Abstract
Recent data highlight the importance of inflammatory markers during human immunodeficiency virus type 1 (HIV) infection. HIV-associated multicentric Castleman disease (HIV-MCD) presents with systemic symptoms attributed to cytokine disarray, and we have previously shown that the use of the anti-CD20 monoclonal antibody rituximab induces clinical remissions. Before and during successful rituximab therapy, 15 plasma cytokines were measured as were adaptive (CD4, CD8, CD19) and innate (CD16/56) immune cell populations and HIV-1 viral loads. A significant reduction from baseline of the CD19 B-cell count, consistent with rituximab's mechanism of action, was observed. Markedly elevated cytokine levels were observed before rituximab therapy, and a reduction from baseline values with rituximab therapy was observed for interleukin (IL)-5, IL-6, and IL-10. Therapies that reduce the inflammatory cytokine response are likely to be successful in a range of diseases, including HIV-MCD, and in the future may be used to guide therapeutic strategies.
Introduction
Multicentric Castleman disease (MCD), a relatively rare lymphoproliferative disorder that presents with fevers, anemia, and multifocal lymphadenopathy, is today most commonly observed in persons infected with HIV.1 A lymph node biopsy typically identifies cells that stain for human herpesvirus 8 (HHV-8) proteins, and the features of Castleman disease may be attributed to the presence of this γ-herpesvirus.2,3 Manifestations of the disease are thought to be related in part to cytokine disarray, in particular increased levels of interleukin-6 (IL-6), a homologue of which is produced by the HHV-8 genome.4,5
Treatment of HIV-MCD had been largely ineffective until a number of case reports6-11 and 2 recent single-arm clinical trials showed that rituximab induces clinical remission in both persons at first presentation12,13 and with pretreated and relapsed HIV-MCD.14 Rituximab-induced B-cell depletion, a likely reservoir of HHV-8, has been postulated to occur via antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity, and apoptosis.15,16
We have previously reported a patient with HIV-MCD treated with rituximab before experiencing a sustained decrease in IL-6.17 Following the clinical outcomes observed,13 we have investigated plasma cytokine changes over time in HIV-MCD before and during rituximab therapy, determining the utility of these pleiotropic molecules as a biomarker in this setting. This has timely relevance because a recent Strategies for Management of Anti-Retroviral Therapy (SMART) trial report examined intermittent versus continuous antiretroviral therapy, showing that levels of specific cytokines and inflammatory markers were strongly related to all-causes mortality.18
Methods
Twenty-four patients with a first presentation of HIV-MCD were recruited between 2003 and 2008, and histologic confirmation of the plasmablastic variant of HIV-MCD was obtained. All patients received rituximab therapy (4 infusions at a standard dose of 375 mg/m2 at weekly intervals). A total of 6 of the 24 patients did not provide baseline data for this study, and this report is thus limited to 18 patients. All 18 patients are alive and their HIV-MCD is currently in remission. This study was approved by all participating institutional ethics review boards, and patient informed consent was obtained in accordance with the Declaration of Helsinki.
Patient demographics, clinical presentation, and hematologic and biochemical markers were recorded and have been previously described.13 Immune subset and HIV-1 viral loads were measured before rituximab therapy and at 1, 3, 6, and 12 months after rituximab therapy as per previously published protocols.19 Briefly, total lymphocyte and subset analyses were performed with the use of whole blood stained with murine anti–human monoclonal antibodies to CD4 (T-helper cells), CD8 (a cytotoxic T-cell marker), CD19 (B cells), and CD16/56 (natural killer [NK] cells; TetraOne; Beckman Coulter, High Wycombe, United Kingdom) and were evaluated on an Epics XL-MCL (Beckman Coulter) multiparametric 4-color flow cytometer. Plasma viral loads (Quantiplex HIV RNA 3.0; Chiron, Halstead, United Kingdom) were recorded with a lower limit of detection of 50 copies/mL.
