Abstract
The balanced manifestation of effector functions and the generation of long-living memory cells is a hallmark of efficient CD8+ T-cell response. Accumulating data pinpoint CD4+ CD25high regulatory T (Treg) cells as a key factor for the inefficiency of CD8+ T-cell responses in viral persistence. Little is known about the effects of Treg cells on the homeostasis of healthy donor CD8+ T cells. The present study demonstrates that Treg cells exert differential effects on CD8+ T-cell subsets. Treg cells inhibited mostly the polyclonal proliferation of CD27− effector cells compared with CD27+ memory CD8+ T cells. Moreover, they inhibited the polyclonal and antigen-driven differentiation of memory cells into functional effectors as defined by IFN-γ secretion and induction of CD160 expression. Finally, Treg cells reduced the apoptosis of memory but not of effector and terminal effector cell populations. These effects were at least in part mediated by a decreased expression of PD-L1, but not of programmed death 1 (PD-1), on CD8+ T cells after activation. Thus, in the setting of a healthy immune system, Treg cells fine-tune the memory/effector cell balance and promote the accumulation of long-living memory cells in case of strong stimulation.
Introduction
Upon antigen encounter, naive and memory CD8+ T cells with relevant specificity are driven into massive proliferation, accompanied by gradual acquisition of cytotoxic effector functions1 and generation of antigen-specific memory cells. Antigen clearance signals the termination of the clonal expansion through apoptosis. Several studies have shown that CD8+ T-cell differentiation stages may be defined by the ordered expression of certain surface receptors. Naive CD8+ T cells coexpress CD45RA isoform, CD28 and CD27 costimulatory receptors, and CCR7 chemokine receptor, while antigen-primed cells gradually lose CD45RA and CD28 expression. Recirculating long-living central memory cells still express CCR7, while more differentiated effector-memory cells are CCR7−. The irreversible loss of CD27 indicates decreased proliferation potential, increased cytotoxicity, and definitive transition toward effector stage. Finally, CD45RA reexpression defines a terminal highly cytotoxic and apoptosis-prone stage.2-5
A hallmark of efficient CD8+ T-cell response is the balance between the prompt manifestation of effector functions and the generation of long-living memory cells.5,6 This balance is fine-tuned by external signals and is strongly dependent on the regulatory mechanisms of the host.7-9 After studies in mice, recent studies in humans have shown that CD4+CD25high regulatory T (Treg) cells constitute a small fraction of CD4+ T cells that plays a role of immune regulator. These cells inhibit the proliferation of T cells stimulated through their T-cell receptor (TCR), and usually exert their suppressive function through contact-dependent mechanisms, though recent data suggest that these cells may also function in a cytokine-dependent manner.10,11
The mechanisms by which Treg cells exert their suppressive function are not fully elucidated. Some groups have described an increased expression of glucocorticoid-induced tumor necrosis factor (GITR) and CTLA-4 on Treg cells, but so far it is not clear whether these molecules are differentiation markers or directly involved in Treg cell functions.12-14
The effects of Treg cells on both CD4+ and CD8+ T-cell responses have been largely investigated in the settings of chronic viral infections.15-20 Accumulating data show that this particular population might be a key factor for the inefficiency of CD8+ T-cell response and viral persistence. Independently from Treg cell studies, the interaction between the programmed death 1 (PD-1) receptor and its ligands was shown to affect the survival capacity of CD8+ T cells, and hence to be critical for the outcome of antiviral responses.21-23 In healthy donors, data regarding the regulation of CD8+ T cells by CD4+CD25high T cells are limited to the proliferation and secretion of cytokines at the level of the whole CD8+ T-cell population.21-23 Comparatively little is known about the effects of Treg cells on the differentiation and homeostasis of CD8+ T lymphocyte subsets. In the present paper, we studied the effects of Treg cells on CD8+ T-cell differentiation induced with polyclonal or antigen-specific stimulation. We looked for their effects on cytokine secretion, effector function, proliferation, and apoptosis of the different CD8+ subpopulations. Finally, we investigated whether the interaction of PD-1 (CD279) and its ligand PD-L1 (CD274) is involved in Treg cell–mediated effects.
Methods
Cell populations
Blood from healthy donors was collected after informed consent was obtained in accordance with the Declaration of Helsinki. All studies received institutional review board approval from the Regional Blood Transfusion Department (Creteil, France). CD8+ and CD4+ T cells were purified by negative separation using RosetteSep CD4- and CD8-enrichment antibody cocktails (StemCell Technologies, Vancouver, BC) according to the manufacturer's instructions. This procedure resulted in a greater than 90% purity of CD8+ and CD4+ T-cell populations as assessed by flow cytometry. Purified CD4+ T cells were subsequently incubated with 2 μL CD25− magnetic beads per 10 × 106 cells for 15 minutes at 4°C, and passed on 2 successive magnetic columns (Miltenyi Biotec, Bergisch-Gladbach, Germany), according to the company's guidelines. The CD25− fraction after the first passage contained fewer than 5% CD25+ cells. The CD25+ fraction after the second passage (referred to as CD4+CD25high) contained more than 93% CD25+ cells; of these, more than 70% expressed CD25 with high intensity. In sorting experiments, purified CD8+ T cells were stained with CD27/CD45RA monoclonal antibodies (mAbs), and the CD27+CD45RA− memory (M) and CD27−CD45RA− effector (E) subsets were sorted under sterile conditions. In some experiments, terminally effector (TE) CD27−CD45RA+ cells were also sorted (Coulter sorter; Beckman Coulter, Marseille, France).
Immunophenotyping studies
Immunophenotyping was performed using 4-color flow cytometry. Cell fractions were incubated with appropriate mAbs for 30 minutes at 4°C, washed, and fixed in phosphate-buffered saline (PBS) 1% formaldehyde. The following murine mAbs were purchased from BD Biosciences (Le Pont Claix, France): anti-CD25–FITC, anti-CD4–PerCP, anti-CD8–PerCP, anti-CD3–APC, anti-CD27–PE, anti–PD-1–PE, anti-PD-L1–PE, anti-CD45RA–APC, anti–IL-2–PE, and anti-IFNγ–FITC. Anti-CD45RA–Cy5 used for sorting was a product of Coulter (Beckman Coulter). FITC-conjugated anti-CD160 mAb was produced in one of our laboratories (Inserm U955). Isotype-matched controls were used in all phenotyping experiments.
