Abstract
Clustering and occupancy of platelet integrin αIIbβ3 (GPIIb-IIIa) generate biologically important signals: conversely, intracellular signals increase the integrins' affinity, leading to integrin activation; both forms of integrin signaling play important roles in hemostasis and thrombosis. Indirect evidence implicates interactions between integrin α and β transmembrane domains (TMDs) and cytoplasmic domains in integrin signaling; however, efforts to directly identify these associations have met with varying and controversial results. In this study, we develop mini-integrin affinity capture and use it in combination with nuclear magnetic resonance spectroscopy to show preferential heterodimeric association of integrin αIIbβ3 TMD tails via specific TMD interactions in mammalian cell membranes in lipid bicelles. Furthermore, charge reversal mutations at αIIb(R995)β3(D723) confirm a proposed salt bridge and show that it stabilizes the TMD-tail association; talin binding to the β3 tail, which activates the integrin, disrupts this association. These studies establish the preferential heterodimeric interactions of integrin αIIbβ3 TMD tails in mammalian cell membranes and document their role in integrin signaling.
Introduction
Integrin-mediated cell adhesion modulates signaling pathways that control many biologic functions,1 and these signals involve both integrin clustering and integrin occupancy2 ; the latter changes integrin conformation, leading to allosteric rearrangements that propagate across the membrane and modify intracellular interactions.3 A second form of integrin signaling, initiated intracellularly, leads to increased affinity for ligands, a process termed integrin activation.1 Both forms of integrin signaling are central to the functions of platelet integrin αIIbβ3 (GPIIb-IIIa) in hemostasis and thrombosis.4 Integrin activation is also essential for functions such as inflammation and assembly of the extracellular matrix. Integrins are noncovalent α-β heterodimeric, type-1 transmembrane (TM) receptors formed from combinations of 18 α and 8 β subunits. Many studies indirectly implicate interactions between integrin α and β TM domains (TMDs) and cytoplasmic domains (tails) in both forms of integrin signaling5-11 ; however, studies to directly identify such interactions have met with contradictory results.
Mutational studies of platelet integrin αIIbβ3 (GPIIb-IIIa), based on sequence alignments, suggested an interaction between β3 Asp723 and αIIb Arg995.5 Charge reversal mutation of either residue resulted in constitutive bidirectional integrin signaling (ie, integrin activation and constitutive phosphorylation of pp125FAK); however, a double-charge reversal in αIIb(R995D)β3(D723R) did not exhibit constitutive signaling, suggesting that a salt bridge between these residues was a defined structural constraint that limited the integrin signaling.5 A spontaneous activating mutation (β3(D723H)) found in patients also suggests the importance of the β3 Asp723 residue for stabilization of the inactive state of the integrin.12 This constraint gained support from elegant protein engineering studies in which clasping the cytoplasmic domains or TMDs together inhibited integrin activation,9 and joining of the α and β TMDs with disulfide bonds limited bidirectional integrin signaling.7 Despite this mutational data, our initial efforts to identify interactions of isolated α and β integrin tails by nuclear magnetic resonance (NMR) spectroscopy were unsuccessful in aqueous solution.13 Two laboratories reported NMR studies of these tails that suggested the existence of such interactions, albeit different structures for the αβ dimer were reported.14,15 Structures of the individual αIIb and β3 TMDs in phospholipid bicelles16,17 show that the β3 TMD adopts an elongated, tilted membrane helix17 and the αIIb TMD folds into a short, straight helix, followed by a surprising backbone reversal that packs Phe992 and Phe993 against the TM helix.16 This structure demonstrated an unexpected complexity in the αIIb TMD and membrane-proximal cytoplasmic domain that was not observed in the studies of cytoplasmic domains in aqueous solution,14,15 thus calling NMR studies that suggested the interactions into question.
Whereas mutational studies and molecular modeling have strongly suggested that integrin αβ TMD interactions are important in regulating integrin signaling,8,10,11,18,19 efforts to identify direct interactions between α and β TMDs (either biochemically or genetically) have had contradictory results.20-23 Indeed, αα and ββ interactions were proposed to make a major contribution to the clustering that occurs in integrin signaling22 ; however, later studies have strongly challenged this idea.8,11 Furthermore, a 20 Å reconstruction of detergent-solubilized integrin αIIbβ3 revealed a cylindrical density interpreted as a TM segment that suggests that the TMDs are associated in a parallel, α-helical coiled-coil24 ; however, as noted above, higher resolution structures of the individual αIIbβ3 TMDs in a lipid environment are not consistent with a purely coiled-coil architecture. In this study, we use a novel mini-integrin affinity capture approach to establish the preferential heteromeric interaction of integrin αIIb and β3 TMDs and cytoplasmic domains in mammalian cells, and show by NMR spectroscopy that heterodimerization takes place analogously in small bicelle model membranes. Thus, we show that integrin TMDs and cytoplasmic domains preferentially interact in a heterodimeric rather than homodimeric fashion in mammalian cell membranes, interaction between the highly conserved β3 Asp723 and αIIb Arg995 stabilizes this interaction, and talin binding to the β3 cytoplasmic domain disrupts it.