Assays of 15 plasma cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, GM-CSF, TNF-α, IFN-α, and IFN-γ) were measured before rituximab therapy and at 1, 3, 6, and 12 months after therapy (according to length of follow-up). For each assay, fluorescence ion digital imaging system (FIDIS) cytokine-coated beads were prepared, divided into aliquots, and placed into a 96-well plate, before the addition of either standards of known concentration or samples to be assayed. The plates were treated with washes interspersed by the addition of biotinylated detector antibody and then streptavidin-RPE solution. The plate was then placed on the XY platform of the Luminex 100 instrument (Austin, TX) for analysis. Data acquisition and analysis were performed by STarStation software (Applied Cytometry, Dinnington, United Kingdom). The mean MFI value and standard curves were produced for each analyte in each well that was converted into a concentration with the use of the standard curve. This value was then multiplied by the dilution coefficient 2 to give the concentration of the analyte in the original serum sample.
Cell marker, HIV-1 viral load, and plasma cytokine levels at each time point (1, 3, 6, and 12 months) were compared with levels before rituximab therapy with the Wilcoxon signed rank test. P values less than .05 were considered significant.
Results and discussion
HIV-MCD presents with systemic symptoms attributed to cytokine disarray. In this cohort of patients with HIV-MCD, one of the largest ever described, the majority had elevated cytokines before rituximab therapy. Both IL-6 (encoded by the HHV-8 genome) and IL-10 have been implicated in the development of HIV-MCD, and these data support the existing body of knowledge that IL-6 and IL-10 are associated with the known aggressive disease features observed. Similarly, normalization of these cytokines are associated with the response observed.
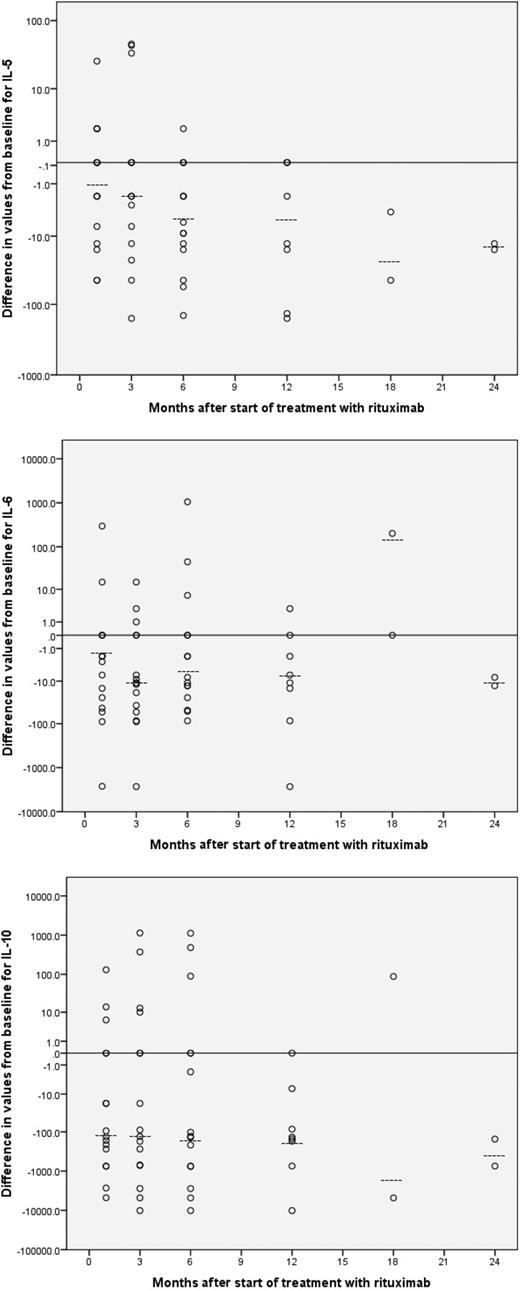
Eighteen patients (16 male, 89%) with histologically confirmed plasmablastic variant of HIV-MCD were enrolled into the study. Median NK cell counts were not found to change significantly compared with values before therapy. Significant reductions in median measurements compared with values before rituximab therapy were observed for CD4, CD8, CD19, and HIV-1 viral load (Table 1). Plasma cytokine profiles for the 18 patients included in this study showed cytokine disarray before rituximab therapy. Inspection of the median values showed significant reductions at 2 or more time points compared with values before rituximab therapy for IL-5, IL-6, and IL-10 (Table 2; Figure 1). No other cytokine showed consistent trends, although there are occasional significant reductions in mean plasma cytokine values compared with values before rituximab therapy for another 3 cytokines, IL-12, IL-17, and IFN-α.