CFSE proliferation assay
Cell cultures were performed in RPMI 1640 (BioWhittaker Europe, Verviers, France) supplemented with 10% human blood group AB serum (BioWest, Nuaille, France), penicillin-streptomycin (100 IU/mL and 100 μg/mL, respectively), 2 mM l-glutamine (all from Gibco BRL, Paisley, United Kingdom), and 1 mM sodium pyruvate, (Sigma-Aldrich, St Louis, MO). CD8+ T cells were washed 3 times in PBS and 3 to 5 × 106 cells/mL were mixed with 1 μM CFSE (Molecular Probes, Eugene, OR) in 1:1 volume ratio. Staining was performed for 8 minutes at 37°C followed by 3 washes in ice-cold complete medium. For anti-CD3 stimulation, 5 μg/mL anti-CD3 mAb (clone UCHT1; Beckman Coulter) was coated on 96-well flat-bottom cell-culture plates overnight at 4°C. CFSE-labeled CD8+ T cells were cultured in the presence or absence of CD4+ T-cell fractions at a total cell concentration of 2.5 × 106/mL and final volume of 200 μL. Previous titration experiments showed that cocultures (CD8/Treg cells) at a ratio of 2:1; 4:1, and 8:1 led to 55%, 40%, and 22% (mean of 3 experiments) inhibition of CD8+ T-cell proliferation, whereas no inhibition was noted in the presence of CD4+CD25−. In further experiments, cocultures were performed at a 4:1 ratio. At the end of culture, cells were washed, additionally stained with CD27/CD8/CD45RA, PD-1/CD8/CD4, or PD-L1/CD8/CD4 combinations, and analyzed by flow cytometry. The percentage of Treg cell–mediated inhibition was calculated as follows: (percentage CFSElow in the presence of Treg cells divided by percentage CFSElow in the absence of Treg cells) multiplied by 100.
Cytokine assays
Procedures were performed as previously described.24 CD8+ T cells were cultured in 48-well plates in the presence or absence of CD4+ T-cell fractions at a concentration of 5 × 105 cells/well. For antigen-specific stimulation experiments, autologous monocytes were added at 5 × 104/well. Overnight stimulation was carried out with plate-bound anti-CD3 (5 μg/mL) or CEF (cytomegalovirus [CMV], Epstein-Barr virus [EBV], and influenza virus) peptide mixture, 5 μg/mL each. At 2 hours after the beginning of culture, 1 μL/mL brefeldin A (5 μg/mL; Sigma-Aldrich) was added to each well. Harvested cells were washed 2 times in PBS, surface-stained with CD3/CD8 mAb, fixed and permeabilized by Cytofix/Cytoperm (BD Pharmingen, San Diego, CA) and stained for intracellular expression of IFN-γ and IL-2 (30 minutes at room temperature).
Apoptosis and transwell studies
CD8+ T cells were cultured in the presence or absence of CD4+ T-cell fractions in 96-well round-bottom plates coated with anti-CD3 at 5 μg/mL. Forty-eight hours later, cells were harvested, washed with cold PBS, suspended in binding buffer (containing 2.5 mM CaCl2), and stained with annexin V–FITC (apoptosis kit; BD Pharmingen) and a combination of CD27-PE/CD8-PerCP/CD45RA-APC mAbs for 20 minutes on ice. At the end of the culture, 300 μL of binding buffer was added, and the samples were analyzed within 30 minutes by flow cytometry while kept on ice. When needed, CD8+ T cells were preincubated with human anti–PD-L1–blocking mAb (16-5983; eBioscience, San Diego, CA), at 10 μg/mL for 15 minutes at 37°C before adding the CD4 cells. For PD-1 apoptosis induction experiments, wells were coated with a combination of anti–CD3 and anti–PD-1 (AF 1086; R&D Systems, Minneapolis, MN) mAbs to obtain final concentrations of 5 μg/mL and 20 μg/mL, respectively. In transwell experiments, CD8+ T cells were seeded at 5 × 105/well in the lower chamber of a Cellstar 24-well plate (Greiner Bio-One, Frickenhausen, Germany) coated with 5 μg/mL anti-CD3 mAb, and were cultured in the presence of Treg cells or CD4+CD25− T cells, added either in direct contact with CD8+ T cells or in the upper chamber (Greiner Bio-One).
Flow cytometry
Cells were analyzed by a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA). At least 20 × 103 CD8+ gated events were collected for cell-surface studies and at least 100 × 103 were collected for cytokine expression studies. Data analysis was performed with CellQuest software (BD Immunocytometry Systems, San Jose, CA). Cells were routinely gated on forward scatter/side scatter (FSC/SSC) plots followed by selection of CD8+ T cells. CD8+ subpopulations were defined on a CD45RA/CD27 plot and analyzed according to the experiment.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 4 software (GraphPad, San Diego, CA). Essentially, the Student paired t test was applied to estimate the effects of Treg cells after a preliminary confirmation of normal data distribution. Notably, equivalent nonparametric tests yielded similar results.
Results
Treg cells inhibit the polyclonal and antigen-specific proliferation of CD8+ T cells
We first studied the effects of Treg cells on polyclonal and antigen-specific induced proliferation of CD8+ T cells. To this end, CD8+, CD4+CD25−, and CD4+CD25high T cells from 11 healthy donors were purified. We verified that CD4+CD25high Treg cells expressed high levels of the transcription factor FoxP3 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). CD8+ T cells were labeled with CFSE and stimulated with plate-bound anti-CD3 mAb for 5 days, either with CD4+CD25− or with Treg cells at a 1:4 (suppressor-responder) ratio previously determined in titration experiments (data not shown). More than 80% of anti-CD3–activated CD8+ T cells proliferated when cultured alone (data not shown) or in the presence of CD4+CD25− cells (Figure 1A). In contrast, CD8 proliferation was significantly reduced in the presence of Treg cells (average inhibition, 39%; range, 22%-77%; Figure 1B). Likewise, Treg cells inhibited antigen-specific proliferation of CD8+ T cells stimulated in the presence of autologous monocytes and CEF peptides: average inhibition was 29% (range, 18%-41%) and 26% (range, 17%-40%) compared with cultures performed in the presence of CD4+CD25− control cells (Figure 1A,B) or alone (data not shown), respectively.
Treg cells inhibit anti-CD3 and CEF-induced proliferation of CD8+ T cells. (A) Representative histograms depicting the CFSE profile of CD8+ T cells from a healthy donor stimulated with coated anti-CD3 mAb for 5 days (left panel) or with CEF mix for 7 days (right panel), in the presence of CD4+CD25− T cells (top row) or Treg cells (bottom row). The percentage of CD8+ T cells that have divided after stimulation (CFSElow) is indicated. (B) Pooled data from 11 donors showing the percentage of inhibition of CD8+ T-cell proliferation after anti-CD3 or CEF stimulation in the presence of Treg cells in comparison with proliferation in the presence of CD4+CD25−. The mean values are depicted with horizontal lines.
Treg cells inhibit anti-CD3 and CEF-induced proliferation of CD8+ T cells. (A) Representative histograms depicting the CFSE profile of CD8+ T cells from a healthy donor stimulated with coated anti-CD3 mAb for 5 days (left panel) or with CEF mix for 7 days (right panel), in the presence of CD4+CD25− T cells (top row) or Treg cells (bottom row). The percentage of CD8+ T cells that have divided after stimulation (CFSElow) is indicated. (B) Pooled data from 11 donors showing the percentage of inhibition of CD8+ T-cell proliferation after anti-CD3 or CEF stimulation in the presence of Treg cells in comparison with proliferation in the presence of CD4+CD25−. The mean values are depicted with horizontal lines.
