the expression of tissue factor, and contributes to the changes in the vascular tone by inducing the release of the vasodilator prostacyclin PGI28 and the vasoconstrictor thromboxane A2.9
To neutralize the damaging effects of MAC, ECs express on their surface CD59, which inhibits the oligomerization of C9 thus preventing the assembly of MAC.10,11 It should be emphasized that CD59 does not provide full protection from an excessive amount of MAC, and, in addition, fails to control the stimulating activity of the cytolytically inactive TCC released in the circulation under conditions of unrestricted C activation. Assembly of this complex starts with the formation of C5b6 complex, which is then stabilized by the binding of C7. However, the trimolecular complex C5b-7 has only a transient ability to insert into the phospholipid bilayer of the cell membrane.12,13 Should this not occur within few milliseconds, C5b-7 still maintains the ability to bind the remaining terminal components and forms a nonlytic terminal complex C5b-9 containing the soluble C regulators S-protein and clusterin (SC5b-9).12,14 Despite the inability to cause cytolysis, SC5b-9 has been shown to bind to ECs and to induce expression of adhesion molecules, production of cytokines, and vascular leakage.15,16
We now report an additional protective mechanism of ECs from the proinflamatory effects of SC5b-9. The protection is provided by C7 expressed on the cell membrane as a functionally active molecule that reacts with activated C5b6 and the remaining late components to form membrane-bound TCC (mTCC). Evidence will also be presented suggesting that this complex, unlike the soluble SC5b-9, fails to stimulate ECs acting as a trap for the assembling TCC and down-regulates the activation of these cells induced by the soluble complex.
Methods
Reagents
Components C5 to C9, goat antisera to C3 and to the late C components from C6 to C9, monoclonal antibodies (mAbs) to human C3, C7, and clusterin were purchased from Quidel (San Diego, CA); mAb to human S-protein was obtained from Chemicon (Billerica, MA). The immunoglobulin G (IgG) was purified from the antisera by affinity chromatography on protein G-Sepharose column (GE Healthcare, Piscataway, NJ). The mAb aE11 recognizing a neoantigen expressed in poly(C9) was from Dako (Glostrup, Denmark) and mAb anti-C7 (WU 4-15) was previously reported.17 Anti-VCAM mAb P3C4 was from Chemicon, anti–E-selectin CBL180 from Cymbus Biotechnology (Southampton, United Kingdom), and anti–ICAM-1 6.5B5 from Dako, and all were used at a final concentration of 5 μg/mL. Anti–vimentin polyclonal and mAb V9 was purchased from Sigma-Aldrich (St Louis, MO).
The following secondary antibodies were used: fluorescein isothiocyanate (FITC)–conjugated F(ab′)2 fragment of goat anti–mouse Ig were from Dako; biotin-labeled rabbit anti–mouse IgG, alkaline-phosphatase (AP)–conjugated goat IgG directed to mouse IgG or rabbit anti–goat IgG, and AP-conjugated streptavidin were purchased from Sigma-Aldrich.
Heparinases I, II, and III were from Sigma-Aldrich. Human recombinant TNF-α, IL-1α, and interferon-γ (IFN-γ) were purchased from PeproTech (Rocky Hill, NJ) and were used at a concentration of 100 ng/mL, 10 U/mL, and 100 U/mL, respectively.
Assembly of soluble and membrane-bound TCC
SC5b-9 was prepared as previously described15 and used at a final concentration of 5 μg/mL.
The mTCC was assembled on human umbilical vein endothelial cells (HUVECs) grown to confluence in 96-well plates by incubating the cells with 2 μg C5b6 for 10 minutes at 37°C and then with 1 μg C8 and 2 μg C9 to a final volume of 100 μL for 2 hours at 37°C. The presence of mTCC was evaluated by enzyme-linked immunosorbent assay (ELISA) on whole cells using mAb aE11. A similar approach was followed to assemble SC5b-9 on affinity-purified mC7 (see below) using C5b-6 and C7-deficient serum as a source of C8, C9, S-protein, and clusterin.
Cells and cell line
HUVECs were isolated by collagenase treatment and cultured in medium 199 supplemented with 20% newborn calf serum (Invitrogen, Carlsbad, CA), 50 μg/mL endothelial growth supplement, 50 μg/mL heparin, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich) as previously published.18 HepG2 cells (hepatocellular carcinoma cell line) and human B-lymphocyte cell line Raji, obtained from the ATCC (Manassas, VA), were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 1% antibiotic-antimycotic solution (Invitrogen).
ELISA
The amount of C3 and C7 released in the supernatant of EC culture and on whole cells was measured by ELISA as previously reported.18-20 The level of IL-8 was measured by ELISA using a commercial kit (Biosource International, Camarillo, CA).
Flow cytometry
HUVECs (5 × 105) were incubated with primary antibodies for 1 hour at 37°C followed by FITC-conjugated goat F(ab′)2 anti–mouse Ig. After fixation with 1% paraformaldehyde (Sigma-Aldrich), the cells were analyzed on a FACSCalibur instrument (BD Biosciences, San Jose, CA) using CellQuest software.