Differences from baseline for IL-5, IL-6, and IL-10 after start of rituximab therapy. Differences from baseline (Time 0) for values of IL-5 (top), IL-6 (middle), and IL-10 (bottom) after commencement of rituximab therapy. Dotted lines denote median values.
Differences from baseline for IL-5, IL-6, and IL-10 after start of rituximab therapy. Differences from baseline (Time 0) for values of IL-5 (top), IL-6 (middle), and IL-10 (bottom) after commencement of rituximab therapy. Dotted lines denote median values.
The presence of IL-6 and IL-10 have been associated with increased autonomous cell proliferation and viability of HHV-8–infected cells, especially in primary effusion lymphoma models.20,21 In HIV-1 infection, IL-10 produced by HHV-8–infected and HIV-tat–induced cell lines has been linked to B-cell activation and polyclonal hypergammaglobulinemia.20-22 These data are consistent with previous findings, which suggested a close relation between elevated IL-10 and symptomatic MCD in patients on highly active antiretroviral therapy (HAART). IL-5 has been implicated in a case report in the cause of non–HIV-MCD,23 and a role in immunosuppression has been postulated, by increasing the production of transforming growth factor β1 (TGF-β1) and enhancing phosphorylation of Smad2 in CD4+ T cells.24
Rituximab appears efficacious in treating HIV-MCD, and its use is associated with a significant reduction in multiple plasma cytokines. In addition to a reduction from baseline in IL-6 we report, for the first time, a significant reduction from baseline of IL-5 and IL-10 with rituximab therapy for HIV-MCD. This study was limited by small patient numbers, a feature in common with all MCD studies, and plasma cytokine levels may not be a true reflection of the tumor microenvironment.
In the SMART case-control analysis,18,25 elevated levels of either IL-6 or D-dimer at study entry were strongly related to all-cause mortality, and increases in both markers were related to the level of HIV-RNA after 1 month. Increases in these markers after randomization were associated with mortality. These findings suggest that HIV-induced activation of inflammatory pathways (and we suggest pathways induced by both HIV and HHV-8 here) has an adverse effect on all-cause mortality and may be used to identify patients at high risk of death. The magnitude of the association appears clinically relevant.
Further work on patients with HIV-MCD treated with rituximab will increase our understanding of the disease processes here and help refine the optimal therapy for this aggressive disease. It appears that plasma cytokines represent a useful biomarker in this setting, and their decrease occurs concomitantly with a response to therapy. This is likely to have utility across a broad range of diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the St Stephen's AIDS Trust and the Hammersmith Hospital Special Trustees, London, United Kingdom.
Authorship
Contribution: M.B. and J.S. designed the research, contributed reagents and analytic tools, and wrote the paper; O.V. and R.S. performed statistics and analyzed the data; P.C. and P.K. performed the cytokine analyses; B.G. and M.N. assisted in study design; and M.B., J.S., B.G., and M.N. helped care for the patients. All authors approved the final version submitted.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Justin Stebbing, Imperial College Healthcare NHS Trust, Charing Cross Hospital, Department of Medical Oncology, 1st Floor/East Wing, Fulham Palace Rd, London W6 8RF, United Kingdom; e-mail: j.stebbing@imperial.ac.uk; or Mark Bower, Imperial College, Chelsea and Westminster Hospital, 369 Fulham Rd, London SW10 9NH, United Kingdom; e-mail: m.bower@imperial.ac.uk.