Treg cells inhibit CD8+ T-cell effector response to polyclonal and antigen-specific stimulation
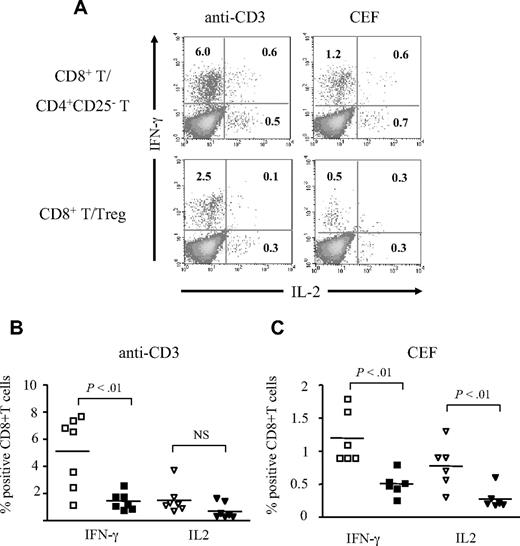
CD8+ T-cell effectors are characterized by an increased capacity of cytokine secretion. We studied the effect of Treg cells on CD8+ T-cell expression of IFN-γ and IL-2 after polyclonal or antigen-specific stimulation. Overnight anti-CD3 stimulation of CD8+ T cells in the presence of Treg cells resulted in a lower expression of intracellular IFN-γ (mean, 1.4%; n = 7) compared with CD8+ T cells stimulated in the presence of CD4+CD25− T cells (mean, 5.1%; P < .01; Figure 2A,B) or alone (mean, 4.2%; P < .05; data not shown). Similarly, Treg cells decreased the percentages of IFN-γ+ CD8+ T cells (mean, 0.5%) stimulated in the presence of CEF peptides compared with CD8+ T cells stimulated in the presence of CD4+CD25− T cells or alone (1.2% and 0.8%, respectively; P < .01 for both comparisons; Figure 2A,B). The effect of Treg cells on IL-2 expression was less pronounced, and was found significant only for CEF-stimulated cells (mean, 0.2% in the presence of Treg cells compared with 0.7% in the presence of CD4+CD25− T cells and 0.5% for CD8+ T stimulated alone; P < .05 for both comparisons) but not for anti-CD3–stimulated CD8+ T cells (mean, 0.8% in the presence of Treg cells compared with 1.5% in the presence of CD4+CD25− T cells and 1.4% for CD8+ T cells stimulated alone, respectively; P > .05; Figure 2A-C; data not shown).
Treg cells inhibit the cytokine expression after polyclonal and antigen-specific stimulation of CD8+ T cells. (A) Representative dot plots of CD8+ T cells secreting IFN-γ and IL-2 after overnight stimulation with coated anti-CD3 (left panels) or CEF (right panels) in the presence of CD4+CD25− T (top row) or Treg cells (bottom row). The percentage of cytokine-expressing CD8+ T cells is indicated. (B,C) Pooled data showing the percentage of CD8+ T cells secreting IFN-γ or IL-2 after overnight stimulation with anti-CD3 (n = 7 donors; B) or CEF (n = 6 donors; C) in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). The P values were calculated using a paired Student t test. The mean values are depicted with horizontal bars.
Treg cells inhibit the cytokine expression after polyclonal and antigen-specific stimulation of CD8+ T cells. (A) Representative dot plots of CD8+ T cells secreting IFN-γ and IL-2 after overnight stimulation with coated anti-CD3 (left panels) or CEF (right panels) in the presence of CD4+CD25− T (top row) or Treg cells (bottom row). The percentage of cytokine-expressing CD8+ T cells is indicated. (B,C) Pooled data showing the percentage of CD8+ T cells secreting IFN-γ or IL-2 after overnight stimulation with anti-CD3 (n = 7 donors; B) or CEF (n = 6 donors; C) in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). The P values were calculated using a paired Student t test. The mean values are depicted with horizontal bars.
Treg cells inhibit the differentiation of effector CD8+ T cells
Next, we evaluated the effects of Treg cells on the differentiation of activated CD8+ T cells. For this, experiments were performed either with total CD8+ T cells or sorted CD8 populations. We defined CD8+ T-cell subsets according to the coexpression of CD45RA and CD27 and further studied the expression of CCR7, CD28, and the cytotoxic CD160 receptor in these subsets (Figure S2). Based on this analysis, CD27hiCD4RA−CD8+ T cells were mostly CCR7+CD28+, while CD27lowCD45RA− CD8+ T cells contained exclusively CCR7− cells, and a variable proportion of CD28− cells. Both subsets were CD160−/low. Therefore, the CD27+CD45RA− subset contained central memory and memory cells in transition to effectors and was defined as “memory” subset (M) thereafter. CD27−CD45RA−CD8+ T cells were invariably CCR7−CD28− and expressed high levels of CD160. These cells may be considered as definitely engaged into effector functions, and we consequently named them effectors (Es). Finally, CD27−CD45RA+CD8+ T cells were also CCR7−CD28−CD160+, and corresponded to terminally differentiated effector cells (TEs).
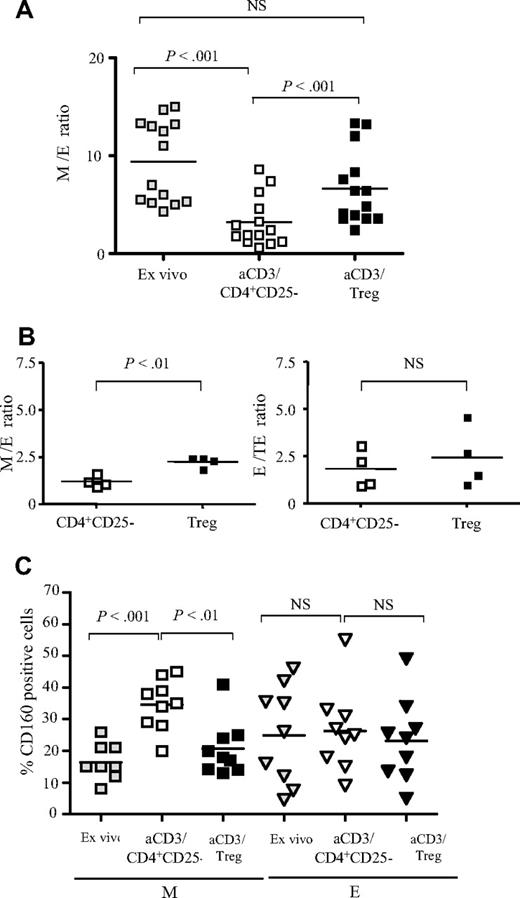
We compared the distribution of M and E CD8+ T cells before (ex vivo analysis) and after 48 hours of anti-CD3 stimulation of total CD8+ T cells in the presence of CD4+CD25− T cells or Treg cells (Figure 3A). We found that anti-CD3 stimulation changed the distribution of CD8+ T-cell subsets significantly, resulting in a decrease of the mean (n = 14 subjects) M/E ratio from 9.4 in nonstimulated conditions to 3.5 after anti-CD3 stimulation of CD8+ T cells alone (data not shown) and 3.2 in the presence CD4+CD25− T cells (P < .001; Figure 3A). In contrast, in the presence of Treg cells, the mean value of the M/E ratio corresponded to 6.7, which was not significantly different from the one obtained for nonactivated CD8+ T cells (P > .5), but was significantly higher than the M/E ratio of CD8+ T cells activated alone or in the presence of CD4+CD25− T cells (P < .001; Figure 3A).