Transmission electron microscopy
The localization of C7 and C3 on HUVECs grown to confluence in 8-chamber culture slides (BD Biosciences) was analyzed using a pre-embedding technique as previously described.21
Immunohistochemical staining
Sections from paraffin-embedded tissue samples obtained from the archives of the Department of Human Pathology (University of Palermo, Palermo, Italy), were analyzed as previously reported.20
Acid strip
The acid strip procedure was performed on HUVECs grown to confluence in 96-well tissue-culture plates (Costar; Corning, Lowell, MA) according to Haigler.22 In other experiments, the cells were incubated in phosphate-buffered saline (PBS) containing 1 or 3 M KCl for 6 minutes at 4°C. The residual cell-bound C7 was evaluated by ELISA on whole cells (see above).
Immunoaffinity chromatography
HUVECs grown to confluence in 75-cm2 flasks (Costar) were washed and lysed with 0.5 M octyl β-D-glucopyranoside (Sigma-Aldrich) in distilled water and protease inhibitor cocktail (Sigma-Aldrich). Cell supernatant or cell lysate was passed through an anti-C7 immunoaffinity column prepared as described by Langeggen et al,19 and bound C7 was eluted with 4 M guanidine-HCl (Sigma-Aldrich) in 0.1 M sodium phosphate, pH 7.4, containing 2 M NaCl.
SDS-PAGE and immunoblotting
Immunopurified products were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions as previously described.20
RNA isolation and Northern blot analysis
Cultured cells were harvested in Omnizol (Euroclone, Milano, Italy), and total RNA was extracted according to the supplier's instructions. The Northern blot analysis was carried out by the method of Maniatis et al23 The specific probe C7 (primer sequence: sense, 5′-ATGTCAGCGCTGGGAGAAACT-3′; antisense, 5′-CAAGGCCTTATGCTGGTGACA-3′24 was 32P-labeled by random priming using Ready-To-Go DNA labeling beads (GE Healthcare) according to the manufacturer's protocol.
Protein sequencing
Protein bands were excised from SDS-PAGE gels stained with Coomassie blue. The spots were in-gel–digested with trypsin,25 and the mixture of recovered tryptic peptides was subjected to nanoelectrospray tandem mass spectrometry (MS/MS) sequencing essentially as described.26 A similar approach was followed to analyze tryptic peptides obtained from affinity-purified C7. Analyses were performed on LTQ mass spectrometer (Thermo Electron; Thermo Fisher Scientific, Waltham, MA) equipped with a nanoelectrospray ion source and online coupled to the high-performance liquid chromatography (HPLC) system. The MS/MS data were searched against the human International Protein Index (IPI) nonredundant database (version 3.19) using SORCERER-SEQUEST version 3.0.3, which was run on the SageN Sorcerer (Thermo Electron). Search results were subjected to statistical filtering using Peptide Prophet and Protein Prophet (version 3.0).26,27 Peptides with a final P value of .9 or more, which in this case equals to a probability of approximately 98% of being correct, were used for all further analyses.
Antibody capture ELISA
ECs detached from culture plates with 5 mM ethylenediaminetetraacetic acid (EDTA) were incubated with goat IgG against human C7, C3, or C6 for 1 hour at 37°C and, after washing, were treated with lysis buffer (see above). The antibody capture ELISA (AC-ELISA) for C7-vimentin complex was performed coating microtiter plates (Corning) with 100 μL protein G (1 μg/mL in carbonate buffer, pH 9.5) overnight at 4°C. After washing and blocking with 2% skimmed milk, 100 μL cell lysate were incubated for 1 hour at 37°C, and the captured proteins were detected using goat IgG anti-vimentin or unrelated antibodies for 1 hour at 37°C and AP-conjugated anti–goat IgG for 45 minutes at 37°C. The reaction was visualized with p-nitrophenil phosphate.
EC leakage
The assay was performed following a procedure previously described.16
Statistical analysis
Results were reported as mean plus or minus standard deviation (SD). The Student t test was used to compare 2 groups of data. Statistical significance was defined as P value less than .05.
Results
ECs express C7 on their surface
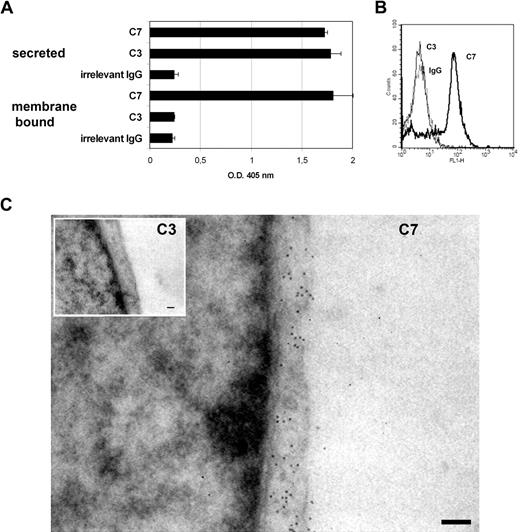
We have previously reported that ECs actively synthesize C7, and they represent a major site of synthesis of this C component.19 While searching for intracellular localization of C7 by immunofluorescence analysis of ECs, we noted that the cell surface stained for this C component. This observation prompted us to find more compelling evidence for the surface expression of C7 and its functional role. Our first approach was to analyze the supernatants of HUVECs grown to confluence in 96-well culture plates for the presence of C7 by ELISA using goat IgG anti-C7 and goat IgG anti-C3, as a control. The data presented in Figure 1A confirmed our previous finding that ECs secrete both C3 and C7, but in addition, they show that HUVECs express a high amount of C7 on their surface, whereas C3 is undetectable. Similar results were obtained using 2 different mAbs to C7 (data not shown) and fluorescence-activated cell sorting (FACS) analysis confirmed the surface expression of C7 on HUVECs (Figure 1B).