Methods
Plasmids, antibody, and cell lines
Tac-αIIbTM, Tac-β3TM were generated by ligation of polymerase chain reaction (PCR)–generated fusion sequences consisting of extracellular domain of Tac and TM tail regions of each integrin subunit (Figure 1A) into pcDNA3.1 (Invitrogen, Carlsbad, CA). For the construction of αIIbTM- TAP (tandem affinity purification), the sequences of the preprotrypsin leader sequence followed by 3 repeats of FLAG sequence (Sigma-Aldrich, St Louis, MO) were first cloned into pcDNA3.1, and then the PCR-generated fusion sequences containing αIIbTM and TAP were ligated. Point mutations were performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Anti–Tac 7G7B6 (ATCC, Manassas, VA), anti–Tac N19 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti–FLAG M2 (Sigma-Aldrich) were obtained commercially. Rabbit polyclonal anti-β3 antibody (Rb8053), mouse monoclonal antibody specific for the human integrin αIIb subunit (PMI-1), activation-specific anti-αIIbβ3 antibody (PAC1), and αIIbβ3 activating antibody (anti-LIBS6) have been described previously.26 Chinese hamster ovary (CHO) cells and CHO cells expressing recombinant αIIbβ3 (A5) were maintained, as described previously.26 Lipofectamine and Lipofectamine Plus reagents (Invitrogen) were used according to the manufacturer's recommendation for transient transfections.
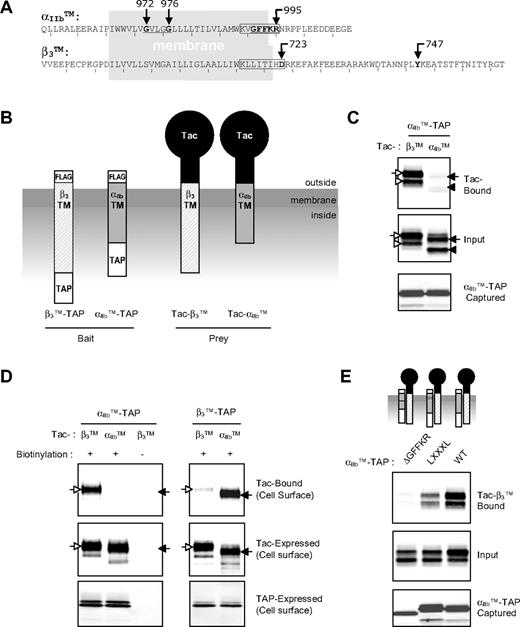
Integrin αIIb and β3 TMD-tail mini-integrins interact with each other via the TMDs. (A) Amino acid sequences of αIIbTM and β3TM used in this study. Sequences in the gray box represent amino acid residues in membrane region. GXXXG motif and GFFKR motif in αIIb are underlined. Point mutations used in this study are indicated with arrows. The previously designated membrane-proximal regions containing hydrophobic stretches in both α and β integrin are boxed.25 (B) Schematic diagram of TMD-tail constructs. For β3TM-TAP and αIIbTM-TAP baits, β3TM and αIIbTM (A) were fused to TAP tag for purification and an N-terminal FLAG tag for detection. Tac-αIIbTM and Tac-β3TM were made by fusion of αIIbTM and β3TM, respectively, with Tac extracellular domain. (C) CHO cells were transiently transfected with αIIbTM-TAP (bait) and Tac-αIIbTM or Tac-β3TM (preys), and cells were lysed and incubated with calmodulin beads to capture the baits. Bound Tac constructs were analyzed by Western blot using anti-Tac antibody (top panels). Expression of Tac preys (middle panel) and captured αIIbTM-TAP (bottom panel) were verified by Western blot using anti-Tac antibody and anti-FLAG antibody, respectively. The arrows indicate mature cell-surface proteins, and the arrowheads incompletely glycosylated intracellular proteins. Open symbols represent Tac-β3TM, and closed symbols represent Tac-αIIbTM. (D) CHO cells were transiently transfected with baits and preys, as indicated, and cell-surface proteins were biotinylated before cell lysis. Ten percent of the lysates were incubated with neutravidin beads to determine the input of biotinylated proteins in the lysates (middle and bottom panels). The remaining lysates were first incubated with calmodulin beads to capture the baits, and the bound proteins were eluted with 10 mM EDTA. The eluates were then incubated with NeutrAvidin beads to capture the biotinylated surface proteins and the presence of Tac preys was analyzed with Western blot using anti-Tac antibody (top panel). (E) αIIbTM-TAP constructs containing deletion of GFFKR motif or mutations of 2 Gly in GXXXG motif to Leu were tested for their binding to Tac-β3 as in (C).
Integrin αIIb and β3 TMD-tail mini-integrins interact with each other via the TMDs. (A) Amino acid sequences of αIIbTM and β3TM used in this study. Sequences in the gray box represent amino acid residues in membrane region. GXXXG motif and GFFKR motif in αIIb are underlined. Point mutations used in this study are indicated with arrows. The previously designated membrane-proximal regions containing hydrophobic stretches in both α and β integrin are boxed.25 (B) Schematic diagram of TMD-tail constructs. For β3TM-TAP and αIIbTM-TAP baits, β3TM and αIIbTM (A) were fused to TAP tag for purification and an N-terminal FLAG tag for detection. Tac-αIIbTM and Tac-β3TM were made by fusion of αIIbTM and β3TM, respectively, with Tac extracellular domain. (C) CHO cells were transiently transfected with αIIbTM-TAP (bait) and Tac-αIIbTM or Tac-β3TM (preys), and cells were lysed and incubated with calmodulin beads to capture the baits. Bound Tac constructs were analyzed by Western blot using anti-Tac antibody (top panels). Expression of Tac preys (middle panel) and captured αIIbTM-TAP (bottom panel) were verified by Western blot using anti-Tac antibody and anti-FLAG antibody, respectively. The arrows indicate mature cell-surface proteins, and the arrowheads incompletely glycosylated intracellular proteins. Open symbols represent Tac-β3TM, and closed symbols represent Tac-αIIbTM. (D) CHO cells were transiently transfected with baits and preys, as indicated, and cell-surface proteins were biotinylated before cell lysis. Ten percent of the lysates were incubated with neutravidin beads to determine the input of biotinylated proteins in the lysates (middle and bottom panels). The remaining lysates were first incubated with calmodulin beads to capture the baits, and the bound proteins were eluted with 10 mM EDTA. The eluates were then incubated with NeutrAvidin beads to capture the biotinylated surface proteins and the presence of Tac preys was analyzed with Western blot using anti-Tac antibody (top panel). (E) αIIbTM-TAP constructs containing deletion of GFFKR motif or mutations of 2 Gly in GXXXG motif to Leu were tested for their binding to Tac-β3 as in (C).
