Abstract
Chronic graft-versus-host disease (cGVHD) is a limiting factor in allogeneic hematopoietic stem cell transplantation (alloHSCT) for the treatment of leukemia and other malignancies. Relative to the process that initiates and promotes cGVHD, the regulation is poorly understood. In this study, we examined the role of naturally occurring regulatory dendritic cells (DCregs) in murine major histocompatibility complex (MHC)-compatible and multiple minor histocompatibility antigen (miHAg)–incompatible model of cGVHD in alloHSCT. DCregs generated from bone marrow in vitro (BM-DCregs) exclusively expressed CD200 receptor 3 (CD200R3), which exerted a suppressive function in the Ag-specific CD4+ T-cell response. CD49+CD200R3+ cells showed similarities in phenotype and function to BM-DCregs, which formally distinguishes them from other leukocytes, suggesting that they are the natural counterpart of BM-DCregs. Treatment of the recipient mice after alloHSCT with the recipient-type CD49+CD200R3+ cells as well as BM-DCregs protected against cGVHD, and the protection was associated with the generation of Ag-specific anergic CD4+ T cells as well as CD4+CD25+Foxp3+ regulatory T cells (Tregs) from donor-derived alloreactive CD4+CD25−Foxp3− T cells. In addition, the depletion of CD49+CD200R3+ cells before alloHSCT enhanced the progression of cGVHD. In conclusion, CD49+CD200R3+ cells act as naturally occurring DCregs to regulate the pathogenesis of cGVHD in alloHSCT mediated through the control of the transplanted alloreactive CD4+ T cells.
Introduction
Allogeneic hematopoietic stem cell transplantation is a curative therapy for a variety of hematologic, neoplastic, and genetic disorders.1-3 However, the application of alloSCT has been limited by the morbidity and mortality of graft-versus-host disease (GVHD).1-3 GVHD is caused by donor T cells that recognize and react to major histocompatibility complex (MHC) and/or minor histocompatibility antigen (miHAg) differences between donor and recipient. Based on different clinical manifestations and histopathology, GVHD can be divided into acute and chronic types.3-5 Acute GVHD (aGVHD) targets the skin, gastrointestinal tract, and liver.3-5 Chronic GVHD (cGVHD) typically results in cutaneous and hepatic fibrosis.3-5 Although aGVHD and cGVHD are similar in some respects, it seems probable that the 2 diseases have different requirements for initiation and pathogenesis mechanisms. Previous studies have suggested that residual host Ag-presenting cells (APCs) are required to initiate aGVHD mediated by donor-derived CD8+ T cells, whereas either donor or host APCs initiated donor-derived CD4+ T cell–mediated cGVHD in murine allogeneic bone marrow (BM) transplantation (alloBMT).4,5 Although progress has been made in understanding the pathophysiology and the potential curative therapy of aGVHD, much less is understood about the effective therapy for cGVHD, primarily because of the obscurity of regulation of the pathogenesis of cGVHD.
Dendritic cells (DCs) are specialized APCs that play a dual role in inducing adaptive immune responses and in maintaining self-tolerance.6-8 DCs consist of heterogeneous subsets, including conventional DCs and plasmacytoid DCs, distinguishable by surface and intracellular phenotypic markers, immunologic function, and anatomic distribution.6 Immature DCs (iDCs) serve as sentinels, recognizing the presence of invading pathogens through various pattern-recognition receptors, and become mature DCs (mDCs) with the up-regulated expression of MHC and costimulatory molecules under inflammatory conditions.6 Consequently, mDCs move via the afferent lymphatics into the T-cell area of secondary lymphoid tissues, where they select rare Ag-specific naive T cells and induce their activation and differentiation into effector cells, thereby initiating primary immune responses.6
Previous studies have shown that tolerogenic DCs generated by the treatment of iDCs with certain immunosuppressive molecules in vitro and their potential natural counterpart CD11clowCD45RBhigh DCs prevented an immune response through the induction of interleukin-10 (IL-10)–producing T regulatory type 1 cells and expansion of naturally occurring thymic-derived CD4+CD25+Foxp3+ regulatory T cells (nTregs).9,10 However, the specialized DC subset that drives the conversion of CD4+CD25−Foxp3− T cells to inducible CD4+CD25+Foxp3+ Tregs (iTregs) remains unclear.11
We have previously reported that regulatory DCs (DCregs) generated from murine BM cells in vitro (BM-DCregs), with a moderately high level of MHC class II but defective in the expression of costimulatory molecules, had a greater capacity to protect mice from various immunopathogenic diseases, including aGVHD and cGVHD, than the classic tolerogenic DCs, mediated through the generation of Ag-specific anergic CD4+ T cells and CD4+CD25+Foxp3+ iTregs from CD4+CD25−Foxp3− T cells.12,13 In the present study, we explored the natural counterpart of BM-DCregs expressing specific markers and the possible role of naturally occurring DCregs in cutaneous cGVHD in MHC-compatible and multiple miHAg-incompatible alloBMT.
Methods
Mice
BALB/c (H-2d) and C57BL/6 (H-2b) mice were purchased from Charles River Breeding Laboratories (Portage, MI). B10.D2 mice (H-2d) were purchased from Jackson Laboratory (Bar Harbor, ME). Ovalbumin (OVA)-specific T-cell receptor (TCR; KJ1-26 clonotype) transgenic Rag2+/+ DO11.10 BALB/c and Rag2−/− DO11.10 BALB/c mice have been described.13,14 All mice were used between 6 and 10 weeks of age and maintained in specific pathogen–free conditions and in accordance with guidelines of the Institutional Animal Care Committee of the RIKEN Institute.
Preparation of DC subpopulations and leukocytes
DCs generated from murine BM cells in vitro (BM-DCs) were prepared as described previously.12,13 Briefly, BM-iDCs were generated by culturing BM cells with murine granulocyte-macrophage colony-stimulating factor (20 ng/mL; Wako Pure Chemicals, Osaka, Japan) for 8 days subsequently stimulated with lipopolysaccharide (LPS, 1 μg/mL; Sigma-Aldrich, St Louis, MO) for 24 hours to prepare BM-mDCs. BM-DCregs precursors (BM-preDCregs) were generated by culturing BM cells with murine granulocyte-macrophage colony-stimulating factor (20 ng/mL), murine IL-10 (20 ng/mL, Wako Pure Chemical Industries), and human transforming growth factor (TGF)-β1 (20 ng/mL, Wako Pure Chemical Industries) for 8 days. After being stimulated with LPS (1 μg/mL) for 24 hours, BM-preDCregs were depleted of CD40+CD80+CD86+ cells with biotinylated monoclonal antibodies (mAbs) to CD40 (3/23), CD80 (16-10A1), and CD86 (GL1; all from BD Biosciences, San Jose, CA) plus Streptavidin Particles Plus-DM (BD Biosciences) to prepare BM-DCregs. CD3+ cells, B220+ cells, or CD11b+ cells were purified from splenocytes with mouse T Lymphocyte Enrichment Set-DM, mouse B Lymphocyte Enrichment Set-DM, or mouse CD11b Magnetic Particles-DM, respectively (all from BD Biosciences). Leukocytes collected from spleen, peripheral blood, and mesenteric lymph nodes (MLNs) were also depleted of FcϵRIα+ basophils15 with biotinylated anti-FcϵRIα mAb (MAR-1, eBioscience, San Diego, CA) plus Streptavidin Particles Plus-DM. CD49b+ FcϵRIα− cells were purified from splenocytes by AutoMACS with a mouse NK Cell Isolation Kit (all from Miltenyi Biotec, Bergisch Gladbach, Germany). These cells were subsequently labeled with biotinylated anti-CD200R3 mAb (6C4H2) plus streptavidin-phycoerythrin (PE; BD Biosciences), and then sorted by AutoMACS with anti-PE Microbeads (Miltenyi Biotec) into CD49b+CD200R3+ cells or CD49b+CD200R3− cells. For the preparation of splenic classic NK cells, CD49b+CD200R3− cells were further depleted of CD11c+ cells using biotinylated anti-CD11c mAbs (HL3, BD Biosciences) plus Streptavidin Particles Plus-DM. Similarly, BM cells were sorted by FACSVantage (BD Biosciences) with allophycocyanin-labeled anti-CD49b mAb (HMα2, BD Biosciences) and PE-labeled anti-FcϵRIα mAb (MAR-1, eBioscience) into CD49b+ FcϵRIα− cells and CD49b+ FcϵRIα+ basophils, respectively. CD11c+ cells were purified from splenocytes by AutoMACS with mouse CD11c (N418) Microbeads (Miltenyi Biotec). In some experiments, cells were pulsed with OVA323-339 peptide (OVAp; 10 μM) for 4 hours, washed, and used for subsequent experiments. KJ1-26+ T cells were purified from splenocytes obtained from Rag2+/+ DO11.10 BALB/c and Rag2−/− DO11.10 BALB/c mice with mouse CD4 T Lymphocyte Enrichment Set-DM (BD Biosciences). Subsequently, KJ1-26+ T cells were sorted into CD25− T cells and CD25+ T cells with high purity (> 98%) by AutoMACS with a mouse CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec).