Treg cells inhibit the maturation of anti-CD3–stimulated CD8+ T cells. (A) Pooled data from 14 donors showing the ratio of M/E CD8+ T cells before (▩), and after 48 hours of stimulation of total CD8+ T cells with coated anti-CD3 in the presence of CD4+CD25− T cells (□) or Treg cells (■). (B) Left panel: M/E ratio resulting from 48 hours of stimulation of sorted M (CD27+CD45RA−) CD8+ T cells with coated anti-CD3 in the presence of CD4+CD25− T cells (□) or Treg cells (■). Right panel: E/TE ratio resulting from the stimulation of sorted E (CD27−CD45RA−) CD8+ T cells in the same conditions. Pooled data from 4 donors are shown. (C) Pooled data from 9 donors showing the expression of CD160 before (gray symbols), and after 48 hours of stimulation of total CD8 T cells with coated anti-CD3 in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols) on gated memory (M) and effector (E) CD8+ T cells. The P values were calculated using a paired Student t test. The mean values are depicted with horizontal bars.
Treg cells inhibit the maturation of anti-CD3–stimulated CD8+ T cells. (A) Pooled data from 14 donors showing the ratio of M/E CD8+ T cells before (▩), and after 48 hours of stimulation of total CD8+ T cells with coated anti-CD3 in the presence of CD4+CD25− T cells (□) or Treg cells (■). (B) Left panel: M/E ratio resulting from 48 hours of stimulation of sorted M (CD27+CD45RA−) CD8+ T cells with coated anti-CD3 in the presence of CD4+CD25− T cells (□) or Treg cells (■). Right panel: E/TE ratio resulting from the stimulation of sorted E (CD27−CD45RA−) CD8+ T cells in the same conditions. Pooled data from 4 donors are shown. (C) Pooled data from 9 donors showing the expression of CD160 before (gray symbols), and after 48 hours of stimulation of total CD8 T cells with coated anti-CD3 in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols) on gated memory (M) and effector (E) CD8+ T cells. The P values were calculated using a paired Student t test. The mean values are depicted with horizontal bars.
Next, we repeated these experiments with sorted M (CD27+CD45RA−) and E (CD27−CD45RA−) CD8+ T cells (n = 4). These populations were stimulated for 48 hours with anti-CD3 antibodies in the presence of either CD4+CD25− or Treg cells. Here again, Treg cells inhibited significantly the maturation of stimulated M to E cells compared with CD4+CD25− T cells (M/E ratio: 2.3 versus 1.2, respectively; P < .01; Figure 3B). In contrast, Treg cells did not inhibit the maturation of E to TE CD8 T cells (E/TE ratio, 1.6 and 2.2 in the presence of CD4+CD25− and Treg cells, respectively; P = .08; Figure 3B). Globally, these results indicate that Treg cells inhibited the differentiation of M to E CD8+ T cells upon polyclonal stimulation.
We have previously shown that the CD160 receptor is expressed by mature circulating or tissue effector cytotoxic CD8+ T cells.24,25 Therefore, we studied CD160 expression on CD8+ T-cell subsets differentiated from activated total CD8 T cells. Polyclonal stimulation of CD8+ T cells cultured either alone or in the presence of CD4+CD25− T cells increased the percentage of CD160+ CD8+ T cells (mean, 35% and 37%, respectively, compared with 21% under nonstimulated conditions; n = 9; P < .01). This effect was abolished in the presence of Treg cells (mean, 23%; data not shown). Detailed analysis revealed that this effect was mainly due to a significant inhibition of the induction of CD160 expression on the M subset: the mean was 21% in the presence of Treg cells compared with 35% in the presence of CD4+CD25− T cells (P < .01), whereas no effect was noted on E CD8+ T cells (mean, 23% compared with 26%, respectively; P > .05; Figure 3C). In addition, we found that Treg cells decreased the expression of surface CD107a, another marker associated with cytotoxic effector CD8+ T cells (data not shown), confirming that Treg cells prevented the maturation of CD8+ T effectors upon polyclonal stimulation.
We concluded that Treg cells might exert differential effects on CD8+ T memory and effector subsets, thus changing their proportions at the overall level. More specifically, Treg cells prevented the differentiation toward E phenotype and promoted the accumulation of M cells.
Treg cells preferentially inhibit the proliferation of E CD8+ T cells
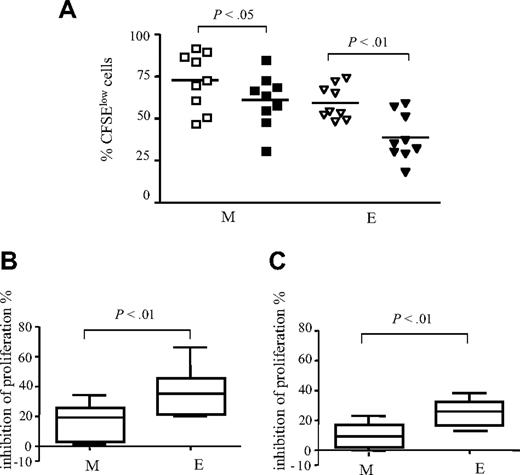
We next investigated whether Treg cells affect the proliferative rate of anti-CD3–activated M and E CD8+ T cells differently. Activation of total CD8+ T cells revealed that in contrast to the E subset, M CD8+ T cells had a higher proliferative rate and a lower susceptibility to Treg cell–mediated inhibition. At day 5, we found that the M subset contained 73% (range, 47%-92%) CFSElow cells in the presence of CD4+CD25− T cells, and 61% (range, 31%-85%) CFSElow cells in the presence of Treg cells (P < .05; Figure 4A). For E CD8+ T cells, these percentages were 59% (range, 48%-74%) and 39% (range, 18%-59%), respectively (P < .01). On the average (n = 9 subjects), Treg cells inhibited the polyclonal proliferation of M and E subsets by 16% and 36%, respectively (P < .01; Figure 4B). Because proliferation is accompanied by differentiation, M and E subsets resulting from activation of CD8+ T cells may be from heterogeneous origin. Therefore, we repeated these experiments with sorted M (CD27+CD45RA−) and E (CD27−CD45RA−) cells. Similarly, we found that Treg cells preferentially inhibited the proliferation of sorted E CD8+ T cells compared with M (mean inhibition, 25.5 versus 8.9, respectively; P < .01; n = 4; Figure 4C).