Presence of C7 on the endothelial cells surface. (A) The amount of C7 and C3 secreted by HUVECs grown to confluence was measured by ELISA in the culture supernatant. The presence of C7 and C3 on the cell membrane was evaluated by ELISA on whole cells using biotin-labeled goat antibodies to C7 or C3 (5 μg/mL), followed by AP-labeled streptavidin. (B) FACS analysis of nonpermeabilized HUVECs for surface expression of mC7 using mAbs anti-C7 (black line), anti-C3 (gray line; 10 μg/mL), and irrelevant IgG (dotted line) followed by FITC-labeled goat anti–mouse IgG. (C) TEM analysis of HUVECs incubated with mAb anti-C3 or anti-C7 (both used at 1:50) followed by biotinylated secondary antibodies and gold-labeled streptavidin. Magnification bar represents 200 nm. One of 3 experiments with similar results is shown.
Presence of C7 on the endothelial cells surface. (A) The amount of C7 and C3 secreted by HUVECs grown to confluence was measured by ELISA in the culture supernatant. The presence of C7 and C3 on the cell membrane was evaluated by ELISA on whole cells using biotin-labeled goat antibodies to C7 or C3 (5 μg/mL), followed by AP-labeled streptavidin. (B) FACS analysis of nonpermeabilized HUVECs for surface expression of mC7 using mAbs anti-C7 (black line), anti-C3 (gray line; 10 μg/mL), and irrelevant IgG (dotted line) followed by FITC-labeled goat anti–mouse IgG. (C) TEM analysis of HUVECs incubated with mAb anti-C3 or anti-C7 (both used at 1:50) followed by biotinylated secondary antibodies and gold-labeled streptavidin. Magnification bar represents 200 nm. One of 3 experiments with similar results is shown.
To further document the presence of C7 on the cell surface, HUVECs grown to confluence in 8-chamber culture slides were incubated with mAbs to human C3 or C7 followed by biotinylated secondary antibodies and gold-labeled streptavidin. The cells were then analyzed under a transmission electron microscope. Colloidal gold particles were clearly visible on EC membrane stained for C7, but not on cells analyzed for C3 (Figure 1C). To further extend these observations, we analyzed human biopsy specimens from normal skin, brain, kidney, endometrium, and decidua by immunoperoxidase and found that goat IgG anti-C7 stained ECs of the blood vessels in all tissues examined (Figure 2).
Expression of C7 on ECs of blood vessels in various tissues. Sections of paraffin-embedded human skin, kidney, brain, endometrium, and decidua were stained for C7 using goat IgG anti–human C7 or control IgG. Bound antibodies were revealed with the LSAB+ horseradish peroxidase (HRP) kit and 3-amino-9-ethylcarbazole (AEC) as chromogen. The sections were counterstained with hematoxylin. Magnification bar represents 30 μm.
Expression of C7 on ECs of blood vessels in various tissues. Sections of paraffin-embedded human skin, kidney, brain, endometrium, and decidua were stained for C7 using goat IgG anti–human C7 or control IgG. Bound antibodies were revealed with the LSAB+ horseradish peroxidase (HRP) kit and 3-amino-9-ethylcarbazole (AEC) as chromogen. The sections were counterstained with hematoxylin. Magnification bar represents 30 μm.
Characterization of membrane C7
The immunoenzymatic detection of a substantial amount of C7 on ECs led us to investigate whether cell-associated C7 may derive from soluble C7 captured by the cells after secretion. To address this issue, a confluent monolayer of ECs was incubated with affinity-purified membrane-bound C7 (mC7) or soluble C7 for 2 hours at 37°C, and bound C7 was revealed by ELISA. Under these experimental conditions, we failed to detect binding of C7 to ECs (data not shown). An alternative possibility was that mC7 was an integral membrane protein or was bound to ECs as a peripheral membrane protein. To this end, HUVECs grown to confluence were kept on ice in medium of progressively lower pH or high ionic strength buffers for the maximal time compatible with their survival (6 minutes), and the residual mC7 was measured by whole cell ELISA. Both treatments failed to remove C7 from the cell surface, suggesting that this C component was bound to the cell membrane via relatively strong interactions (data not shown). The possible binding of mC7 to the cell glycocalix was excluded, because we failed to see any reduction in mC7 expression after the treatment with a mixture of heparinases I, II, and III (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) that remove cell-surface glycosaminoglycans.
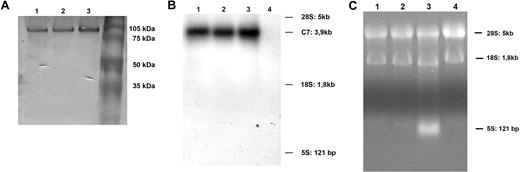
Analysis of cell-associated C7 extracted from HUVECs by SDS-PAGE under reducing conditions, and Western blot analysis, revealed a band of mC7 with an electrophoretic mobility similar to that of C7 secreted by ECs or purified from human serum (Figure 3A) suggesting that the 3 molecules have the same molecular weights. The structural similarity of soluble and mC7 was also supported by the finding of one single message for C7 on Northern blot analysis of mRNA extracted from HUVECs, that synthesize both soluble and cell-bound C7. This band had an electrophoretic mobility similar to the one obtained with HepG2 cells, that produce only the soluble form of C7, and was specific because it was not seen in the track containing mRNA from Raji cells that do not synthesize this C component (Figure 3B,C).