Semiquantitative RT-PCR
Total RNA from cells (106) was extracted with TRIzol (Invitrogen, Carlsbad, CA), and cDNA was synthesized with oligo(dT)20 as a primer using the SuperScript III First-Strand Synthesis System for reverse-transcribed polymerase chain reaction (RT-PCR) kit (Invitrogen). PCR analysis was performed using AccuPower PCR PreMix (BIONEER, Daejeon, Korea) with a pair of specific primers for Cd200r1 (5′-atg ttt tgc ttt tgg aga-3′ and 5′-cta gat tcc aat ggc cga-3′), Cd200r2 (5′-atg cat gct ttg ggg agg-3′ and 5′-tca tgt tct ggt aac att-3′), Cd200r3 (5′-atg cat gct ttg ggg agg-3′ and 5′-cta cgt agg agc caa tgt-3′), Cd200r4 (5′-atg cat gct ctg ggg agg-3′ and 5′-tca tgt tct ggc aaa att-3′), Gzmb (5′-aca aca tca aag aac agg aga aga c-3′ and 5′-gct ttt cat tgt ttt ctt tat cca g-3′), Mcpt8 (5′-ggc ata cat aag gtt taa tga cag c-3′ and 5′-agt cac cct tac cag tag cct tat t-3′), Tgfb1 (5′-cca tct atg aga aaa cca aag aca t-3′ and 5′-gag ctg aag caa tag ttg gta tcc-3′), and Actb (5′-tcc tgt ggc atc cat gaa act-3′ and 5′-gaa gca ctt gcg gtg cac gat-3′). Conditions for the reactions were 30 seconds of denaturation at 94°C, 30 seconds of annealing at 58°C, and 1 minute of elongation at 72°C for 25 cycles (Actb) or 30 cycles (Cd200r1, Cd200r2, Cd200r3, Cd200r4, Gzmb, Mcpt8, Tgfb1).
Quantitative TaqMan RT-PCR
cDNA was synthesized with random hexamers as a primer as described in “Semiquantitative RT-PCR”. Expression levels of Cd200r1, Cd200r2, Cd200r3, and Cd200r4 were measured by real-time PCR (7300 Real Time PCR System, Applied Biosystems, Foster City, CA) using TaqMan universal PCR Master Mix (Applied Biosystems) and TaqMan probes mix for Cd200r1, Cd200r2, Cd200r3, and Cd200r4 (Applied Biosystems) after normalization for the expression of Actb (Applied Biosystems).
Retrovirus-mediated gene transfer
The full-length Cd200, Cd200r1, Cd200r2, Cd200r3, and Cd200r4 were amplified by PCR as described in “Semiquantitative RT-PCR” with a pair of specific primers for Cd200 (5′-cgc gga tcc gcc gcc atg ggc agt ctg gta-3′ and 5′-cgc gaa ttc tta ttt cat tct ttg cat-3′), Cd200r1 (5′-cgc gga tcc gcc gcc atgt ttt gct ttt gg-3′ and 5′-cgc gga tcc cta gat tcc aat ggc cga-3′), Cd200r2 (5′-tc gga tcc gcc gcc atg cat gct ttg ggg agg act ccg-3′ and 5′-tc gaa ttc atg ttc tgg taa cat ttc tct tc-3′), Cd200r3 (5′-cgc gga tcc gcc gcc atg cat gct ttg ggg-3′ and 5′-cgc gga tcc cta cgt agg agc caa tgt-3′), and Cd200r4 (5′-tc gga tcc gcc gcc atg cat gct ctg ggg agg att ccg-3′ and 5′-tc gaa ttc atg ttc tgg caa aat ttc tct tc-3′), and each of the 5′- and 3′-primers was also tagged (indicated in italics) with BamH1 and EcoR1sites for Cd200, Cd200r2, and Cd200r4 or a BamH1 site for Cd200r1 and Cd200r3, respectively. The PCR product was subcloned into pCR4-TOPO using a TA TOPO Cloning Kit for Sequencing (Invitrogen), and the nucleotide sequence was confirmed with an ABI3100xl automated sequencer (Applied Biosystems) and the fluoresceinated dye terminator cycle sequencing method. After restriction enzyme digestion, the DNA sequence was cloned into the sites of the pMX-internal ribosome entry site-green fluorescent protein (GFP) vector16 and transfected into a retroviral packaging cell line, Phoenix,16 with LipofectAMIN Plus Reagent (Invitrogen). The culture supernatant of Phoenix after 24 hours of culture was collected and centrifuged at 8000g for 16 hours at 4°C to concentrate the virus. The retroviral vector was transfected into RBL2H3 cells together with DOTAP Liposomal Transfection Reagent (Roche Diagnostics, Mannheim, Germany) by centrifugation at 2000g for 1 hour at 32°C, and transfectants were subsequently collected for examining GFP expression using FACSVantage. Similarly, BM-iDCs were spin-infected with supernatants containing retrovirus carrying CD200R3-internal ribosome entry site-GFP mixed with polybrene (1 μg/mL; Sigma-Aldrich) on days 2, 3, and 4 according to a previous report.17 Subsequently, cells were assayed for the expression CD200R3 and GFP and sorted into CD200R3+GFP+ and CD200R3−GFP+BM-iDCs using FACSVantage.