Treg cells preferentially inhibit the accumulation of E CD8+ T cells. Total CD8+ T cells were stimulated for 5 days with anti-CD3 mAb in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). (A) Percentages of CFSElow M and E CD8 T-cell subsets (n = 9). (B) Pooled data from 9 donors: mean percentages of inhibition of the proliferation of CD8+ T-cell M and E subsets. (C) Sorted M and E CD8+ T cells were stimulated in the presence of Treg cells or CD4+CD25− cells. Pooled data from 5 donors: mean percentages of inhibition of the proliferation are shown. The mean values are depicted with horizontal lines. P values were calculated using a paired Student t test.
Treg cells preferentially inhibit the accumulation of E CD8+ T cells. Total CD8+ T cells were stimulated for 5 days with anti-CD3 mAb in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). (A) Percentages of CFSElow M and E CD8 T-cell subsets (n = 9). (B) Pooled data from 9 donors: mean percentages of inhibition of the proliferation of CD8+ T-cell M and E subsets. (C) Sorted M and E CD8+ T cells were stimulated in the presence of Treg cells or CD4+CD25− cells. Pooled data from 5 donors: mean percentages of inhibition of the proliferation are shown. The mean values are depicted with horizontal lines. P values were calculated using a paired Student t test.
Globally, these results confirmed that Treg cells prevent the accumulation of E cells, due to specific effects on CD8 subsets.
Treg cells inhibit preferentially the apoptosis of M CD8+ T cells
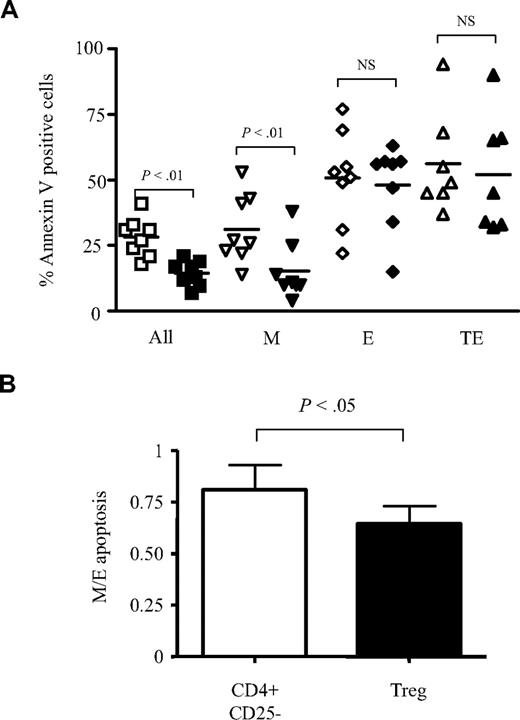
Proliferation in response to strong stimulation is always accompanied by activation-induced cell death (AICD) that terminates clonal expansion. Therefore, we investigated whether Treg cells could similarly protect the various CD8+ T-cell subsets from AICD related to anti-CD3 mAb stimulation. To this end, the apoptosis rate of total CD8+ T cells from 8 healthy donors stimulated in vitro alone, or in the presence of either CD4+CD25− T cells or Treg cells was quantified by annexin V staining. At day 2, the mean percentage of anti-CD3–activated CD8+ annexin V+ T cells was 23% (range, 14%-36%; data not shown). In the presence of CD4+CD25− T cells, the rate of CD8+ T-cell apoptosis increased to 28% (range, 18%-41%), while Treg cells decreased this rate to 15% (range, 7%-21%; P < .01; Figure 5A). Analyses of the apoptosis rates of M, E, or TE anti-CD3–activated CD8+ T cells showed that Treg cells significantly inhibited M cell apoptosis (mean, 15% compared with 28% in the presence of CD4+CD25− T cells; P < .01), whereas no significant effects were noted for E and TE subsets (Figure 5A). These results suggested that M and E CD8+ T cells exhibit a different sensitivity to Treg cell–mediated modulation of apoptosis. However, because these experiments were performed with total CD8 T cells, we cannot rule out cell-cell interactions between different subsets. Therefore, new experiments were performed on sorted M and E T cells. We found in the presence of CD4+CD25− T cells a 19% decrease of apoptosis rate among M compared with E CD8+ T cells. This effect was up to 36% in the presence of Treg cells (P < .05; Figure 5B).
Treg cells preferentially inhibit the apoptosis of CD27+CD45RA− M CD8+ T cells. (A) Total CD8+ T cells were stimulated for 48 hours with anti-CD3 antibodies in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). Percentages of annexin V+ cells on total and gated M, E, and TE CD8+ T cells are shown (n = 8). (B) Sorted M and E CD8+ T cells were stimulated in the presence of CD4+CD25− T cells or Treg cells. In each condition, percentages of annexin V+ cells were estimated at 48 hours. Histograms represent the ratio of annexin V+ M and E cells observed in CD4+CD25− (□) and Treg cell cocultures (■). P values were calculated using a paired Student t test.
Treg cells preferentially inhibit the apoptosis of CD27+CD45RA− M CD8+ T cells. (A) Total CD8+ T cells were stimulated for 48 hours with anti-CD3 antibodies in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). Percentages of annexin V+ cells on total and gated M, E, and TE CD8+ T cells are shown (n = 8). (B) Sorted M and E CD8+ T cells were stimulated in the presence of CD4+CD25− T cells or Treg cells. In each condition, percentages of annexin V+ cells were estimated at 48 hours. Histograms represent the ratio of annexin V+ M and E cells observed in CD4+CD25− (□) and Treg cell cocultures (■). P values were calculated using a paired Student t test.
Treg cells inhibit the apoptosis of M CD8+ T cells by affecting the PD-1/PD-L1 signaling pathway
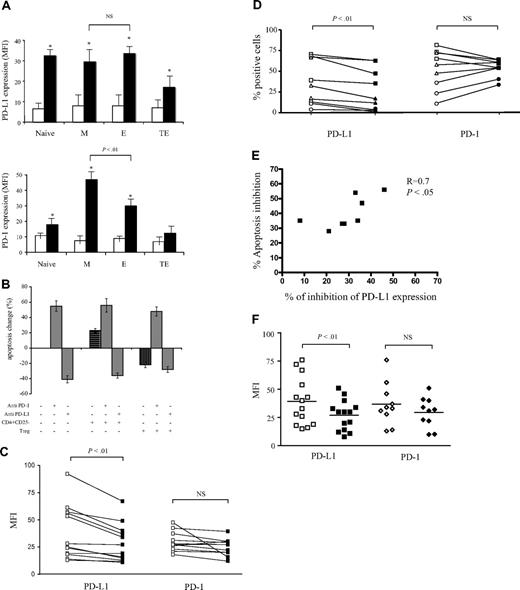
As the interaction between PD-1 and its ligand PD-L1 has been recently recognized as a key regulator of activated CD8+ T-cell survival,21-23 we investigated whether this pathway might be involved in Treg cell–mediated protection of M CD8+ T cells from apoptosis. First, we studied the expression of these molecules on anti-CD3–stimulated total CD8+ T cells. Both PD-1 and its ligand were up-regulated upon activation, with a maximum at 48 hours, followed by a decrease to the initial levels (data not shown). While PD-L1 expression increased uniformly and significantly in all CD8+ subsets compared with baseline levels (P < .01), PD-1 was induced preferentially on the M and E subsets (P < .01). Moreover, PD-1 expression was significantly higher on activated M compared with activated E CD8+ T cells (mean fluorescence intensity [MFI] 47 vs 30; P < .01; Figure 6A). Based on this differential expression, PD-1 seemed a suitable target for subset-specific regulation of the apoptosis rate.