Affinity capture
Twenty-four hours after transfection, cells were lysed with CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.4, 1% CHAPS, 150 mM NaCl, 2 mM CaCl2, and EDTA [ethylenediaminetetraacetic acid]–free protease inhibitor mixture [Roche, Basel, Switzerland]) and clarified by centrifugation at 14 000 rpm for 15 minutes, and then the clarified lysates were incubated with calmodulin Sepharose (GE Healthcare, Piscataway, NJ) for 2 hours at 4°C. Bound proteins were eluted with sodium dodecyl sulfate (SDS) reducing sample buffer, subjected to SDS–polyacrylamide gel electrophoresis (PAGE), and analyzed by Western blot. For capturing biotinylated intact integrin subunits, A5 cells expressing TAP constructs were detached and surface proteins were biotinylated using EZ-Link Sulfo-NHS-Biotin (Thermo Scientific, Rockford, IL) according to the manufacturer's recommendation. The cells were washed twice with Tris-buffered saline (TBS; pH 8.4), and incubated for 30 minutes at 37°C with TBS containing 5 mM EDTA. Resulting cells were washed with HEPES-buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4) before being lysed with CHAPS lysis buffer. Lysates were incubated with calmodulin-beads to capture TAP constructs for 2 hours, bound proteins were eluted with 10 mM EDTA, and then the eluates were further incubated with NeutrAvidin agarose resin (Thermo Scientific) overnight to capture biotinylated proteins. Bound proteins were eluted with SDS reducing sample buffer, subjected to SDS-PAGE, and analyzed by Western blot.
NMR spectroscopy
Peptides encompassing human integrin αIIb(Ala958-Pro998) and β3(Pro685-Phe727) residues, respectively, including β3(Cys687Ser), were prepared as described previously.16,17 In addition, an αIIb peptide incorporating Arg995Ala and a β3 peptide with an Asp723Ala substitution were prepared analogously. The peptides were reconstituted in 385 mM 1,2-dihexanoyl-sn-glycero-3-phosphocholine, 83 mM 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-choline, 41 mM 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-l-serine], 25 mM HEPES·NaOH, pH 7.4, 6% D2O, and 0.02% wt/vol NaN3. Transverse relaxation optimized–heteronuclear single quantum coherence (TROSY-HSQC) NMR experiments were conducted on a cryoprobe-equipped Bruker Avance 700 spectrometer at 23°C.
Flow cytometry
PAC1-binding assay and fibronectin-binding assay were performed essentially as described.26,27 In brief, 1 day after transfection, suspended cells were incubated with 7G7B6 (anti-Tac) in combination with either PAC1 or biotinylated glutathione S-transferase-FN9-11, followed by staining with fluorescein isothiocyanate (FITC)–conjugated anti–mouse immunoglobulin (Ig) G and with R-phycoerythrin (PE)–conjugated anti–mouse IgM or PE-conjugated streptavidin. Five minutes before analysis, propidium iodide (PI) was added, and PI-negative live cells were analyzed on FACSCalibur (BD Biosciences, San Jose, CA). The data were analyzed using MATLAB R2007a software to calculate the geometric means and generate dot plots.
Cell imaging
A5 cells expressing Tac-αIIbTM or Tac-αIIb were detached and stained with anti–Tac N19 antibody and PAC1 in the presence or absence of anti-LIBS6, as indicated, followed by staining with FITC-conjugated anti–rabbit IgG and rhodamine-conjugated anti–mouse IgM. The stained cells were fixed, mounted in Prolong Gold (Invitrogen), and observed under a Nikon Eclipse TE2000-U inverted microscope. Images were acquired using 60×/1.4 NA oil-objective lens with 1.5× intermediate magnification, a Coolsnap HQ camera (Photometrics, Tucson, AZ), and QED InVivo imaging software (Media Cybernetics, Silver Spring, MD) at room temperature. Postacquisition image analysis was performed using ImageJ (National Institutes of Health, Bethesda, MD).