Characterization of mC7. (A) Western blot analysis of affinity-purified C7 from human normal serum (track 1), from cell supernatant (track 2), or from cell lysate (track 3). (B) Northern blot analysis of mRNA purified from 2 preparations of HUVECs (track 1 and 2), HepG2 (positive control; track 3), and Raji cells (negative control; track 4). (C) Ethidium bromide image of 2 preparations of HUVECs (track 1 and 2), HepG2 (track 3), and Raji cells (track 4). One of 3 experiments with similar results is shown.
Characterization of mC7. (A) Western blot analysis of affinity-purified C7 from human normal serum (track 1), from cell supernatant (track 2), or from cell lysate (track 3). (B) Northern blot analysis of mRNA purified from 2 preparations of HUVECs (track 1 and 2), HepG2 (positive control; track 3), and Raji cells (negative control; track 4). (C) Ethidium bromide image of 2 preparations of HUVECs (track 1 and 2), HepG2 (track 3), and Raji cells (track 4). One of 3 experiments with similar results is shown.
To obtain further information on the molecular structure of mC7, cell-bound C7 was purified by affinity chromatography and analyzed by a MS-based shotgun proteomics approach.27 Tryptic peptides spanning regions of the entire sequence of soluble C7 were identified (Table 1 and Table S1), with the exception of a minor number of fragments either too long or too short to be compatible with MS analysis. This result, together with the electrophoretic mobility data, shows that mC7 is structurally indistinguishable from soluble C7 and therefore excludes the presence of transmembrane protein domains in mC7. Interestingly, sequence analysis of the affinity-purified material from HUVECs with an antibody to C7 revealed vimentin as a major contaminant copurified with C7 (Table 1 and Table S1). The presence of vimentin on the surface of HUVECs was documented by FACS analysis on cells grown to confluence and detached with EDTA (Figure 4A). The formation of a complex between C7 and vimentin was confirmed by analysis of affinity purified mC7 by SDS-PAGE under reducing conditions, followed by Western blot analysis, using polyclonal antibodies to C7 and vimentin. As shown in Figure 4B, the antibodies revealed 2 distinct bands corresponding to vimentin (50 kDa) and C7 (105 kDa), respectively. Copurification of vimentin with C7 was also supported by the immunoenzymatic analysis of the complex extracted from HUVECs. To this purpose, cells grown to confluence were incubated with goat IgG anti-C7 and then lysed with n-octyl pyranoside. The cell extract containing IgG bound to surface-expressed C7 was added to wells coated with Protein G to allow binding of IgG, and the presence of vimentin in the complex was revealed by specific antibodies. The results presented in Figure 4C clearly show that vimentin was bound to mC7 forming a molecular complex. Similar experiments were performed incubating the cells with goat IgG to C3 or C6, and in both cases we failed to detect bound vimentin, suggesting that this molecule interacts selectively with C7 and does not bind to C3, C6, or IgG.
Membrane-bound C7 is anchored to vimentin on the cell surface. (A) FACS analysis of nonpermeabilized HUVECs for surface expression of vimentin using mAb anti-vimentin (black line) or unrelated IgG (gray line) (10 μg/ml) followed by RPE-labeled secondary antibody. (B) Western blot of C7 affinity-purified from cell lysate analyzed with polyclonal anti-C7 (1:1000) (track 1) or anti-vimentin (1:100) (track 2) followed by AP-conjugated anti–goat antibodies. (C) ECs were incubated with goat IgG to C7, or with goat IgG to C3 or C6, as controls, and then lysed. The expression of C7/vimentin complex in the cell lysate was documented by AC-ELISA using protein G–coated wells to trap the complex containing antibodies to the C components in the cell lysate and goat IgG anti-vimentin to reveal the presence of this protein in the complex. The results are expressed as mean (± SD) of triplicate determinations of 3 separate experiments. *P < .01 versus controls.
Membrane-bound C7 is anchored to vimentin on the cell surface. (A) FACS analysis of nonpermeabilized HUVECs for surface expression of vimentin using mAb anti-vimentin (black line) or unrelated IgG (gray line) (10 μg/ml) followed by RPE-labeled secondary antibody. (B) Western blot of C7 affinity-purified from cell lysate analyzed with polyclonal anti-C7 (1:1000) (track 1) or anti-vimentin (1:100) (track 2) followed by AP-conjugated anti–goat antibodies. (C) ECs were incubated with goat IgG to C7, or with goat IgG to C3 or C6, as controls, and then lysed. The expression of C7/vimentin complex in the cell lysate was documented by AC-ELISA using protein G–coated wells to trap the complex containing antibodies to the C components in the cell lysate and goat IgG anti-vimentin to reveal the presence of this protein in the complex. The results are expressed as mean (± SD) of triplicate determinations of 3 separate experiments. *P < .01 versus controls.