Treg cells inhibit the apoptosis of anti-CD3–stimulated CD8+ T cells by regulating PD-L1 expression levels. (A) Expression levels of PD-L1 (top histograms) and PD-1 (bottom histograms) at day 0 (□) and after 48 hours (■) of anti-CD3 stimulation were compared on the indicated CD8+ T-cell subsets. *Significant change (P < .05) compared with nonstimulated control. (B) Treg cells and anti–PD-L1–blocking mAb inhibit CD8+ T-cell AICD but do not prevent anti–PD-1–induced apoptosis. Pooled data from 7 separate experiments where CD8+ T cells were stimulated for 48 hours with coated anti-CD3 mAb alone, in the presence of CD4+CD25− T cells or in the presence of Treg cells. Blocking anti–PD-L1 or agonist anti–PD-1 antibodies were added as indicated. The percentage of apoptosis change was calculated on the basis of annexin V+ cells (mean ± SD) detected when CD8+ T cells were stimulated alone. (C) Pooled data from 12 donors showing the expression of PD-1 and PD-L1 on anti-CD3–stimulated CD8+ T cells in the presence of CD4+CD25− T cells (□) or Treg cells (■). (D) M (squares), E (triangles), or TE (circles) CD8+ T-cell subsets were sorted from 3 different donors and stimulated in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). Percentages of PD-1 and PD-L1 expression in each subset are shown. P values were calculated using a paired Student t test. (E) Correlation between the inhibition of PD-L1 expression on CD8 T cells and the inhibition of CD8 T-cell apoptosis in the presence of Treg cells. (F) Pooled data from 14 donors showing the expression of PD-1 and PD-L1 on anti-CD3–stimulated CD4+CD25− T cells (□) or Treg cells (■).
Treg cells inhibit the apoptosis of anti-CD3–stimulated CD8+ T cells by regulating PD-L1 expression levels. (A) Expression levels of PD-L1 (top histograms) and PD-1 (bottom histograms) at day 0 (□) and after 48 hours (■) of anti-CD3 stimulation were compared on the indicated CD8+ T-cell subsets. *Significant change (P < .05) compared with nonstimulated control. (B) Treg cells and anti–PD-L1–blocking mAb inhibit CD8+ T-cell AICD but do not prevent anti–PD-1–induced apoptosis. Pooled data from 7 separate experiments where CD8+ T cells were stimulated for 48 hours with coated anti-CD3 mAb alone, in the presence of CD4+CD25− T cells or in the presence of Treg cells. Blocking anti–PD-L1 or agonist anti–PD-1 antibodies were added as indicated. The percentage of apoptosis change was calculated on the basis of annexin V+ cells (mean ± SD) detected when CD8+ T cells were stimulated alone. (C) Pooled data from 12 donors showing the expression of PD-1 and PD-L1 on anti-CD3–stimulated CD8+ T cells in the presence of CD4+CD25− T cells (□) or Treg cells (■). (D) M (squares), E (triangles), or TE (circles) CD8+ T-cell subsets were sorted from 3 different donors and stimulated in the presence of CD4+CD25− T cells (open symbols) or Treg cells (filled symbols). Percentages of PD-1 and PD-L1 expression in each subset are shown. P values were calculated using a paired Student t test. (E) Correlation between the inhibition of PD-L1 expression on CD8 T cells and the inhibition of CD8 T-cell apoptosis in the presence of Treg cells. (F) Pooled data from 14 donors showing the expression of PD-1 and PD-L1 on anti-CD3–stimulated CD4+CD25− T cells (□) or Treg cells (■).
Accordingly, anti–PD-1 mAb increased significantly the apoptosis rate of activated CD8+ T cells (by a mean of 55%; n = 6 experiments), while anti–PD-L1 mAb reduced this rate (by a mean of 41%) compared with control conditions (Figure 6B). These data suggest that the CD8+ T-cell AICD rate might be a consequence of PD-1/PD-L1 “cis” interactions. Coculture of anti-CD3–activated CD8+ T cells with CD4+CD25− T cells induced apoptosis and did not affect the sensitivity of CD8+ T cells to anti–PD-1 mAb or revert the blocking effect of anti–PD-L1 antibodies. These results confirmed that, in our conditions, the AICD rate was principally regulated through CD8+/CD8+ T-cell interactions. In contrast, coculture with Treg cells significantly reduced the apoptosis rate of CD8+ T cells (by a mean of 22%; P < .05), mimicking the effect of PD-L1–blocking mAb. This inhibitory effect was not amplified by addition of anti–PD-L1 antibody, indicating that Treg cell and anti–PD-L1 effects were not cumulative. Importantly, in the presence of Treg cells, CD8+ T cells remained sensitive to anti–PD-1–induced apoptosis (Figure 6B). These results strongly suggest that Treg cells might preferentially affect PD-L1 rather than PD-1 expression on activated CD8+ T cells.
To test the latter hypothesis, we compared the expression of PD-L1 and PD-1 on CD8+ T cells activated either in the presence of Treg cells or CD4+CD25− T cells. We found that Treg cells significantly decreased PD-L1 expression on activated CD8+ T cells compared with CD4+CD25− T cells (average MFI, 23.4 and 30.7, respectively; P < .01; Figure 6C). PD-1 expression under these conditions did not differ significantly (average MFI, 39 and 47, respectively; P > .05; Figure 6C). These results showed that Treg cells affect the apoptosis rate of CD8+ T cells by reducing the expression of PD-L1 on activated CD8+ T cells. This effect was confirmed in experiments performed with sorted M, E, and TE T cells. After polyclonal activation, we found a significant decrease in PD-L1 but not PD-1 percentages of expressing cells in the presence of Treg cells compared with CD4+CD25− T cells (P < .01; n = 3; Figure 6D).
Finally, we found a significant correlation between the decrease of PD-L1 expression and apoptosis inhibition of CD8+ T cells in the presence of Treg cells (Figure 6E).