Results
Integrin αIIb and β3 TMDs mediate heterodimeric interactions in mammalian cell membranes
To test whether the TMDs of αIIb and β3 interact with each other, we constructed an αIIb mini-integrin containing the TMD and cytoplasmic tail of αIIb (Figure 1A) joined to an N-terminal flag tag for detection and a C-terminal TAP tag28 for rapid and efficient purification (Figure 1B). We expressed this αIIbTM-TAP bait in combination with preys comprising the extracellular domain of the Tac (interleukin-2 receptor α) joined to the TMD and tail of αIIb (Tac-αIIbTM) or β3 (Tac-β3TM; Figure 1B). The cells were lysed, and baits were captured using calmodulin beads. In the reaction, approximately 20% of expressed baits were usually captured. We detected bound prey by Western blotting with anti-Tac antibody and found that αIIbTM bait bound preferentially to Tac-β3TM rather than Tac-αIIbTM (17-fold more in quantification; Figure 1C). This interaction required the β3 TMD, because a prey containing the Tac extracellular and TMD joined to the β3 tail failed to bind to the αIIbTM bait (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). We noted multiple bands for each Tac construct and enzymatic deglycosylation and cell-surface biotinylation experiments showed that the top band observed in Figure 1C is a glycosylated form found predominantly on the cell surface, whereas the bottom band is a nonglycosylated intracellular form (Figure S1B,C). When we surface labeled the cells, we again found that αIIbTM-TAP bait interacts with Tac-β3TM (Figure 1D left panels) and only the top band was observed. Conversely, bait containing the TMD and tail of β3 preferentially bound the Tac-αIIbTM rather than Tac-β3TM on the cell surface, although subtle ββ homomeric interactions were also observed (Figure 1D right panels). Thus, we observed preferential heteromeric interactions between αIIb and β3 TMD-tail in both cell surface and intracellular membranes.
The foregoing results showed preferential heteromeric interactions between baits and preys containing the TMDs and cytoplasmic domains of integrin αIIbβ3, and that the interaction required the β3 TMD. To test the role of the αIIb TMD, we changed 2 Gly in the αIIb TMD GXXXG motif to Leu (Figure 1A). This motif is buried in the hydrophobic core of the lipid bilayer and is predicted to be important in αβ TMD packing.8,19 As a second test of specificity, we deleted the membrane-proximal GFFKR motif; both this deletion and the Leu substitutions activate integrin αIIbβ3.8 Both of these mutations markedly reduced interaction of αIIb bait with β3 prey (Figure 1E). Thus, the bait-prey interactions detected in this study are mediated by specific packing interactions of the TMDs. The strong preference for heteromeric αβ interactions in mammalian cell membranes is in sharp contrast to the homodimeric and trimeric interactions previously observed in detergent micelles,21 in electrophoresis,21,22 and in the Escherichia coli inner membrane.23 Schneider and Engelman20 did observe promiscuous weak heterodimeric interactions of central hydrophobic 17-residue fragments of the integrin TMDs using transcriptional repression of LacZ by dimer formation in E coli membranes; those authors observed extensive homo-oligomerization as well. Thus, the present data provide the first direct evidence for the heteromeric association of αIIbβ3 TMD-tails in mammalian cell membranes.
Integrin αIIb and β3 TMD interaction is stabilized by interaction of αIIb Arg995 and β3 Asp723
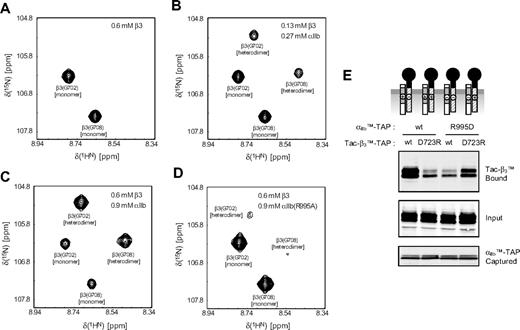
The plasma membrane is a complex environment; that is, it contains many proteins and lipids. Whereas this complexity is representative of the environment of integrin αIIbβ3 in blood platelets,29 it raises the possibility of indirect αIIb-β3 interactions. Using NMR spectroscopy, we therefore assessed the association of peptides corresponding to αIIb(Ala958-Pro998) and β3(Pro685-Phe727), which contain the TMDs16,17 and possible cytosolic heterodimerization sites in the controlled lipid environment of small bicelle model membranes. Within this environment, the presence of the αIIb peptide led to the appearance of a second set of NMRs for the β3 TMD residues in addition to the previously observed monomeric signals,16,17 as exemplified for β3(G702) and β3(G708) (Figure 2A-C). This second set of resonances arises from αIIb-β3 association (heterodimerization), which creates new chemical environments for all αIIbβ3 TMD resonances (Figure S2A) as a consequence of direct αIIb-β3 contacts and indirect, propagated effects. For example, β3(G708) is expected to form the dimerization interface, whereas β3(G702) is peripheral and most likely experiences predominantly indirect, next-neighbors changes. To test the existence of the proposed αIIb(R995)-β3(D723) salt bridge and verify the specificity of the αIIb-β3 interaction, wild-type αIIb TMD peptide was substituted by αIIb(R995A).5 This mutant αIIb peptide, whose backbone fold is indistinguishable from wild-type αIIb (Figure S2B), failed to induce a significant second set of β3 signals (Figure 2D), demonstrating that heterodimerization weakened considerably. Analogously, mutant β3(D723A) peptide did not induce a second set of αIIb resonances in contrast to wild-type peptide (data not shown). Thus, in the defined model membrane environment of phospholipid bicelles, specific TM αIIb-β3 interactions, leading to heterodimerization, were observed and were dependent on αIIb(R995) and β3(D723) electrostatic interactions.
