In this issue of Blood, Otsu and colleagues provide evidence that high concentrations of bone marrow–derived MSCs have the capacity to induce endothelial cell apoptosis in culture and inhibit angiogenesis and tumor growth in murine melanomas.
Initial interest in bone marrow–derived mesenchymal stems cells (MSCs) focused on their potential for restoring organ function by engraftment and differentiation.1,2 More recent work has focused on the immunomodulatory properties of MSCs on T cells, B cells, natural killer cells, and neutrophils, all of which may have value in treating inflammatory disorders including acute lung injury, acute kidney injury, and inflammatory bowel disease.3,4 There is also clinical data that MSCs may have activity in treating acute graft-versus-host disease.5 In addition to their effects on immune responses, MSCs produce endothelial and epithelial growth factors that might promote tissue repair. However, because of their capacity to release angiogenic growth factors, such as vascular endothelial growth factor and basic fibroblast growth factor, there has been concern that MSCs might favor the development or growth of tumors in patients by stimulating angiogenesis.6 There has also been concern that MSCs have the potential to become neoplastic.3,6 Thus, the observation in the current article that bone marrow–derived MSCs can inhibit growth of melanoma tumors in mice is interesting and potentially important.
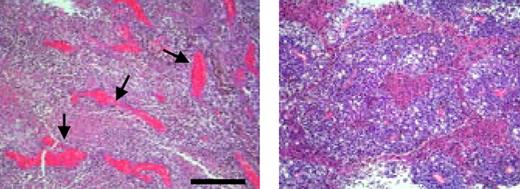
The antiangiogenic effects of MSCs injected into melanoma are illustrated by the histologic sections that show blood vessels in an untreated (left panel) and MSC-inoculated (right panel) tumor. There is markedly reduced vascular density in the MSC-treated tumors. Bar equals 300 μm. See the complete figure in the article beginning on page 4197.
The antiangiogenic effects of MSCs injected into melanoma are illustrated by the histologic sections that show blood vessels in an untreated (left panel) and MSC-inoculated (right panel) tumor. There is markedly reduced vascular density in the MSC-treated tumors. Bar equals 300 μm. See the complete figure in the article beginning on page 4197.
Using an in vitro Matrigel angiogenesis assay, Otsu et al found that MSCs migrated to capillaries, established gap junction intercellular communications, and induced a marked increase in reactive oxygen species (ROS) in the cultured endothelial cells, resulting in endothelial cell apoptosis and capillary degeneration.7 Direct inoculation of MSCs into subcutaneous melanomas in C57BL/6 mice induced apoptosis in the microcirculation of the tumors, findings that were associated with marked inhibition of tumor growth. Control experiments with mouse lung fibroblasts had no effect. There was a remarkable decrease in vascular density in the MSC-inoculated melanoma tumors. Molecular markers of endothelium and tissue levels of hemoglobin in the tumors were also reduced. Based on the in vitro studies, the mechanism for inducing endothelial cell apoptosis may be explained by the generation of ROS, since ROS inhibitors blocked MSC-induced endothelial cytotoxicity in the Matrigel capillaries. The investigators demonstrated transfer of mitochondria from the MSCs to the endothelial cells, raising the possibility that MSC mitochondria may have been the source for ROS in the endothelial cell. Work from another group indicated that intravenously injected MSCs can home to highly vascular Kaposi sarcoma tumors in athymic nude mice and markedly reduce tumor growth, an effect that depends on cell to cell contact and Akt inhibition.8
Although the current study is interesting and well done, there are some issues that will require further study. The authors needed to use a high concentration of MSCs to endothelial cells (1:1) in order to induce apoptosis in capillaries in the Matrigel assay. It was not clear what the ratio of MSCs to tumor cells was in the mouse melanoma experiments, but the effect was only reported with direct injection into the tumors, not with intravenous delivery. Thus, we do not know if the MSCs would home to the melanoma tumors if delivered in the systemic circulation, and if the effect would be transient or sustained with repeated delivery of the MSCs. Further experiments will be needed to assess how MSCs would perform in other mouse tumor models, especially on highly vascular, rapidly growing tumors that are highly dependent on active angiogenesis for ongoing growth and metastatic potential. Finally, the in vivo effects of MSCs could involve immune responses that were not evaluated in this study.
Despite these limitations, there are several important implications from these studies. First, more preclinical studies on the capacity of MSCs to control tumor growth are warranted. Second, since transfection of MSCs is straight forward, the antitumor capacity of MSCs might be up-regulated with genes that could enhance their antitumor properties,6 including genes that induce apoptosis by the generation of ROS. Third, the ability of MSCs to home to tumors needs to be better defined at a molecular level, including more dose- and time-dependent studies with intravenous delivery. Fourth, since MSCs have now been isolated from bone marrow, placenta, amniotic fluid, and fetal tissues,3 investigators should consider testing the relative efficacy of MSCs derived from different sources for their antitumor and antiangiogenesis properties. Finally, in considering MSCs for the treatment of acute and chronic inflammatory disorders, physicians and scientists will need to be concerned that under some conditions, MSCs might impair tissue repair by reducing microcirculatory blood flow, especially if high concentrations of MSCs are retained in a single organ.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health