Flow cytometry
Cells were stained with fluorescein-conjugated mAbs to mouse CD3ϵ (145-2C11), CD11b (M1/70), CD11c (HL3), CD40 (3/23), CD49b (HMα2), CD80 (16-10A1), CD86 (GL1), H-2Kd (SF1-1.1), I-A/I-E (M5/114.15.2), B220 (RA3-6B2), Thy1.2 (30-H2), isotype-matched control mAb (all from BD Biosciences), Foxp3 (FJK-16s; eBioscience), CD25 (7D5), mPDCA-1 (JF05-1C2.4.1; both from Miltenyi Biotec), and mouse DO11.10 TCR (KJ1-26; Invitrogen). In another experiment, cells were stained with biotinylated mAbs to CD200R1 (13H6E3), CD200R2 (B2C2F3), CD200R3 (6C4H2), and CD200R4 (8B3C9), which were generated in our laboratory as described in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), plus streptavidin-PE. Fluorescence staining was analyzed with a FACSCalibur flow cytometer and CELLQuest Software (both from BD Biosciences).
Priming of CD4+ T cells in vitro
Rag2−/−KJ1-26+ T cells (2 × 106) were cultured with the irradiated (15 Gy from a 137Cs source, Gammacell 40 Exactor; MDS Sciex, Concord, ON) OVAp/APCs (2 × 105) in the presence or absence of human TGF-β1 (0.1 ng/mL), anti-TGF-β mAb (1D11, 5 μg/mL), anti-CD200R3 mAb (6A5A8-F7, 5 μg/mL), or control Ig (5 μg/mL) in 6-well flat plates (BD Biosciences) for 6 days, and used in subsequent experiments.
APC assay
Rag2−/−KJ1-26+ T cells (5 × 104) were cultured in 96-well flat-bottomed plates (BD Biosciences) with the irradiated syngeneic OVAp/APCs (6.25 × 102-5 × 103) in the presence or absence of anti-CD200R3 mAb (6A5A8-F7) or control Ig (each 5 μg/mL) for 3 days. We also used irradiated syngeneic hemolyzed splenocytes (5 × 104) cultured in the presence of OVAp (1 μM). Alternatively, huIgFc, CD200ext-huIgFc, CD200R1ext-huIgFc, CD200R2ext-huIgFc, CD200R3ext-huIgFc, CD200R4ext-huIgFc, or CTLA-4ext-huIgFc (R&D Systems, Minneapolis, MN; 5 μg/mL) was added to the culture. [3H]Thymidine (GE Healthcare, Little Chalfont, United Kingdom) incorporation was measured on day 3 for the last 18 hours.
Cell stimulation
CD11c+DCs, CD49b+CD200R3+ cells or CD49b+CD200R3− cells (5 × 105) were cultured with or without LPS (1 μg/mL) or CpG oligodeoxynucleotide (ODN) 1668 (0.1 μM; Hokkaido System Science, Sapporo, Japan) for 24 hours in 24-well plates (BD Biosciences). The culture supernatants were collected and stored at −80°C until assayed for cytokines or morphology was examined by phase-contrast microscopy.
Enzyme-linked immunosorbent assay
Culture supernatants and serum were assayed using enzyme-linked immunosorbent assay kits for mouse IL-12p70, tumor necrosis factor (TNF)-α, IL-10, interferon (IFN)-α, and IFN-γ (all from BioSource International, Camarillo, CA).
Cytotoxicity assay
CD11c+DCs, CD49b+CD200R3− cells, or CD49b+CD200R3+ cells (6.25 × 103-5 × 104) were cultured in 96-well round bottom plates (BD Biosciences) with Na251CrO4 (NEN; Life Science Products, Frederick, CO)-labeled YAC-1 cells (5 × 103) for 4 hours. Radioactivity of the supernatants was measured, and the percentage specific lysis was calculated. The value of spontaneous release was less than 10% of the total release.
Models for cGVHD and the evaluation
The induction and the evaluation of cGVHD were performed according to previous reports4,5 with some modifications.13 In brief, recipient BALB/c mice (10 per group) received a single dose (800 cGy) of lethal total body irradiation (TBI). Three hours after the TBI, all recipients received T cell–depleted BM cells (107/mouse) and CD4+CD25− T cells (2 × 106/mouse) obtained from B10.D2 mice through a tail vein. The day of transplantation was designated day 0. Subsequently, the transplanted recipient mice received repetitive intravenous injections of BM-DCregs, CD11c+ DCs, and CD49b+CD200R3+ cells (5 × 105/mouse) obtained from BALB/c mice on days 2, 9, and 16 after transplantation. Alternatively, huIgFc or CD200R3ext-huIgFc (250 μg/mouse) was intravenously administered on days 2, 4, 6, 8, and 10. For the depletion experiments in vivo, the recipient mice were intravenously injected with anti-CD200R3 mAb (6C4H2) or control Ig (each 500 μg/mouse) on days −7, −5, −3, and −1. Recipients were monitored once every day from the day of transplantation to the indicated days after transplantation to determine the incidence and severity of cutaneous cGVHD as well as mobility, diarrhea, and weight loss. The following scoring system for cutaneous cGVHD was used: 0 indicates healthy appearance; 1, skin lesions with alopecia less than 1 cm2 in area; 2, skin lesions with alopecia 1 to 2 cm2 in area; 3, skin lesions with alopecia more than 2 cm2 in area. Incidence was expressed as the percentage of mice that showed clinical manifestations. In another experiment, recipients were killed on day 30 after allogeneic transplantation to obtain spleen and serum.
Adoptive transfer
Generation of CD4+CD25+Foxp3+ Tregs from CD4+CD25−Foxp3− T cells in vivo was performed according to previous reports.13 In brief, Rag2−/−KJ1-26+ T cells (107/mouse) were intravenously transferred with or without OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (106/mouse) into BALB/c mice. After 8 days, CD4+ T cells were purified from the recipient mice as described in “Preparation of DC subpopulations and leukocytes,” and the expression of CD25 and Foxp3 among gated KJ1-26+ T cells was analyzed by flow cytometry. In another experiment, Rag2−/−KJ1-26+ T cells and Rag2−/−KJ1-26+CD25+ T cells were isolated from CD4+ T cells and used for subsequent experiments.
Analysis of Treg function
CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with the irradiated CD11c+ DCs (5 × 103) obtained from BALB/c mice in the presence or absence of CD4+CD25+ T cells (6.25 × 103-5 × 104) obtained from B10.D2 mice or the transplanted recipient mice in 96-well round bottom plates. Similarly, Rag2−/−KJ1-26+ T cells (2 × 104) were cultured with the irradiated splenocytes (2 × 104) plus anti-CD3ϵ mAb (1 μg/mL) in the presence or absence of Rag2+/+KJ1-26+CD25+ T cells obtained from Rag2+/+ DO11.10 BALB/c mice or Rag2−/−KJ1-26+CD25+ T cells obtained from the adoptively transferred mice (2.5 × 103-2 × 104) in 96-well round bottom plates. The proliferation was evaluated on day 3 based on [3H]thymidine incorporation.
Statistical analysis
Data are the mean plus or minus SEM. All analyses for statistically significant differences were performed with analysis of variance. P values less than .01 were considered significant.