In vitro αIIb-β3 TM heterodimerization. (A-C) Spectral region of TROSY-HSQC spectra showing Gly702 and Gly708 of the 2H/13C/15N-labeled β3 TM segment in the absence and presence of unlabeled αIIb TM peptide. One set of signals corresponds to the monomeric signals (A). The signal intensity ratios of monomeric-to-heterodimeric signals, but not their positions, depend on the αIIb and β3 peptide concentrations, demonstrating slow exchange kinetics on the NMR timescale between the 2 species. As evidenced by the disappearance of the monomer signals at higher peptide-to-bicelle ratios (C), heterodimerization is predominant. (D) In the presence of mutant αIIb(R995A) peptide, heterodimerization is weakened. The signal intensity ratio of heterodimer-to-monomer signals drops from 1.56 to 0.14 (C,D), and the heterodimeric signals are shifted compared with the interaction with wild-type αIIb peptide. (E) The effects of charge reversal mutations in the membrane-proximal regions of αIIb or β3 on the TMD interaction were analyzed, as in Figure 1.
In vitro αIIb-β3 TM heterodimerization. (A-C) Spectral region of TROSY-HSQC spectra showing Gly702 and Gly708 of the 2H/13C/15N-labeled β3 TM segment in the absence and presence of unlabeled αIIb TM peptide. One set of signals corresponds to the monomeric signals (A). The signal intensity ratios of monomeric-to-heterodimeric signals, but not their positions, depend on the αIIb and β3 peptide concentrations, demonstrating slow exchange kinetics on the NMR timescale between the 2 species. As evidenced by the disappearance of the monomer signals at higher peptide-to-bicelle ratios (C), heterodimerization is predominant. (D) In the presence of mutant αIIb(R995A) peptide, heterodimerization is weakened. The signal intensity ratio of heterodimer-to-monomer signals drops from 1.56 to 0.14 (C,D), and the heterodimeric signals are shifted compared with the interaction with wild-type αIIb peptide. (E) The effects of charge reversal mutations in the membrane-proximal regions of αIIb or β3 on the TMD interaction were analyzed, as in Figure 1.
To assess whether αIIb(R995)-β3(D723) electrostatic interaction also takes place in cell membranes, we introduced charge reversal mutations in these residues creating αIIb(R995D) bait and β3(D723R) prey. The combination of αIIb(R995D) bait with β3 prey, or of β3(D723R) prey with αIIb bait, resulted in marked reduction in the interaction (Figure 2E). The αβ interaction was largely rescued when a combination of αIIb(R995D) bait and β3(D723R) prey was used (Figure 2E). Thus, both NMR in synthetic membranes and affinity capture in mammalian cell membranes confirm the importance of the αIIb(R995)-β3(D723) interaction in stabilizing the association of the αIIbβ3 TMD-tails. Our previous inability to detect specific αIIb(Arg995)-β3(Asp723) contacts in aqueous solution13 indicates their weakness in the absence of a lipid milieu that provides a lowered dielectric constant and reduced solvent water concentration.
Talin binding to the β3 tail disrupts the interaction of the αIIb and β3 TMD-tails
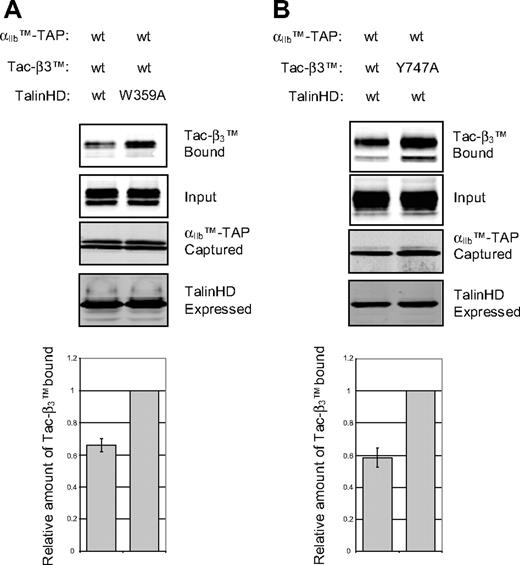
Talin binding to integrin β cytoplasmic domains is a final step in integrin activation.30,31 Transfection of cells with the talin head domain (THD) inhibits Förster resonance energy transfer (FRET) between donors and acceptors fused to the cytoplasmic tails of αL and β2 integrins,6 indicating a change in the orientation and/or distance between the tails. Having developed a direct measure of the association of the integrin α and β TMD-tails, we asked whether talin binding can inhibit the interaction. THD reduced the αIIbTM-TAP-Tac-β3TM interaction by 40% relative to a β3 binding-defective mutant THD, THD(W395A)32 (Figure 3A). Conversely, THD did not block binding of Tac-β3TM(Y747A), a mutation that inhibits talin binding,32 to αIIbTM-TAP (Figure 3B). In the absence of THD, Tac-β3TM(Y747A) also showed increased association with αIIbTM-TAP, probably due to the presence of endogenous talin in the CHO cells (data not shown). Thus, talin binding to the β3 tail inhibits the interaction of the αIIb and β3 TMD-tails. The partial dissociation of the αβ complex by THD may be due to variation in the ratio of THD to Tac-β3TM and αIIbTM-TAP on a per cell basis in these triple transfection experiments.
Talin binding to the β3 tail inhibits the αβ TMD-tail interaction. (A) αIIbTM-TAP and Tac-β3TM were cotransfected with THD or THD(W359A) mutant into CHO cells, and their interaction was analyzed, as described in Figure 1C. Ratio of the amount of Tac-β3TM bound to αIIbTM-TAP in presence of wild-type THD to that in the presence of THD W359A is shown as a bar graph. The data are the mean ± SE of 4 experiments. (B) αIIbTM-TAP and THD were cotransfected with Tac-β3TM or Tac-β3TM(Y747A) mutant into CHO cells and their interaction was analyzed, as described in panel A. The data are mean ± SE of 3 experiments.