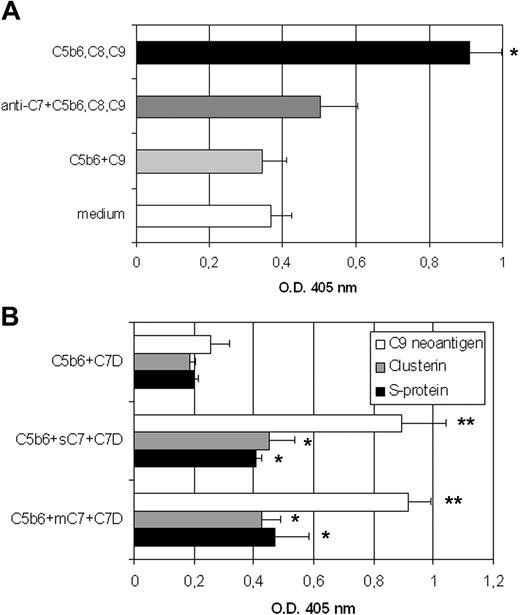
The molecular similarity of soluble and mC7 led us to investigate the ability of cell-associated C7 to contribute to the assembly of TCC. To address this issue, the cells were incubated with C5b6, C8, and C9, and the formation of TCC on the cell membrane (mTCC) was revealed using the mAb aE11 that selectively recognizes a neoepitope on polymerized C9. Under these conditions, TCC containing C9 dimers (Figure S2) was easily assembled on mC7, and its formation was prevented by incubating the cells with goat IgG anti-C7 before addition of C5b6 and the remaining late components C8 and C9 (Figure 5A). To assess the incorporation of S-protein and clusterin into mTCC, affinity purified mC7 or soluble C7 were incubated with C5b6 and C7-deficient serum (C7D), as a source of the remaining late C components, S-protein, and clusterin. The results shown in Figure 5B indicate that both C regulators were incorporated both into mTCC and soluble TCC as evaluated by ELISA using specific antibodies.
mC7 retains the ability to participate in the assembly of the TCC. (A) HUVECs grown to confluence in 96-well plates were incubated with the mixture of C5b6, C8, and C9 in the absence or in the presence of polyclonal anti-C7 antibodies (20 μg/mL), or C5b6 and C9 as a negative control. The assembly of mTCC was revealed by mAbs anti-C9 neoantigen. Values are mean (± SD) of triplicate determinations of 3 separate experiments (*P < .05 vs complete cell culture medium). (B) Analysis of SC5b-9 formed by incubating C5b6 with C7-deficient serum (C7D) in the presence of either mC7 or soluble C7. The mixture of C5b6 and C7-deficient serum in the absence of C7 served as control. The formation of the complex was documented by ELISA using goat IgG anti-C5 as a capture reagent and mAb aE11 to reveal exposure of C9 neoantigen. The presence of clusterin or S-protein into the complex was evaluated as above except that mAbs anti-clusterin or anti–S-protein were used as revealing reagents (**P < .01; *P < .05 vs control).
mC7 retains the ability to participate in the assembly of the TCC. (A) HUVECs grown to confluence in 96-well plates were incubated with the mixture of C5b6, C8, and C9 in the absence or in the presence of polyclonal anti-C7 antibodies (20 μg/mL), or C5b6 and C9 as a negative control. The assembly of mTCC was revealed by mAbs anti-C9 neoantigen. Values are mean (± SD) of triplicate determinations of 3 separate experiments (*P < .05 vs complete cell culture medium). (B) Analysis of SC5b-9 formed by incubating C5b6 with C7-deficient serum (C7D) in the presence of either mC7 or soluble C7. The mixture of C5b6 and C7-deficient serum in the absence of C7 served as control. The formation of the complex was documented by ELISA using goat IgG anti-C5 as a capture reagent and mAb aE11 to reveal exposure of C9 neoantigen. The presence of clusterin or S-protein into the complex was evaluated as above except that mAbs anti-clusterin or anti–S-protein were used as revealing reagents (**P < .01; *P < .05 vs control).
The complex assembled on membrane C7 down-regulates the proinflammatory effect of the soluble terminal complex
Previous data from our own group have shown that the cytolytically inactive complex is able to stimulate ECs inducing proinflammatory effects.15,16,18 This observation prompted us to ascertain whether the TCC assembled on mC7 (mTCC) might have similar stimulating activity on ECs. Titration curves were established to find the concentrations of C5b6, C8, and C9 required for the assembly of the maximal amount of mTCC on HUVECs as assessed by the binding of mAb aE11. The mTCC formed under these conditions was then evaluated for the ability to stimulate ECs as compared with the activity of SC5b-9. As expected, SC5b-9 at the concentration of 5 μg/mL was effective in inducing expression of adhesion molecules, release of IL-8, and bovine serum albumin (BSA) leakage on a monolayer of HUVECs, whereas mTCC failed to do so (Figure 6A-C). Next, we examined the possibility that assembly of mTCC on the cell surface before addition of SC5b-9 might control the stimulating effect induced by the soluble complex. To this end, mTCC was allowed to assemble on a confluent monolayer of ECs incubated with C5b6, C8, and C9 for 2 hours at 37°C, and the cells were then exposed to SC5b-9. Under these conditions, SC5b-9 was still able to bind to mTCC-bearing ECs, suggesting that mTCC does not prevent SC5b-9 binding (data not shown). However, the stimulating activity of SC5b-9 was totally inhibited and was not significantly different from that of mTCC or medium alone (Figure 6A-C). To investigate if mC7 expression is altered at inflammatory sites, HUVECs were stimulated with proinflammatory cytokines (IL-1α, TNF-α, and IFN-γ), and the presence of mC7 was evaluated by ELISA. The results (Figure S3) indicate that all these proinflammatory mediators increased the expression of mC7 on the cell membrane. The maximal inhibitory effect obtained with the full complex decreased to 40 and 15% with the assembly of mC5b-8 and mC5b-7, respectively, and was not seen when HUVECs were treated with goat IgG anti-C7 (data not shown). Moreover, a concentration of C5b6, C8, and C9 4-fold lower than that used for the assembly of the highest amount of complex (Figure 7A) was still effective in inhibiting the stimulatory activity of SC5b-9 suggesting that low amount of mTCC was sufficient to down-regulate the proinflammatory effect of SC5b-9 (Figure 7B).