Results
Identification of CD200R3 as a specific functional molecule for BM-DCregs
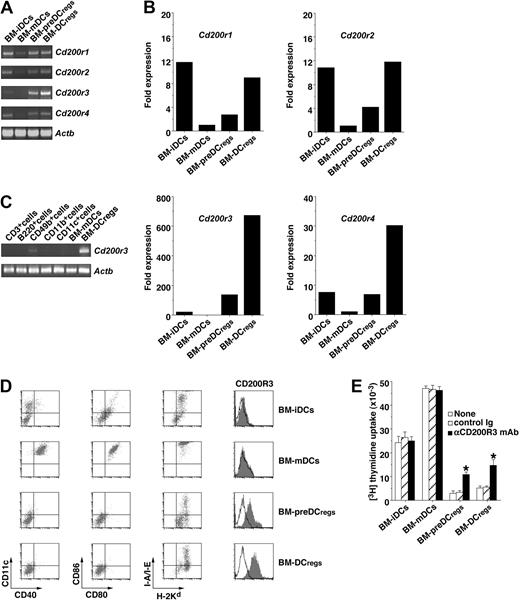
To identify the molecule specifically expressed on BM-DCregs, we screened the transcripts of the potential immunoregulatory cell surface molecules increased in BM-DCregs compared with BM-mDCs, and identified CD200R family members as candidates (Figure 1A-C). Transcription of Cd200r3, but not other Cd200rs, was specifically up-regulated in both BM-DCregs and BM-preDCregs compared with both BM-iDCs and BM-mDCs (Figure 1A-C). To analyze the cell surface expression of CD200Rs, we generated mAbs, which specifically recognized each CD200R (Figure S1). BM-preDCregs and BM-DCregs exclusively expressed CD200R3, whereas BM-iDCs and BM-mDCs did not express this molecule (Figure 1D).
Identification of CD200R3 as a specific molecule for BM-DCregs. (A-C) The transcriptional expression of Cd200rs in BM-DCs (A,B) and leukocytes (C) was measured by RT-PCR (A,C) and real-time PCR (B). The results of RT-PCR for Actb demonstrate the loading of equal amounts of DNA on the gel (A,C). The expression of Cd200rs determined by real-time PCR was normalized to that of Actb, and the data are as the comparative fold increase compared with BM-mDCs (B). (D) The expression of cell surface molecules on BM-DCs was analyzed by flow cytometry, and data are represented by a dot plot and a histogram. Data are representative of 4 replicate experiments (A-D). (E) Rag2−/−KJ1-26+ T cells (5 × 104) were cultured with the indicated BM-DCs (5 × 103) in the presence or absence of anti-CD200R3 mAb or control Ig (each 5 μg/mL) for 3 days, and the proliferative response was measured. *P < .01 compared with each of the BM-DCs alone. Data are mean ± SEM, and the results are combined from 4 replicate experiments.
Identification of CD200R3 as a specific molecule for BM-DCregs. (A-C) The transcriptional expression of Cd200rs in BM-DCs (A,B) and leukocytes (C) was measured by RT-PCR (A,C) and real-time PCR (B). The results of RT-PCR for Actb demonstrate the loading of equal amounts of DNA on the gel (A,C). The expression of Cd200rs determined by real-time PCR was normalized to that of Actb, and the data are as the comparative fold increase compared with BM-mDCs (B). (D) The expression of cell surface molecules on BM-DCs was analyzed by flow cytometry, and data are represented by a dot plot and a histogram. Data are representative of 4 replicate experiments (A-D). (E) Rag2−/−KJ1-26+ T cells (5 × 104) were cultured with the indicated BM-DCs (5 × 103) in the presence or absence of anti-CD200R3 mAb or control Ig (each 5 μg/mL) for 3 days, and the proliferative response was measured. *P < .01 compared with each of the BM-DCs alone. Data are mean ± SEM, and the results are combined from 4 replicate experiments.
We examined the role of CD200R3 in the ability of BM-preDCregs and BM-DCregs to stimulate Ag-specific CD4+ T cells using Rag2−/−OVA-specific TCR (KJ1-26 clonotype) transgenic mice (DO11.10 mice), which lack CD25+Foxp3+ nTregs (Rag2−/−KJ1-26+ T cells).13,14 The ability of OVAp-pulsed BM-preDCregs (OVAp/BM-preDCregs) and OVAp/BM-DCregs to stimulate Rag2−/−KJ1-26+ T cells was much less than that of OVAp/BM-iDCs or OVAp/BM-mDCs (Figure 1E). Anti-CD200R3 mAb partly abrogated the reduced activation of Rag2−/−KJ1-26+ T cells by OVAp/BM-preDCregs and OVAp/BM-DCregs (Figure 1E).
To address the immunoregulatory function of CD200R3, we fused the extracellular domain of CD200R3 with human IgFc (CD200R3ext-huIgFc). Although CD200ext-huIgFc bound to CD200R1-GFP-RBL2H3 cells and CD200R2-GFP-RBL2H3 cells to a lesser degree, it did not bind to CD200R3-GFP-RBL2H3 cells or CD200R4-GFP-RBL2H3 cells (Figure S2A top panel), and similar results were observed when the binding of each fusion protein to CD200-GFP-RBL2H3 cells was tested (Figure S2A bottom panel). On the other hand, CD200R3ext-huIgFc as well as CD200ext-huIgFc and CTLA-4ext-huIgFc, but not other CD200Rsext-huIgFc, suppressed the activation of Rag2−/−KJ1-26+ T cells by OVAp/BM-mDCs (Figure S2B).
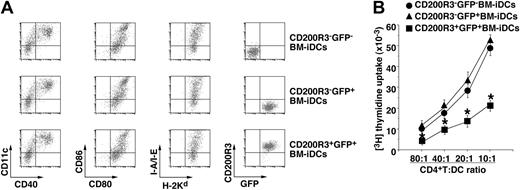
We also examined the effect of the ectopic expression of CD200R3 on the ability of BM-iDCs to stimulate Rag2−/−KJ1-26+ T cells. OVAp/CD200R3+GFP+ BM-iDCs generated by retroviral transfection showed the reduced activation of Rag2−/−KJ1-26+ T cells compared with OVAp/CD200R3−GFP−BM-iDCs and OVAp/CD200R3−GFP+BM-iDCs despite the similar levels of MHC and costimulatory molecules (Figure 2). These results indicate that CD200R3 acts as a suppressive molecule in the Ag-specific response of CD4+ T cells.
Suppressive function of CD200R3 expressed on DCs in the Ag-specific CD4+ T-cell response. (A) The expression of cell surface molecules on retroviral transfected BM-iDCs was analyzed by flow cytometry, and data are represented by a dot plot. Data are representative of 4 replicate experiments. (B) Rag2−/−KJ1-26+ T cells (5 × 104) were cultured with OVAp/CD200R3−GFP−BM-iDCs, OVAp/CD200R3−GFP+BM-iDCs, or OVAp/CD200R3+GFP+BM-iDCs (6.25 × 102-5 × 103) for 3 days, and the proliferative response was measured. * P < .01 compared with OVAp/CD200R3−GFP−BM-iDCs. Data are mean ± SEM, and the results are combined from 4 replicate experiments.
Suppressive function of CD200R3 expressed on DCs in the Ag-specific CD4+ T-cell response. (A) The expression of cell surface molecules on retroviral transfected BM-iDCs was analyzed by flow cytometry, and data are represented by a dot plot. Data are representative of 4 replicate experiments. (B) Rag2−/−KJ1-26+ T cells (5 × 104) were cultured with OVAp/CD200R3−GFP−BM-iDCs, OVAp/CD200R3−GFP+BM-iDCs, or OVAp/CD200R3+GFP+BM-iDCs (6.25 × 102-5 × 103) for 3 days, and the proliferative response was measured. * P < .01 compared with OVAp/CD200R3−GFP−BM-iDCs. Data are mean ± SEM, and the results are combined from 4 replicate experiments.