Talin binding to the β3 tail inhibits the αβ TMD-tail interaction. (A) αIIbTM-TAP and Tac-β3TM were cotransfected with THD or THD(W359A) mutant into CHO cells, and their interaction was analyzed, as described in Figure 1C. Ratio of the amount of Tac-β3TM bound to αIIbTM-TAP in presence of wild-type THD to that in the presence of THD W359A is shown as a bar graph. The data are the mean ± SE of 4 experiments. (B) αIIbTM-TAP and THD were cotransfected with Tac-β3TM or Tac-β3TM(Y747A) mutant into CHO cells and their interaction was analyzed, as described in panel A. The data are mean ± SE of 3 experiments.
It is notable that mutations in the αIIb GXXXG motif, located near the outer leaflet of the membrane, and mutations that disrupt the salt bridge or talin binding, which would act near the inner leaflet of the membrane, both decrease the TMD-tail association. These results raise the intriguing possibility that both outer membrane interaction (including the αIIb GXXXG motif) and inner membrane interaction (involving αIIb(F992,933 and R995) and β3(D723) are required for stabilization of the TMD interface and the inactive state of the integrin.
The TMD-tail of αIIb or β3 activates integrin αIIbβ3
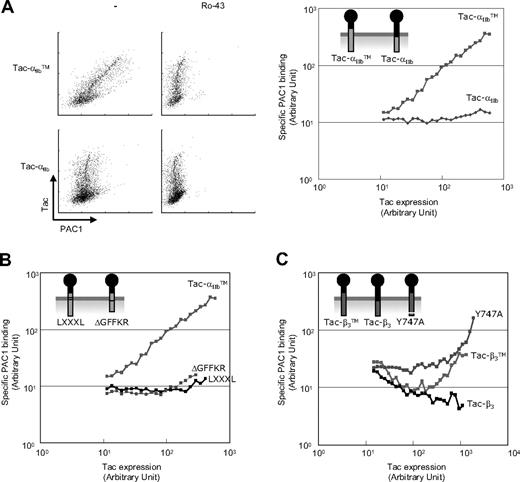
We reasoned that should these TMD-tails interact with native αIIbβ3, then they might disrupt the interaction of the endogenous αIIb and β3, leading to integrin activation. Indeed, overexpression of Tac-αIIbTM induced a dose-dependent increase in the binding of an activation-specific anti-αIIbβ3, PAC1,33 whereas a construct lacking the αIIb TMD (Tac-αIIb) exhibited no such effect (Figure 4A). The Tac-αIIbTM failed to activate α5β1 (Figure S3), supporting the integrin class specificity of interactions of integrin TMDs.34 Additional evidence for specificity of this effect was provided by mutants that disrupt the TMD-tail interaction of integrin αIIb and β3; Tac-αIIbTM constructs in which we deleted the GFFKR motif or mutated the 2 Gly in the GXXXG motif failed to activate αIIbβ3 (Figure 4B).
TMD-tail mini-integrins activate native integrin αIIbβ3. (A) Tac-αIIbTM or Tac-αIIb was transfected into CHO cells expressing αIIbβ3 (A5 cells). Twenty-four hours after transfection, cells were stained with PAC1 (activation-specific anti-αIIbβ3 antibody) and 7G7B6 (anti-Tac antibody) in the presence or absence of a selective inhibitor of ligand binding to αIIbβ3, Ro-43-5054, and then analyzed by flow cytometry to generate the dot plots shown (left). Geometric means of PAC1 binding in cells expressing different quantities of Tac constructs were plotted as larger red dots. Specific PAC1 binding was calculated by subtracting the geometric means of PAC1 binding in the presence of Ro-43-5054 from that in the absence of Ro-43-5054 in each range, and plotted as a line graph (right). (B) The effects of Tac-αIIbTMΔGFFKR and Tac-αIIbTMLXXXL on αIIbβ3 activation were analyzed, as in panel A. (C) The effects of Tac-β3TM, Tac-β3, and Tac-β3TM(Y747A) on αIIbβ3 activation were analyzed, as in panel A. The activating effects of each Tac constructs were tested at least 3 times, and representative data are shown.
TMD-tail mini-integrins activate native integrin αIIbβ3. (A) Tac-αIIbTM or Tac-αIIb was transfected into CHO cells expressing αIIbβ3 (A5 cells). Twenty-four hours after transfection, cells were stained with PAC1 (activation-specific anti-αIIbβ3 antibody) and 7G7B6 (anti-Tac antibody) in the presence or absence of a selective inhibitor of ligand binding to αIIbβ3, Ro-43-5054, and then analyzed by flow cytometry to generate the dot plots shown (left). Geometric means of PAC1 binding in cells expressing different quantities of Tac constructs were plotted as larger red dots. Specific PAC1 binding was calculated by subtracting the geometric means of PAC1 binding in the presence of Ro-43-5054 from that in the absence of Ro-43-5054 in each range, and plotted as a line graph (right). (B) The effects of Tac-αIIbTMΔGFFKR and Tac-αIIbTMLXXXL on αIIbβ3 activation were analyzed, as in panel A. (C) The effects of Tac-β3TM, Tac-β3, and Tac-β3TM(Y747A) on αIIbβ3 activation were analyzed, as in panel A. The activating effects of each Tac constructs were tested at least 3 times, and representative data are shown.