Because PD-L1 is also induced on activated CD4+ T cells, we further compared its expression on the CD4+ T cells from cocultures set with nonregulatory (CD4+CD25−) or with Treg cells. After 48 hours, CD4+ T cells from cocultures set with CD4+CD25− T cells expressed a significantly higher level of PD-L1 compared with CD4 T cells from cocultures set with Treg (MFI, 43.7 and 27.6, respectively; P < .001; Figure 6F). Globally, these results show that besides regulating the expression of PD-L1 on CD8+ T cells, Treg cells may reduce apoptosis by presenting a lower level of PD-L1 compared with non-Treg CD4+ T cells.
Treg cell effect on PD-L1 expression is not exclusively contact dependent
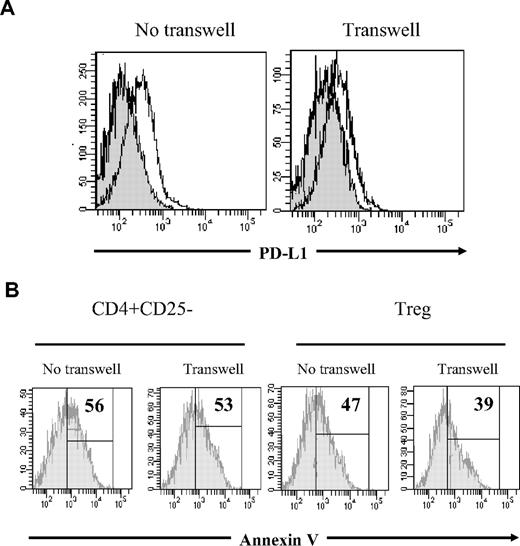
To elucidate some of the mechanisms underlying Treg effects, we asked whether the observed apoptosis inhibition of M CD8+ T cells required cell contact or was cytokine mediated. To this end, CD8+ T cells were cultured in the lower chamber of transwell plates coated with anti-CD3 mAb. Treg or CD4+CD25− T cells were either mixed directly with CD8+ T cells or added in the upper chamber, separated by a semipermeable membrane. As shown in Figure 7A, PD-L1 expression on CD8+ T cells decreased in the presence of Treg cells, either cultured in contact or separated by a semipermeable membrane. These results demonstrated that Treg cells might regulate PD-L1 expression during cell contact as well as in a cytokine-dependent manner. Accordingly, Treg cells decreased significantly the rate of M CD8+ T-cell apoptosis even when separated from activated CD8+ T cells by a semipermeable membrane, whereas CD4+CD25− T cells did not prevent AICD of CD8+ T-cell populations regardless of the culture conditions (Figure 7B for one representative experiment).
Treg cell effect on M CD8+ T-cell apoptosis is rather cytokine dependent. (A) Histogram overlays representing PD-L1 expression on CD8+ T cells stimulated with anti-CD3 for 48 hours in the presence of CD4+CD25− (open histogram) or Treg cells (gray histogram), either mixed in the lower chamber of a transwell plate (left) or separated by a semipermeable membrane (right). (B) Annexin V staining of anti-CD3–activated M CD8+ T cells. Cocultures of CD8+ T cells with either CD4+CD25− T cells (left) or Treg (right) were set in transwell plates as follows: CD8+ and CD4+ T cells were either mixed in the lower chamber, or separated by a semipermeable membrane. Histograms represent CD27+CD45RA−CD8+-gated cells. Percentage of annexin V+ cells is shown, based on negative control. One representative experiment of 2 with similar results is shown. The numbers correspond to the percentage of annexin V+ cells based on negative control staining.
Treg cell effect on M CD8+ T-cell apoptosis is rather cytokine dependent. (A) Histogram overlays representing PD-L1 expression on CD8+ T cells stimulated with anti-CD3 for 48 hours in the presence of CD4+CD25− (open histogram) or Treg cells (gray histogram), either mixed in the lower chamber of a transwell plate (left) or separated by a semipermeable membrane (right). (B) Annexin V staining of anti-CD3–activated M CD8+ T cells. Cocultures of CD8+ T cells with either CD4+CD25− T cells (left) or Treg (right) were set in transwell plates as follows: CD8+ and CD4+ T cells were either mixed in the lower chamber, or separated by a semipermeable membrane. Histograms represent CD27+CD45RA−CD8+-gated cells. Percentage of annexin V+ cells is shown, based on negative control. One representative experiment of 2 with similar results is shown. The numbers correspond to the percentage of annexin V+ cells based on negative control staining.
Altogether, these results indicate that the PD-1/PD-L1 pathway is involved in the regulation of anti-CD3–stimulated CD8+ T-cell expansion. We demonstrate that Treg cell effects on PD-L1 expression and AICD of M CD8+ T cells are cytokine-mediated rather than contact-dependent.
Discussion
In this study, we investigated the role of Treg cells on the proliferation, functional differentiation, and apoptosis of healthy donor CD8+ T-cell subsets. First, we found that Treg cells inhibited the proliferation and effector functions of CD8+ T cells under polyclonal and antigen-specific stimulation. Second, we demonstrated that Treg cells affect homeostasis of CD8+ T-cell subsets differentially by inhibiting mainly the proliferation of E but not CD27+ M cells. At the same time, Treg cells inhibited the polyclonal and antigen-driven differentiation of M into E CD8+ T cells. Third, Treg cells preferentially inhibited the apoptosis of CD27+ CD8 T cells by decreasing their expression of PD-L1, but not of PD-1. Thus, Treg cells finely influence the behavior of activated M CD8+ T-cell subsets and interfere with regulatory mechanisms of CD8+ T-cell homeostasis such as the PD-1/PD-L1 pathway.
To our knowledge, we show for the first time specific Treg cell effects on distinct CD8+ T-cell populations. Accumulating data indicate that memory CD8 subsets are part of a differentiation continuum, rather than separate lineages.26,27 Expression of the CD45RA isoform is restricted to naive and/or “reverted” memory T cells, and terminally differentiated effectors.2 Expression of CD27 promotes the survival of activated and memory CD8+ T cells.28 Furthermore, the ability of activated E cells to reexpress CD27 is limited within a certain number of divisions, and the definite loss of this receptor indicates a truly advanced differentiation state.3
The systematic analysis of M and E subsets in conditions of polyclonal stimulation showed that Treg cell effects are more complicated than a simple inhibition of cell proliferation. Indeed, Treg cells inhibited preferentially the accumulation of E compared with M cells and the apoptosis of this latter subset. Based on these results, it can be predicted that in the context of strong CD8+ T-cell activation, the presence of Treg cells would maintain and/or increase the pool of memory CD8+ T cells and limit effector-cell differentiation, resulting in a conserved ratio of memory-effector T cells. Accordingly, Suvas et al reported that HSV-1–specific effector CD8+ T cells remained activated for a longer period of time in CD25+-depleted animals.29
The functional differentiation of CD8+ T cells is characterized by the concomitant expression of cytokines and cytotoxic molecules. In our hands, Treg cells significantly inhibited the intracellular expression of IFN-γ after both polyclonal and antigen-specific stimulation. Accordingly, Treg cells inhibited the expression of secretion of cytotoxic granules detected by surface expression of CD10730 (data not shown) and CD160, a marker of circulating highly cytotoxic CD8+ T cells.31,32 At the same time, the effect of Treg cells on IL-2 expression was not significant in conditions of TCR polyclonal stimulation. Keeping in mind that IL-2 is the predominant cytokine secreted by M CD8+ T cells, in line with their highly proliferative and self-renewing capacity,5 this result confirmed that Treg cells differentially affect M and E CD8+ T cells.