Identification of naturally occurring DCregs
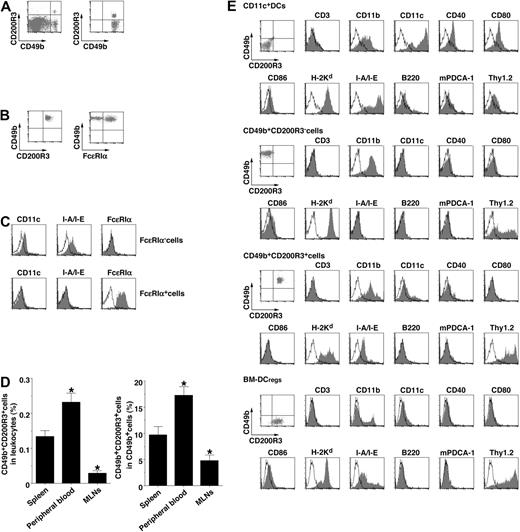
To clarify the naturally occurring DCregs expressing CD200R3 in vivo, we performed a cytofluoremetric analysis of splenocytes. Approximately 0.2% of splenocytes expressed CD200R3 (Figure 3A). In accordance with the transcriptional expression (Figure 1C), CD49b+ cells expressed CD200R3 (Figure 3A), whereas other leukocytes did not express this molecule (data not shown).
Phenotypic identification of naturally occurring CD49b+ CD200R3+ DCregs. (A) The expression of CD49b and CD200R3 on splenocyes (left panel) and among gated CD49b+ cells (right panel) was analyzed by flow cytometry, and data are represented by a dot plot. (B) The expression of FcϵRIα on CD49b+CD200R3+ cells purified from splenocyes was analyzed by flow cytometry, and data are represented by a dot plot. (C) The expression of CD11c, I-A/I-E, and FcϵRIα among gated FcϵRIα− cells and FcϵRIα+ cells in CD49b+CD200R3+ cells purified from splenocyes was analyzed by flow cytometry, and data are represented by a histogram. Data are representative of 4 replicate experiments (A-C). (D) CD49b and CD200R3 on leukocytes (left panel) and CD49b+ cells (right panel) from spleen, peripheral blood, and MLNs depleted of FcϵRIα+ cells were analyzed by flow cytometry, and data are percentage positive cells. *P < .01 compared with splenocytes. Data are mean ± SEM, and the results are combined from 4 replicate experiments. (E) The expression of cell surface molecules on CD11c+ DCs, CD49b+CD200R3− cells, CD49b+CD200R3+ cells, and BM-DCregs was analyzed by flow cytometry, and data are represented by a dot plot and a histogram. Data are representative of 4 replicate experiments.
Phenotypic identification of naturally occurring CD49b+ CD200R3+ DCregs. (A) The expression of CD49b and CD200R3 on splenocyes (left panel) and among gated CD49b+ cells (right panel) was analyzed by flow cytometry, and data are represented by a dot plot. (B) The expression of FcϵRIα on CD49b+CD200R3+ cells purified from splenocyes was analyzed by flow cytometry, and data are represented by a dot plot. (C) The expression of CD11c, I-A/I-E, and FcϵRIα among gated FcϵRIα− cells and FcϵRIα+ cells in CD49b+CD200R3+ cells purified from splenocyes was analyzed by flow cytometry, and data are represented by a histogram. Data are representative of 4 replicate experiments (A-C). (D) CD49b and CD200R3 on leukocytes (left panel) and CD49b+ cells (right panel) from spleen, peripheral blood, and MLNs depleted of FcϵRIα+ cells were analyzed by flow cytometry, and data are percentage positive cells. *P < .01 compared with splenocytes. Data are mean ± SEM, and the results are combined from 4 replicate experiments. (E) The expression of cell surface molecules on CD11c+ DCs, CD49b+CD200R3− cells, CD49b+CD200R3+ cells, and BM-DCregs was analyzed by flow cytometry, and data are represented by a dot plot and a histogram. Data are representative of 4 replicate experiments.
Recent studies have shown that CD200R3 was also expressed on FcϵRIα+ basophils.15,18 CD49b+CD200R3+ cells purified from splenocytes consisted of FcϵRIα− cells and FcϵRIα+ basophils (Figure 3B); and CD49b+CD200R3+ FcϵRIα− cells, but not CD49b+CD200R3+ FcϵRIα+ basophils, expressed CD11c and I-A/I-E (Figure 3C). In addition, CD49b+CD200R3+ FcϵRIα− cells (referred to as CD49b+CD200R3+ cells) were found in peripheral blood as well as spleen, whereas this cell subset rarely existed in MLNs (Figure 3D) because of a low frequency of CD49b+ cells in LNs under steady-state conditions.19 CD49b+CD200R3+ cells and BM-DCregs showed similar levels of CD11c, H-2Kd, I-A/I-E, and Thy1.2 with a lack of CD3, CD40, CD80, CD86, B220, and mPDCA-1 known as a marker of plasmacytoid DCs, different from CD11c+ DCs and classic NK cells (CD3−B220−CD11c−CD49b+CD200R3− cells; Figure 3E).
Functional characterization of naturally occurring DCregs
We examined the characteristic features of CD49b+CD200R3+ cells. CD49b+CD200R3+ cells exhibited a distinct transcriptional expression of Cd200r3, Gzmb, Mcpt8,20 and Tgfb1 from CD11c+ DCs, NK cells, and basophils (Figure 4A). Stimulation with CpG ODN 1668 induced the morphologic change of CD49b+CD200R3+ cells, but not NK cells, with dendrites resembling those of CD11c+ DCs (Figure 4B). Relative to CD11c+ DCs, which secreted IL-12p70, TNF-α, IL-10, and IFN-α on activation with LPS or CpG ODN, CD49b+CD200R3+ cells produced mostly IL-10 and some proinflammatory cytokines similar to BM-DCregs in our previous reports,21,22 and NK cells did not produce much of either. NK cells efficiently killed YAC-1 cells, whereas neither CD49b+CD200R3+ cells nor CD11c+ DCs were able to kill YAC-1 cells (Figure 4D). On the other hand, OVAp/CD49b+CD200R3+ cells induced less Ag-specific activation of Rag2−/−KJ1-26+ T cells than OVAp/CD11c+ DCs, whereas OVAp/NK cells failed to activate them (Figure 4E) because of a lack of MHC class II (Figure 3E).
Functional characterization of naturally occurring CD49b+CD200R3+ DCregs. (A) The transcriptional expression of the selected genes in CD11c+ DCs, CD49b+CD200R3− cells, CD49b+CD200R3+ cells, CD49b+FcϵRIα− cells, and CD49b+FcϵRIα+ cells was measured by RT-PCR. The results of RT-PCR for Actb demonstrate the loading of equal amounts of DNA on the gel. (B) Morphology of CD11c+ DCs, CD49b+CD200R3− cells, and CD49b+CD200R3+ cells was examined by phase-contrast microscopy (400×) after stimulation with or without CpG ODN (0.1 μM) for 24 hours. Images of micrographs were acquired using a 10 × 20 eyepiece (40 × 0.55 NA objective lenses with phosphate-buffered saline) on an Olympus model CKX41 microscope (Olympus, Yokohama, Japan). An Olympus model DP12-2 camera was used along with JPEG Preview 3.0.9 software (Apple, Cupertino, CA) and Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA) to capture and process the still images. Data are representative of 4 replicate experiments (A,B). (C) CD11c+ DCs, CD49b+CD200R3− cells, or CD49b+CD200R3+ cells (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) or CpG ODN (0.1 μM) for 24 hours, and the culture supernatants were analyzed for cytokine production. *P < .01 compared with CD11c+ DCs. (D) The cytotoxicity of CD11c+ DCs, CD49b+CD200R3− cells, or CD49b+CD200R3+ cells (6.25 × 103-5 × 104) against YAC-1 cells (5 × 103) was analyzed by a 4-hour 51Cr release assay. *P < .01 compared with CD11c+ DCs. (E) Rag2−/−KJ1-26+ T cells (5 × 104) were cultured with OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (6.25 × 102-5 × 103) for 3 days, and the proliferative response was measured. *P < .01 compared with OVAp/CD11c+ DCs. Data are mean ± SEM, and the results are combined from 4 replicate experiments (C-E).