Expression of the free β3 cytoplasmic domain inhibited integrin activation (Figure 4C) probably due to sequestration of endogenously expressed talin.33 Addition of the β3 TMD to the cytoplasmic domain led to reversal of the inhibitory effect and integrin activation at higher levels of expression (Figure 4C), possibly resulted from combination of inhibitory effect by sequestering talin and activating effect by binding to the TMDs of the intact integrin. Furthermore, the β3(Y747A) mutation, which blocks talin binding,32 increased the ability of the β3 TMD-tail to activate integrin αIIbβ3 and reduced the inhibitory effect (Figure 4C). Thus, overexpression of the TMD tail of either αIIb or β3 activated the native integrin, and activation depended on the presence of the TMDs.
TMD-tail of αIIb interacts with native αIIbβ3 integrin by binding the β3 subunit
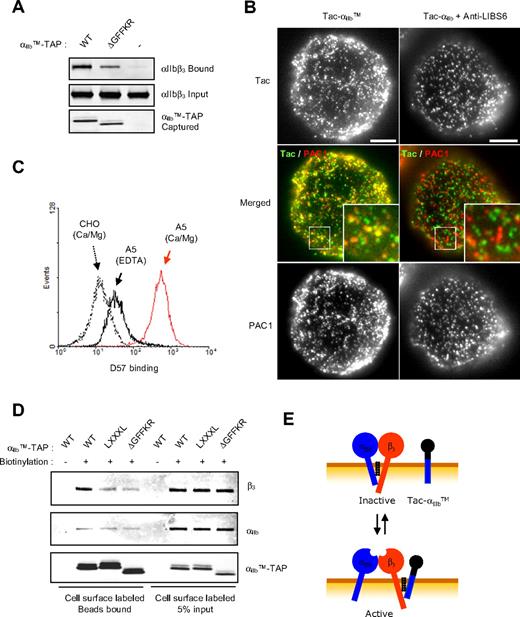
To examine the interaction of αIIb TMD-tail with native αIIbβ3, we captured the αIIbTM-TAP bait, resulting in isolation of native αIIbβ3 (Figure 5A), whereas deletion of the GFFKR motif inhibited the association (Figure 5A). We also examined the association of Tac-αIIbTM with activated αIIbβ3 in cells. Most PAC1-positive clusters of activated αIIbβ3 also contained the Tac-αIIbTM (Figure 5B). Cells transfected with Tac-αIIb, which lacks the TMDs, failed to stain with PAC1; however, when the αIIbβ3 was activated with an activating antibody, anti-LIBS6,35 Tac-αIIb was not associated with the clusters of activated integrin (Figure 5B). Thus, the αIIb TMD tail interacts with native αIIbβ3, and this physical association activates the integrin.
αIIb TMD-tail binds to the β3 subunit of native integrin αIIbβ3. (A) αIIbTM-TAP, αIIbTMΔGFFKR-TAP, or empty vector was transfected into A5 cells, and after 24 hours, cells were lysed and incubated with calmodulin beads to capture TAP constructs. β3 was detected by Western blot using anti-β3 antibody as representative of the bound αIIbβ3 (top). Expression level of β3 in lysates (middle) and captured TAP constructs (bottom) was assessed by Western blot. (B) A5 cells transfected with Tac-αIIbTM or Tac-αIIb were detached and stained with anti-Tac antibody and PAC1. In the merged image, green and red represent Tac and active integrin, respectively. The 2.7-fold digital enlargement of areas in small rectangles is shown in insets. Bar = 5 μm. (C) A5 cells were incubated for 30 minutes at 37°C with TBS (20 mM Tris, pH 8.4, 150 mM NaCl) containing either 1 mM Ca/Mg or 5 mM EDTA, and stained with αIIbβ3 complex-specific antibody (D57). D57 binding was measured by flow cytometry and plotted as histogram. (D) A5 cells transfected with TAP constructs were detached, and surface proteins were biotinylated and treated with EDTA to dissociate αIIbβ3 complex before the cells were lysed. Five percent of the lysates were incubated with neutravidin beads to determine the input of biotinylated proteins in the lysates (lanes 5-8). The remaining lysates were first incubated with calmodulin beads to capture TAP tag, and the bound proteins were eluted with 10 mM EDTA. The eluates were further incubated with NeutrAvidin beads to capture the biotinylated protein (lanes 1-4). Anti-αIIb antibody (PMI-1), anti-β3 antibody (Rb8053), and anti-FLAG antibody (M2) were used for the Western blot, as indicated. Schematic procedure for this experiment is shown in Figure S4. (E) Model of how the αIIb TMD-tail induced αIIbβ3 activation. αIIb TMD-tail interacts with TMD-tail region of β3 in the native integrin and competes for the heterodimeric interaction between αIIb and β3, resulting in rearrangement of the TMD and activation.