TCC assembled on mC7 down-regulates the proinflammatory effects of SC5b-9. (A) Expression of adhesion molecules. HUVECs were stimulated with SC5b-9 either alone or in the presence of the mTCC. The expression of the adhesion molecules was evaluated by ELISA on whole cells using mAbs to vascular adhesion molecule-1 (VCAM-1; 12 hours after stimulation), intercellular adhesion molecule-1 (ICAM-1), or E-selectin (both 4 hours after stimulation). Values are mean (± SD) of triplicate determinations of 3 separate experiments (*P < .05 vs complete cell- culture medium). (B) Synthesis of IL-8. The amount of IL-8 in supernatant of HUVECs stimulated for 12 hours with SC5b-9, in the presence or in the absence of mTCC, or TNF-α, as a positive control, or complete cell-culture medium alone, was evaluated by ELISA. One of 3 experiments with similar results is shown. (C) Endothelial leakage assay. HUVECs grown to confluence on the insert of the Transwell were treated with SC5b-9 in the presence or in the absence of mTCC. The FITC-BSA amount collected in the lower chamber after 30 minutes was measured by the Infinte200 (Tecan, Männedorf, Switzerland). Values are mean (± SD) of triplicate determinations of 4 separate experiments (**P < .01 vs complete cell-culture medium).
TCC assembled on mC7 down-regulates the proinflammatory effects of SC5b-9. (A) Expression of adhesion molecules. HUVECs were stimulated with SC5b-9 either alone or in the presence of the mTCC. The expression of the adhesion molecules was evaluated by ELISA on whole cells using mAbs to vascular adhesion molecule-1 (VCAM-1; 12 hours after stimulation), intercellular adhesion molecule-1 (ICAM-1), or E-selectin (both 4 hours after stimulation). Values are mean (± SD) of triplicate determinations of 3 separate experiments (*P < .05 vs complete cell- culture medium). (B) Synthesis of IL-8. The amount of IL-8 in supernatant of HUVECs stimulated for 12 hours with SC5b-9, in the presence or in the absence of mTCC, or TNF-α, as a positive control, or complete cell-culture medium alone, was evaluated by ELISA. One of 3 experiments with similar results is shown. (C) Endothelial leakage assay. HUVECs grown to confluence on the insert of the Transwell were treated with SC5b-9 in the presence or in the absence of mTCC. The FITC-BSA amount collected in the lower chamber after 30 minutes was measured by the Infinte200 (Tecan, Männedorf, Switzerland). Values are mean (± SD) of triplicate determinations of 4 separate experiments (**P < .01 vs complete cell-culture medium).
Low amount of mTCC was sufficient to down-regulate the proinflammatory effect of SC5b-9. (A) mTCC was assembled on mC7 incubating HUVECs with increasing amount of C5b-6 (0.5 μg [a], 1 μg [b], 2 μg [c], and 3 μg [d] to a final volume of 100 μL). C8 and C9 were added to C5b6 at a molar ratio of 1:2:2, respectively. The assembly of mTCC was revealed by ELISA on whole cells using anti-C9 neoantigen. (B) HUVECs were stimulated with SC5b-9 (5 μg/mL) either alone or in the presence of mTCC assembled using the same quantity of C5b6, C8, and C9 as used above. The expression of VCAM-1 was evaluated by ELISA on whole cells as indicated in the legend to Figure 6A. Values are mean (± SD) of triplicate determinations of 3 separate experiment (*P < .05; **P < .01 vs complete cell-culture medium).
Low amount of mTCC was sufficient to down-regulate the proinflammatory effect of SC5b-9. (A) mTCC was assembled on mC7 incubating HUVECs with increasing amount of C5b-6 (0.5 μg [a], 1 μg [b], 2 μg [c], and 3 μg [d] to a final volume of 100 μL). C8 and C9 were added to C5b6 at a molar ratio of 1:2:2, respectively. The assembly of mTCC was revealed by ELISA on whole cells using anti-C9 neoantigen. (B) HUVECs were stimulated with SC5b-9 (5 μg/mL) either alone or in the presence of mTCC assembled using the same quantity of C5b6, C8, and C9 as used above. The expression of VCAM-1 was evaluated by ELISA on whole cells as indicated in the legend to Figure 6A. Values are mean (± SD) of triplicate determinations of 3 separate experiment (*P < .05; **P < .01 vs complete cell-culture medium).