Functional characterization of naturally occurring CD49b+CD200R3+ DCregs. (A) The transcriptional expression of the selected genes in CD11c+ DCs, CD49b+CD200R3− cells, CD49b+CD200R3+ cells, CD49b+FcϵRIα− cells, and CD49b+FcϵRIα+ cells was measured by RT-PCR. The results of RT-PCR for Actb demonstrate the loading of equal amounts of DNA on the gel. (B) Morphology of CD11c+ DCs, CD49b+CD200R3− cells, and CD49b+CD200R3+ cells was examined by phase-contrast microscopy (400×) after stimulation with or without CpG ODN (0.1 μM) for 24 hours. Images of micrographs were acquired using a 10 × 20 eyepiece (40 × 0.55 NA objective lenses with phosphate-buffered saline) on an Olympus model CKX41 microscope (Olympus, Yokohama, Japan). An Olympus model DP12-2 camera was used along with JPEG Preview 3.0.9 software (Apple, Cupertino, CA) and Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA) to capture and process the still images. Data are representative of 4 replicate experiments (A,B). (C) CD11c+ DCs, CD49b+CD200R3− cells, or CD49b+CD200R3+ cells (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) or CpG ODN (0.1 μM) for 24 hours, and the culture supernatants were analyzed for cytokine production. *P < .01 compared with CD11c+ DCs. (D) The cytotoxicity of CD11c+ DCs, CD49b+CD200R3− cells, or CD49b+CD200R3+ cells (6.25 × 103-5 × 104) against YAC-1 cells (5 × 103) was analyzed by a 4-hour 51Cr release assay. *P < .01 compared with CD11c+ DCs. (E) Rag2−/−KJ1-26+ T cells (5 × 104) were cultured with OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (6.25 × 102-5 × 103) for 3 days, and the proliferative response was measured. *P < .01 compared with OVAp/CD11c+ DCs. Data are mean ± SEM, and the results are combined from 4 replicate experiments (C-E).
Naturally occurring DCregs protect the recipients of allogeneic BMT from cutaneous cGVHD
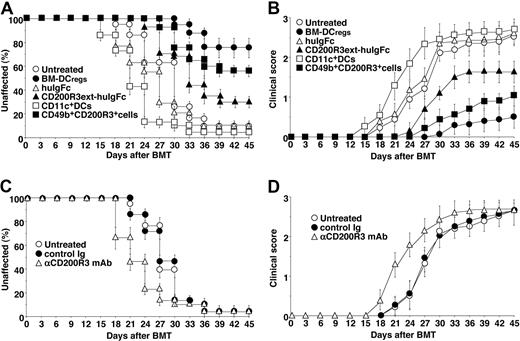
Recipient BALB/c mice transplanted with MHC-compatible (H-2d) and miHAg-incompatible BM cells and CD4+CD25− T cells obtained from B10.D2 mice showed clinical features of cutaneous cGVHD, including skin lesions with alopecia, lower mobility, diarrhea, and weight loss,13 until 33 days after alloBMT (Figure 5). Treatment with the recipient-type CD11c+ DCs slightly enhanced the incidence and severity of cutaneous cGVHD compared with the untreated recipient mice (Figure 5A,B). In contrast, treatment with the recipient-type CD49b+CD200R3+ cells as well as BM-DCregs13 significantly suppressed the incidence and severity of cutaneous cGVHD, whereas CD200R3ext-huIgFc showed a partial protective effect, compared with the untreated recipient mice (P < .01; Figure 5A,B).
Naturally occurring CD49b+CD200R3+ DCregs protect mice from the incidence and severity of cutaneous cGVHD. (A,B) The irradiated recipient BALB/c mice (10 per group) were transplanted with T cell–depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice and then treated with or without the recipient-type BM-DCregs, CD11c+ DCs, CD49b+CD200R3+ cells, huIgFc, or CD200R3ext-huIgFc after the transplantation. The incidence (A) and severity (B) of cutaneous cGVHD were monitored for 45 days after the transplantation. *P < .01 compared with the untreated recipient mice. (C,D) The irradiated recipient BALB/c mice that had received anti-CD200R3 mAb or control Ig (10 per group) were transplanted with T cell–depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice. The incidence (C) and severity (D) of cutaneous cGVHD were monitored for 45 days after the transplantation. *P < .01 compared with the untreated recipient mice. Data are mean ± SEM, and the results are combined from 3 replicate experiments (A-D).
Naturally occurring CD49b+CD200R3+ DCregs protect mice from the incidence and severity of cutaneous cGVHD. (A,B) The irradiated recipient BALB/c mice (10 per group) were transplanted with T cell–depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice and then treated with or without the recipient-type BM-DCregs, CD11c+ DCs, CD49b+CD200R3+ cells, huIgFc, or CD200R3ext-huIgFc after the transplantation. The incidence (A) and severity (B) of cutaneous cGVHD were monitored for 45 days after the transplantation. *P < .01 compared with the untreated recipient mice. (C,D) The irradiated recipient BALB/c mice that had received anti-CD200R3 mAb or control Ig (10 per group) were transplanted with T cell–depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice. The incidence (C) and severity (D) of cutaneous cGVHD were monitored for 45 days after the transplantation. *P < .01 compared with the untreated recipient mice. Data are mean ± SEM, and the results are combined from 3 replicate experiments (A-D).
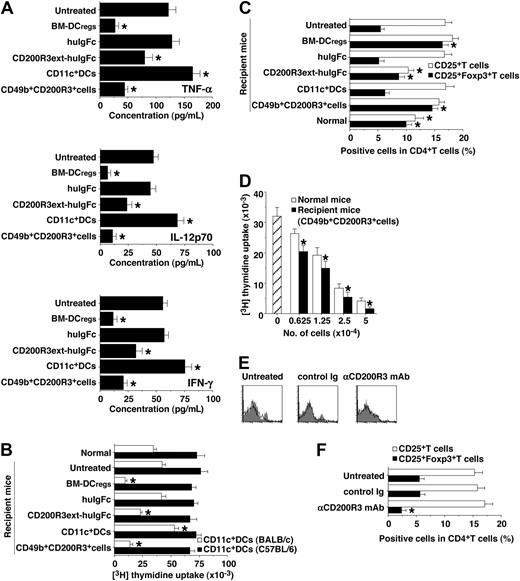
We also examined serum levels of TNF-α, IL-12p70, and IFN-γ in the transplanted recipient mice (Figure 6A). Treatment with the recipient-type CD11c+ DCs enhanced serum production of these proinflammatory cytokines compared with levels in the untreated recipient mice. In contrast, treatment with the recipient-type CD49b+CD200R3+ cells and BM-DCregs13 as well as CD200R3ext-huIgFc significantly inhibited serum levels of these proinflammatory cytokines.