The balance between CD8+ T-cell subsets is the net result of their specific proliferation/death rates. Therefore, Treg cells might influence the proportion of subsets either by inhibiting their proliferation and/or apoptosis. While most studies investigate the effects of Treg cells on cell proliferation, data on CD8+ T-cell apoptosis are limited. In a recent study, Longhi et al showed that Treg cells did not change the apoptosis rate of CD8+ cells as measured by annexin V expression.33 However, in our hands, while the apoptosis rate of memory cells decreased in the presence of Treg cells, no such differences or even increased apoptosis were detected at the level of E and TE cells. Thus, these observations further reinforce the need to evaluate Treg cell effects at the subpopulation level as shown here.
The differential effects of Treg cells on M and E cells could be the consequence of some phenotypic differences between these subsets. In agreement with others,23 we have detected a significantly higher expression of PD-1 on the CD27+CD8+ memory subset after activation. This indicated that in spite of a potentially long life span, memory CD8+ T cells are much more sensitive to negative regulation. Numerous recent studies in animal models and in humans have pinpointed the PD-1/PD-L1 pathway as crucial for the outcome of a virus-specific response, independent of CD4+ T-cell help.34-36 Sekaly et al have demonstrated that in patients with HIV infection, PD-1 expression is more frequent within the HIV-specific memory CD27+CD8+ T-cell subset, and that these cells are much more prone to “exhaustion.”23 Thus, PD-1/PD-L1 signaling appears as a key mechanism of regulation of activated CD8+ T cells. Interestingly, we found that Treg cells significantly decreased PD-L1 but not PD-1 expression on memory CD8+ T cells, resulting in a decreased apoptosis rate. Moreover, in conditions of anti-CD3 stimulation, Treg cells themselves expressed less PD-L1 than activated nonregulatory CD4 (Figure 6F). However, we cannot rule out that Treg cells might affect other pathways (cytokine secretion or decreased expression of other death molecules) of CD8+ T-cell death. Globally, these results showed that activation of CD8+ T cells in the context of Treg cells results in a lower level of PD-L1 molecules available for interacting with activated PD-1high memory cells.
Transwell experiments indicated that the effects of Treg cells on PD-L1 expression and M CD8+ T-cell apoptosis are rather cytokine dependent. These results are in accordance with the reported cytokine-mediated regulation of PD-L1 expression.35,37 Numerous studies on the mechanism of Treg cell function have been conducted in various models. In some animal models, suppression is reversed by neutralizing anti–IL-10 or TGFβ antibodies, indicating a possible effect of these cytokines in vivo.38-40 In contrast, in humans, the regulation of CD8+ T-cell response by Treg cells appears to be mainly contact dependent.19,33,41,42 Nevertheless, other authors did not exclude a contact-independent mechanism involvement in the inhibition of CD8+ proliferation.33 Accordingly, our results demonstrate that the Treg cell–induced inhibition of M CD8+ T-cell AICD is at least in part mediated through soluble factors. (Figure 7).
Our findings may have significant physiologic implications because protective immunity is more efficiently conferred by memory CD8+ T cells.5 In acute viral infections, a vigorous CD8+ T-cell response leads to viral clearance. However, many viruses lead to persistent infection despite detectable CD8+ T-cell responses, a condition in which Treg cells may be involved. In these situations, it is not clear whether Treg cells are “friends” or “foes,” leading to a suppression of tissue damage mediated by virus-specific effector cells or inhibition of host immunity.43 The accumulation of Treg cells in the settings of uncontrolled infection, as well as their ability to strongly inhibit the antigen-specific proliferation and effector functions, have been demonstrated in several human and animal model infections: HIV,15,16,19,20 hepatitis B virus (HBV),15,16,19,20,44,45 hepatitis C virus (HCV),17,45,46 tuberculosis,47 and simian immunodeficiency virus (SIV).48 Lack of control of viral replication by specific CD8+ T cells has been explained by a skewed differentiation program resulting either in accumulation of immature and poorly functional effectors as shown in HIV+ subjects,49-51 or in excessive differentiation and exhaustion of the effector potential, typical of active CMV.52 Because E CD8+ T cells are the most readily responding primed cells, they seem the logical target for Treg cell inhibition. The study of Kinter et al19 demonstrates a direct inhibitory effect of Treg cells on perforin+ HIV-specific CD8+ T cells, which correspond to differentiated effectors. The effects of human Treg cells on the generation of CD8+ memory T cells, however, have not been demonstrated. Although studies in mice suggest that Treg cells participate in the regulation of CD8+ T-cell memory, deleting Treg cells alone has little or transitory impact on memory T-cell homeostasis, suggesting that its regulation is far more complex.29,53,54 A recent study by Sharma et al suggested the critical role of CD25 (IL-2Rα) expression in this control.55 Further evidence of the differential impact of Treg cells on memory and effector T cells is provided by a recent study in SIV-infected sooty mangabies. In this model, the best immunologic correlate of CD4 T-cell loss (ie, of progressive infection) is the expansion of effector cells, which was inversely correlated with the level of Treg cells.56
In summary, our data show that Treg cells fine-tune the memory-effector cell balance and promote the accumulation of long-living memory cells. These effects are at least in part mediated by PD-1/PD-L1 interactions. How and why this homeostatic mechanism may be perturbed in the settings of persistent viral infections warrants further investigation. It is for certain that the simple depletion of Treg cells or blocking of the PD-1/PD-L1 interaction would not always have a positive impact on a hampered CD8+ T-cell response to virus.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from SIDACTION, Inserm, and Agence Nationale Recherche contre le SIDA et les Hepatites Virales of Paris, France (ANRS). M.N. was funded by a fellowship from the French Ministry of Research. J.-D.L. was funded by a fellowship from SIDACTION. M.C. is funded by ANRS.
Authorship
Contribution: M.N. and J.-D.L. performed the experiments, analyzed data, and wrote the paper; M.C. performed the sorting experiments; A.B. analyzed data and wrote the paper; and Y.L. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yves Lévy, Service d'Immunologie, CHU Henri Mondor, Creteil France, 51 Avenue du Mal de Lattre de Tassigny, 94010 Creteil, France; e-mail: yves.levy@hmn.aphp.fr.
References
Author notes
*M.N. and J.-D.L. contributed equally to this study.