Discussion
C7 is a critical component of the terminal pathway of C activation, and its main function is to contribute to the formation of MAC and the cytolytically inactive SC5b-9 involved in host defense against pathogens and also in the promotion of inflammation. We have previously reported that fluid-phase C7, the only late C component that is not synthesized in the liver,28 may have a regulatory function restricting the assembly of the terminal complex.29 The data presented in this paper suggest the possibility that C7 expressed on the cell membrane has an additional role acting as a trap for the late C components on ECs and as a regulator of the excessive proinflammatory stimulation induced by SC5b-9.
An unexpected finding was that C7 was highly expressed on the surface of ECs, while C3 was undetectable, although the 2 C components are secreted by these cells at comparable levels.19 This observation was surprising, because all C components including C7, with the exception of several C regulators and receptors, circulate in the blood and are present in the extravascular fluid as soluble molecules. The only other C component recently shown to be synthesized by the ECs of decidual vessels in human placenta and expressed on their surface is C1q, which acts as a bridge between endovascular trophoblast and decidual endothelial cells (DECs).20 However, the expression of C1q is restricted to DECs in pregnancy, whereas C7 is more widely distributed on ECs from various tissues, including skin, brain, kidney, endometrium, and decidua, suggesting a physiologic role for cell-bound C7 in vivo.
A possible explanation for the ability of ECs to secrete soluble C7 and to express this component on their surface is that the 2 molecules may be, at least in part, structurally different. However, in this case, one would expect 2 RNA messages in ECs, but the finding of one single message for C7 in the Northern blot analysis pattern of mRNA extracted from these cells ruled out this possibility, although we cannot exclude relatively small differences between transcript sizes. This conclusion is further supported by the observation that mC7 and soluble C7 examined by SDS-PAGE under reducing conditions had the same electrophoretic mobility suggesting that they also had similar molecular weights. Analysis of mC7 by MS-based shotgun proteomics provided more direct information on the molecular structure of this molecule suggesting high similarity between soluble and membrane-associated C7. These data indicate that mC7 is a peripheral membrane protein rather than a transmembrane molecule. Failure of cells to release mC7 after incubation in low pH or high ionic strength medium or after heparinase treatment is compatible with a relatively strong interaction of this C7 component with the cell surface. In this regard, mC7 differs from mC1q, because the latter is easily removed from the cell membrane under similar experimental conditions.20
Unexpectedly, sequence analysis of mC7 revealed the presence of vimentin that coprecipitated with this molecule during the purification procedure. Vimentin is a major structural component of intermediate filaments in many cell types and interacts with other cytoskeleton proteins to promote cell contraction, migration, stiffness, and proliferation.30 The formation of a vimentin-mC7 complex was confirmed by the detection of vimentin in C7 affinity-purified from the cell extract and analyzed by SDS-PAGE and by a sandwich assay using an antibody to C7 to trap mC7 and an antibody to vimentin to detect this protein in the complex. Binding to vimentin was selective for C7 because we failed to document complex formation of this intermediate filament molecule with C3, that is also produced by ECs, or to C6, used as a control. Although vimentin is regarded primarily as an intracellular protein, its presence on the cell surface has been documented on tumor cells,31 lymphocytes,32 and ECs33 and has been confirmed in the present study by FACS analysis of HUVECs. The finding that mC7 is not released from the cell surface by lowering the pH or by increasing the ionic strength of the buffer suggests that mC7 establishes a strong interaction with vimentin, although the 2 molecules can be dissociated from the complex under reducing conditions.
Since membrane-bound and soluble C7 share a similar molecular structure, the question arises whether the cell-associated molecule is captured from the medium after secretion. Failure of C7 added to the culture medium to bind to EC surface tends to exclude this possibility and rather supports the concept that this C component is placed on the cell surface during biosynthesis. Vimentin found to coimmunoprecipitate with mC7 extracted from EC membrane with antibody to C7 is likely to provide the binding site for this C component. This conclusion is supported by the finding that C7 is highly expressed on the cell membrane only when the cells reorganize vimentin along the surface after reaching confluence.34 Soluble C7 is also released by ECs after reaching confluence19 and is likely to represent the portion of synthesized C7 unbound to vimentin.
Cell-associated C7 maintains the ability to bind C5b6, C8, and C9, forming mTCC on the cell surface, as shown by ELISA. The complex assembled on ECs is cytolytically inactive and contains S-protein and clusterin resembling soluble SC5b-9. The conditions in which mTCC may be formed are acute situations, such as sepsis, associated with unrestricted C activation leading to the release of C activation products, including SC5b-9.35 Excessive C activation is also responsible for the consumption of C7 and the generation of activated C5b6 from the acute phase proteins C5 and C6.36 The C5b6 complex produced in excess is then available for mC7 and initiates the assembly of the full complex on the cell surface.