Naturally occurring CD49b+CD200R3+ DCregs induce donor-derived alloreactive CD4+CD25+Foxp3+ Tregs in the transplanted recipient mice. (A-D) The transplanted recipient BALB/c mice were prepared as described in Figure 5A and B. Subsequently, serum and donor-derived CD4+ T cells were collected from each transplanted recipient mouse on day 30 after transplantation. (A) Serum production of TNF-α, IL-12p70, and IFN-γ was measured by enzyme-linked immunosorbent assay. *P < .01 compared with the untreated group. (B) Donor-derived CD4+ T cells (2 × 105) were cultured with the irradiated CD11c+ DCs (2 × 104) obtained from BALB/c or C57BL/6 mice for 3 days, and the proliferative response was measured. *P < .01 compared with normal mice. (C) The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are percentage positive cells. *P < .01 compared with the untreated recipient mice. (D) CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with CD11c+ DCs from BALB/c mice (5 × 103) in the presence of a graded dose (6.25 × 103-5 × 104) of CD4+CD25+ T cells obtained from normal B10.D2 mice (normal mice) or the recipient mice treated with CD49b+CD200R3+ cells for 3 days, and the proliferative response was measured. *P < .01 compared with normal mice. Data are expressed as mean plus or minus SEM, and the results are combined from 3 replicate experiments (A-D). (E,F) The transplanted recipient BALB/c mice were prepared as described in Figure 5C and D. (E) The expression of CD200R3 among gated CD49b+ cells was analyzed on day 0 by flow cytometry, and data are represented by a histogram. Data are representative of 3 replicate experiments. (F) Donor-derived CD4+ T cells were collected from each transplanted recipient mouse on day 30 after transplantation. The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are percentage positive cells. *P < .01 compared with the untreated recipient mice. Data are mean ± SEM, and the results are combined from 3 replicate experiments.
Naturally occurring CD49b+CD200R3+ DCregs induce donor-derived alloreactive CD4+CD25+Foxp3+ Tregs in the transplanted recipient mice. (A-D) The transplanted recipient BALB/c mice were prepared as described in Figure 5A and B. Subsequently, serum and donor-derived CD4+ T cells were collected from each transplanted recipient mouse on day 30 after transplantation. (A) Serum production of TNF-α, IL-12p70, and IFN-γ was measured by enzyme-linked immunosorbent assay. *P < .01 compared with the untreated group. (B) Donor-derived CD4+ T cells (2 × 105) were cultured with the irradiated CD11c+ DCs (2 × 104) obtained from BALB/c or C57BL/6 mice for 3 days, and the proliferative response was measured. *P < .01 compared with normal mice. (C) The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are percentage positive cells. *P < .01 compared with the untreated recipient mice. (D) CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with CD11c+ DCs from BALB/c mice (5 × 103) in the presence of a graded dose (6.25 × 103-5 × 104) of CD4+CD25+ T cells obtained from normal B10.D2 mice (normal mice) or the recipient mice treated with CD49b+CD200R3+ cells for 3 days, and the proliferative response was measured. *P < .01 compared with normal mice. Data are expressed as mean plus or minus SEM, and the results are combined from 3 replicate experiments (A-D). (E,F) The transplanted recipient BALB/c mice were prepared as described in Figure 5C and D. (E) The expression of CD200R3 among gated CD49b+ cells was analyzed on day 0 by flow cytometry, and data are represented by a histogram. Data are representative of 3 replicate experiments. (F) Donor-derived CD4+ T cells were collected from each transplanted recipient mouse on day 30 after transplantation. The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are percentage positive cells. *P < .01 compared with the untreated recipient mice. Data are mean ± SEM, and the results are combined from 3 replicate experiments.
To clarify the mechanism responsible for the regulation of cutaneous cGVHD, we analyzed donor-derived CD4+ T cells obtained from the transplanted recipient mice. Approximately 98% of spleen mononuclear cells had disappeared by day 5 after TBI, whereas spleen CD4+ T cells were apparently detected in the recipient mice 5 days after alloBMT, indicating that a large proportion of CD4+ T cells observed in the irradiated recipients of allogeneic transplantation were mainly of donor origin.13 Donor-derived CD4+ T cells obtained from the recipient mice treated with recipient-type CD11c+ DCs showed a more vigorous response to the recall stimulation with the recipient-type CD11c+ DCs than those obtained from the untreated recipient mice (Figure 6B). In contrast, donor-derived CD4+ T cells obtained from the recipient mice treated with the recipient-type CD49b+CD200R3+ cells and BM-DCregs13 as well as CD200R3ext-huIgFc were hyporesponsive to the recall stimulation with the recipient-type CD11c+ DCs, whereas similar allogeneic response was observed when they were stimulated with CD11c+ DCs obtained from MHC-mismatched strains (H-2b; Figure 6B), indicating that this suppression occurred in an allogeneic Ag-specific manner.
We also examined the generation of donor-derived CD4+CD25+Foxp3+ T cells in the transplanted recipient mice. Flow cytometric analysis showed that CD4+CD25+ T cells were increased, whereas CD4+CD25+Foxp3+ T cells were decreased in number in both the untreated and the recipient mice treated with the recipient-type CD11c+ DCs compared with normal mice (Figure 6C). In contrast, the recipient mice treated with the recipient-type CD49b+CD200R3+ cells and BM-DCregs13 had a larger proportion of CD4+CD25+Foxp3+ T cells than the untreated recipient mice (Figure 6C), and CD4+CD25+ T cells obtained from the recipient mice treated with the recipient-type CD49b+CD200R3+ cells showed greater suppression of the allogeneic activation of CD4+CD25− T cells than donor-type CD4+CD25+ nTregs (Figure 6D). On the other hand, depletion of CD200R3+ cells by anti-CD200R3 mAb (Figure 6E) before alloBMT not only reduced the generation of CD4+CD25+Foxp3+ T cells (Figure 6F) but also enhanced the progression of cutaneous cGVHD (P < .01, Figure 5C,D).