αIIb TMD-tail binds to the β3 subunit of native integrin αIIbβ3. (A) αIIbTM-TAP, αIIbTMΔGFFKR-TAP, or empty vector was transfected into A5 cells, and after 24 hours, cells were lysed and incubated with calmodulin beads to capture TAP constructs. β3 was detected by Western blot using anti-β3 antibody as representative of the bound αIIbβ3 (top). Expression level of β3 in lysates (middle) and captured TAP constructs (bottom) was assessed by Western blot. (B) A5 cells transfected with Tac-αIIbTM or Tac-αIIb were detached and stained with anti-Tac antibody and PAC1. In the merged image, green and red represent Tac and active integrin, respectively. The 2.7-fold digital enlargement of areas in small rectangles is shown in insets. Bar = 5 μm. (C) A5 cells were incubated for 30 minutes at 37°C with TBS (20 mM Tris, pH 8.4, 150 mM NaCl) containing either 1 mM Ca/Mg or 5 mM EDTA, and stained with αIIbβ3 complex-specific antibody (D57). D57 binding was measured by flow cytometry and plotted as histogram. (D) A5 cells transfected with TAP constructs were detached, and surface proteins were biotinylated and treated with EDTA to dissociate αIIbβ3 complex before the cells were lysed. Five percent of the lysates were incubated with neutravidin beads to determine the input of biotinylated proteins in the lysates (lanes 5-8). The remaining lysates were first incubated with calmodulin beads to capture TAP tag, and the bound proteins were eluted with 10 mM EDTA. The eluates were further incubated with NeutrAvidin beads to capture the biotinylated protein (lanes 1-4). Anti-αIIb antibody (PMI-1), anti-β3 antibody (Rb8053), and anti-FLAG antibody (M2) were used for the Western blot, as indicated. Schematic procedure for this experiment is shown in Figure S4. (E) Model of how the αIIb TMD-tail induced αIIbβ3 activation. αIIb TMD-tail interacts with TMD-tail region of β3 in the native integrin and competes for the heterodimeric interaction between αIIb and β3, resulting in rearrangement of the TMD and activation.
Consistent with the findings described above, a peptide containing a partial sequence of αIIb TMD (Trp968-Lys989) can bind and activate αIIbβ336 ; however, this effect was ascribed to a homodimeric interaction of the αIIb peptide with the full-length αIIb. To assess which subunit of full-length αIIbβ3 binds to the αIIb TMD-tail, we used EDTA to dissociate the subunits and verified the dissociation by the loss of binding of a complex-specific antibody (Figure 5C). Because the EDTA treatment can affect only cell surface–expressed integrin, we surface biotinylated cells expressing αIIbβ3 in combination with αIIbTM-TAP to exclude the intracellular integrin from this assay, treated the cells with EDTA to dissociate the αIIbβ3 on cell surface, and then captured αIIbTM-TAP with immobilized calmodulin (Figure S4). Biotinylated surface proteins were then affinity purified with NeutrAvidin beads and detected by antibody against αIIb (PMI-1) and β3 (Rb8053). We found that β3 subunit was captured by αIIbTM-TAP (Figure 5D top, lane 2), and the LXXXL and ΔGFFKR mutations markedly reduced the association (Figure 5D top, lanes 3,4). In sharp contrast, only nonspecific (as judged by equal interactions of wt, LXXXL, or ΔGFFKR baits and by the ratio of captured [Figure 5D lanes 2-4] to input of the cell surface–expressed integrin subunits [Figure 5D lanes 6-8] were observed with the αIIb subunit (Figure 5D middle panel). This result shows that the αIIbTM-TAP preferentially captures full-length integrin β3 in the presence of an equimolar amount of native full-length αIIb. Thus, the unpaired αIIb TMD-tail induces equilibrium shift in favor of activation of integrin αIIbβ3 by interacting with the β3 subunit and separating the TMD-tail interaction of the intact integrin (Figure 5E).
Discussion
Bidirectional TM integrin signaling is central to integrin-mediated biological functions. Mutational studies suggested that this signaling involves heterodimeric interactions of the TMD-tails; frustratingly, efforts to directly demonstrate these interactions produced conflicting results. In this study, we took advantage of the high efficiency and rapidity of TAP tag purification to show preferential heterodimeric interactions of the αIIb and β3 TMD-tails in mammalian cell membranes. Previous studies in E coli inner cell membranes showed primarily homodimeric interactions of the αIIb TMDs; the variance from our results is most likely ascribable to the fact that we used a full-length cytoplasmic domain and included the GFFKR motif, shown in this study to be critical for stabilization of the association of the TMD-tail mini-integrins. Similarly, previous studies in detergent micelles suggested primarily homomeric αIIb and β3 TMD-tail interactions21 ; however, phospholipid bicelles are a more accurate representation of the bulk mammalian plasma membrane because they contain lipids in a bilayer arrangement. Indeed, embedding in dodecylphosphocholine micelles distorts the helical structure of the β3 membrane-proximal domain,17 a region important in regulating integrin signaling. The remarkable agreement of biochemical and biophysical methods in identifying heterodimeric associations, combined with previous mutational data, provides compelling evidence for preferential heterodimeric TMD-tail interactions of αIIbβ3 in the lipid environment and the mammalian cell membrane. Furthermore, we now provide direct proof that talin binding disrupts the TMD-tail association and that an αIIb(R995)β3(D723) interaction stabilizes it. Moreover, the new approaches described in this study now enable analysis of TMD-tail interactions among many type I membrane proteins, such as tyrosine kinase growth factor receptors, immunoreceptors, or cytokine receptors that transduce signals as homo- and heteromultimers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (Bethesda, MD) to M.H.G. (HL70784 and AR27214) and T.S.U. (HL089726). C.K. and T.-L.L. are recipients of postdoctoral fellowships from the American Heart Association (Dallas, TX).
National Institutes of Health
Authorship
Contribution: C.K. designed and performed the mammalian cell experiments and wrote the paper; T.-L.L. designed and performed the NMR experiments; T.S.U. designed the NMR experiments and edited the paper; and M.H.G. designed the mammalian cell experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Mark H. Ginsberg, Department of Medicine, University of California San Diego, 9500 Gilman Dr, La Jolla, CA 92093; e-mail: mhginsberg@ucsd.edu.