Data collected over the last 2 decades have provided evidence for the ability of cytolytically inactive TCC to stimulate the expression of adhesion molecules,7 to induce the release of IL-8,37 and also to promote vascular leakage.16 This is not the case of the complex assembled on mC7, that proved to be totally ineffective in this respect. The failure of mTCC to stimulate ECs may be attributed to the type of interaction that the complex establishes with the cell membrane. While mTCC is assembled on cell-bound C7 that acts as a trap for the other components, soluble SC5b-9 binds to ECs through both S protein38 and polymerized C9.18
We were surprised to find out that mTCC not only failed to activate the cells, but also down-regulated the activation signal of SC5b-9 resulting in reduced expression of adhesion molecules, release of IL-8, and endothelial cell permeability. The inability of goat IgG anti-C7 to induce an inhibitory effect suggests that the activity of mTCC as a down-regulator does not depend solely on C7. C9 plays a critical role since the inhibition obtained with mC5b-8 was only 40% that of mTCC and further decreased to approximately 15% with mC5b-7 suggesting also the involvement of C8. The contribution of vimentin to this effect is unclear, but published data suggest that vimentin may act as a potential regulator of transcription, interacting and sequestering transcriptional determinants such as p5339 and menin.40
SC5b-9 released in limited amount at the inflammatory site after local C activation is beneficial and promotes inflammation. Conversely, excessive amount of circulating SC5b-9 formed as a result of unrestricted C activation in patients with sepsis and other acute phase situations can be harmful. These are the conditions that favor the formation of free C5b613 and in which mC7 may act as a trapping molecule promoting the assembly of mTCC preventing in this way the damaging effect of SC5b-9. A point to emphasize is that CD59 expressed on ECs efficiently prevents the assembly of MAC, but has no effect on SC5b-9, that can only be controlled by mTCC. Interestingly, the protective activity of mTCC does not require high concentration of C5b6, C8, and C9 and can still be seen with a relatively low amount of C components.
In conclusion, we have shown that ECs express surface C7 bound to vimentin as a trapping molecule for the assembly of mTCC. This complex does not induce activation of ECs, but rather down-regulates the pro-inflammatory effect of circulating SC5b-9.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Claudio Gamboz and Bernd Bodenmiller for excellent technical assistance and Marco Paluello for editing the manuscript.
This work was supported by the European Network of Excellence (NoE) “EMBIC” within FP6 (contract no. LSHN-CT-2004-512040), Associazione Italiana per la Ricerca sul Cancro (AIRC), and COFIN 2007 provided by Ministero dell'Istruzione, Università e Ricerca (MIUR).
Authorship
Contribution: F.B. performed most of the experiments and contributed to the design of the research project, the analysis of the results, and the writing of the manuscript; L.R. performed immunoaffinity purification, Western blot analysis, and contributed to the preparation of the manuscript; R.B. contributed to the establishment of the primary culture of endothelial cells and to the preparation of the manuscript and figures; A.D. performed the purification of SC5b-9 and reviewed the manuscript; C.T. performed the immunohistochemical analysis of tissue samples and reviewed the manuscript; E.B. performed the Northern blot analysis and reviewed the manuscript; P.P. performed the MS analysis and reviewed the manuscript; P.M. performed Western blot analysis and reviewed the manuscript; C.P. analyzed the data and reviewed the manuscript; R.W. provided the C7 mAb (WU 4-15), contributed to analysis of the data, and reviewed the manuscript; and F.T., the group leader, contributed to the design of the project, the analysis of the results, and the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Tedesco, Department of Life Sciences, University of Trieste, via Fleming 22, 34127 Trieste, Italy; e-mail: tedesco@units.it and tedesco@univ.trieste.it.




![Figure 7. Low amount of mTCC was sufficient to down-regulate the proinflammatory effect of SC5b-9. (A) mTCC was assembled on mC7 incubating HUVECs with increasing amount of C5b-6 (0.5 μg [a], 1 μg [b], 2 μg [c], and 3 μg [d] to a final volume of 100 μL). C8 and C9 were added to C5b6 at a molar ratio of 1:2:2, respectively. The assembly of mTCC was revealed by ELISA on whole cells using anti-C9 neoantigen. (B) HUVECs were stimulated with SC5b-9 (5 μg/mL) either alone or in the presence of mTCC assembled using the same quantity of C5b6, C8, and C9 as used above. The expression of VCAM-1 was evaluated by ELISA on whole cells as indicated in the legend to Figure 6A. Values are mean (± SD) of triplicate determinations of 3 separate experiment (*P < .05; **P < .01 vs complete cell-culture medium).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/15/10.1182_blood-2008-03-146472/7/m_zh80160933820007.jpeg?Expires=1768574680&Signature=xOYmte8BuevB7Aq8VezXfdwY08Wdr4Te2MNArkmYoTTuq9aBEhKNLU1PJgbxi-UOmkNUcsUfZD~SHSDLvnUx57KpXwmjdSKvc1b0NcjnNmjh3SGPVZpu2fylZSdLwUnvoL2NE4iDIv9Sz51CGB9MdlbJO9v6n8XXIg1AZouHyS6BPCc8JGt099kgLMRyCD~NbsHKRFeYv7Acb1lII84~4W6smesG5y9kxHFGW7W71AsR-59VqXs9WULUXLzaY97i6Y7PVEGxVxMwfjSI0ToLG2BjXD0sVrKam8tWhOXPklzh~FTSqFtIR8WCcAOlUdgAXpwsKR1eC11F5BLQOfOenA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