Conversion of CD4+CD25−Foxp3− T cells to CD4+CD25+Foxp3+ iTregs by naturally occurring DCregs
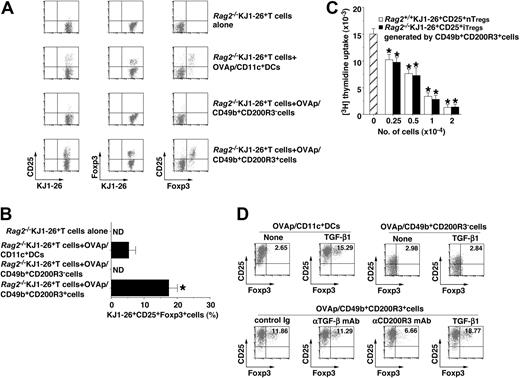
To clarify the role of the recipient-type CD49b+CD200R3+ cells in the production of alloreactive donor-derived CD4+CD25+Foxp3+ Treg in the transplanted recipient mice, we examined the ability of CD49b+CD200R3+ cells to generate CD4+CD25+Foxp3+ iTregs from CD4+CD25−Foxp3− T cells using the adoptive transfer experiment. The adoptive transfer of Rag2−/−KJ1-26+CD25−Foxp3− T cells alone or Rag2−/−KJ1-26+CD25−Foxp3− T cells plus OVAp/NK cells did not induce Rag2−/−KJ1-26+CD25+Foxp3+ T cells (Figure 7A,B). On the other hand, OVAp/CD49b+CD200R3+ cells generated Rag2−/−KJ1-26+CD25+Foxp3+ T cells from Rag2−/−KJ1-26+CD25−Foxp3− T cells more than OVAp/CD11c+ DCs (Figure 7A,B). In addition, Rag2−/−KJ1-26+CD25+ T cells generated by OVAp/CD49b+CD200R3+ cells exerted the equivalent regulatory function to KJ1-26+CD25+ nTregs isolated from Rag2+/+ DO11.10 mice (Figure 7C), indicating that they could be iTregs. We also showed that OVAp/CD49b+CD200R3+ cells generated Rag2−/−KJ1-26+CD25+Foxp3+ T cells from Rag2−/−KJ1-26+CD25−Foxp3− T cells in vitro, and this conversion was partly blocked by anti-CD200R3 mAb, but not anti–TGF-β mAb (Figure 7D). Furthermore, exogenous TGF-β1 enhanced the generation of Rag2−/−KJ1-26+CD25+Foxp3+ T cells by OVAp/CD49b+CD200R3+ cells, which was similar to OVAp/CD11c+ DCs plus TGF-β1 (Figure 7D).
Generation of CD4+CD25+Foxp3+ iTregs from CD4+CD25−Foxp3− T cells by naturally occurring CD49b+CD200R3+ DCregs. (A-C) Rag2−/−KJ1-26+ T cells (107/mouse) were transferred with or without OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (106/mouse) into mice. On day 8 after the adoptive transfer, Rag2−/−KJ1-26+ T cells were isolated from the recipient mice. (A,B) The expression of CD25 and Foxp3 among gated KJ1-26+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (A) and are percentage positive cells (B). *P < .01 compared with Rag2−/−KJ1-26+ T cells and OVAp/CD11c+ DCs. Data are representative of 4 replicate experiments (A). Data are mean ± SEM, and the results are combined from 4 replicate experiments (B). (C) Rag2−/−KJ1-26+ T cells (2 × 104) were cultured with splenocytes (2 × 104) plus anti-CD3ϵ mAb (1 μg/mL) in the presence of a graded dose (2.5 × 103-2 × 104) of Rag2+/+KJ1-26+CD25+ T cells or Rag2−/−KJ1-26+CD25+ T cells obtained from the adoptively transferred mice for 3 days, and the proliferative response was measured. *P < .01 compared with Rag2−/−KJ1-26+ T cells. Data are mean ± SEM, and the results are combined from 4 replicate experiments. (D) Rag2−/−KJ1-26+ T cells (2 × 106) were cultured with OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (2 × 105) in the presence or absence of TGF-β1 (0.1 ng/mL), anti–TGF-β mAb, anti-CD200R3 mAb, or control Ig (each 5 μg/mL) for 6 days, and the expression of CD25 and Foxp3 among gated KJ1-26+ T cells was analyzed by flow cytometry. Data are represented by a dot plot, and numbers represent the percentage of CD25+Foxp3+ cells among gated KJ1-26+ T cells. Data are representative of 4 replicate experiments.
Generation of CD4+CD25+Foxp3+ iTregs from CD4+CD25−Foxp3− T cells by naturally occurring CD49b+CD200R3+ DCregs. (A-C) Rag2−/−KJ1-26+ T cells (107/mouse) were transferred with or without OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (106/mouse) into mice. On day 8 after the adoptive transfer, Rag2−/−KJ1-26+ T cells were isolated from the recipient mice. (A,B) The expression of CD25 and Foxp3 among gated KJ1-26+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (A) and are percentage positive cells (B). *P < .01 compared with Rag2−/−KJ1-26+ T cells and OVAp/CD11c+ DCs. Data are representative of 4 replicate experiments (A). Data are mean ± SEM, and the results are combined from 4 replicate experiments (B). (C) Rag2−/−KJ1-26+ T cells (2 × 104) were cultured with splenocytes (2 × 104) plus anti-CD3ϵ mAb (1 μg/mL) in the presence of a graded dose (2.5 × 103-2 × 104) of Rag2+/+KJ1-26+CD25+ T cells or Rag2−/−KJ1-26+CD25+ T cells obtained from the adoptively transferred mice for 3 days, and the proliferative response was measured. *P < .01 compared with Rag2−/−KJ1-26+ T cells. Data are mean ± SEM, and the results are combined from 4 replicate experiments. (D) Rag2−/−KJ1-26+ T cells (2 × 106) were cultured with OVAp/CD11c+ DCs, OVAp/CD49b+CD200R3− cells, or OVAp/CD49b+CD200R3+ cells (2 × 105) in the presence or absence of TGF-β1 (0.1 ng/mL), anti–TGF-β mAb, anti-CD200R3 mAb, or control Ig (each 5 μg/mL) for 6 days, and the expression of CD25 and Foxp3 among gated KJ1-26+ T cells was analyzed by flow cytometry. Data are represented by a dot plot, and numbers represent the percentage of CD25+Foxp3+ cells among gated KJ1-26+ T cells. Data are representative of 4 replicate experiments.
Discussion
cGVHD is an increasingly frequent complication of alloBMT.3-5 Despite a better understanding of the suppression of aGVHD, how cGVHD is regulated remains unclear. Here we report the crucial role of naturally occurring CD49b+CD200R3+ DCregs in the regulation of cutaneous cGVHD in an MHC-compatible and miHAg-incompatible model of murine alloBMT possibly mediated through the generation of alloreactive CD4+CD25+Foxp3+ Tregs from donor-derived CD4+CD25−Foxp3− T cells.
We showed that BM-DCregs exhibited an extremely higher transcriptional and cell surface expression of CD200R3 than their conventional counterparts, suggesting that CD200R3 is a specific molecule for BM-DCregs. On the other hand, the defective activation of Ag-specific CD4+ T cells by BM-DCregs was partly abrogated by anti-CD200R3 mAb. In addition, the analysis with CD200R3ext-huIgFc and CD200R3-transduced BM-iDCs revealed that CD200R3 directly regulated the activation of Ag-specific CD4+ T cells. BM-DCregs had relatively high levels of MHC class II and extremely low levels of costimulatory molecules, and this unique expression profile could deliver a sufficient antigenic signal plus a poor costimulation to Ag-specific CD4+ T cells, which could be involved in the induction of clonal T-cell anergy.12,23 Therefore, CD200R3 and the default Ag-presentation machinery could mediate the T-cell regulatory function of BM-DCregs.
It has been suggested that binding of CD200 to CD200R imparts a unidirectional negative signal to CD200R-expressing leukocytes, whereas there seems to be no direct effect on cells expressing CD200, which does not contain signaling motifs or docking domains in its short cytoplasmic tail.24 In accordance with recent reports,15,25 CD200 could not be a ligand for CD200R3 and CD200R4, although CD200R1 and CD200R2 could be a high and low affinity receptor for CD200, respectively. We also observed that CD200R3ext-huIgFc not only specifically bound to CD4+ T cells and the 2B4 T-cell line but also suppressed intracellular signaling events involving ERK, p38, JNK, and IkBα when they were stimulated with mAbs to CD3ϵ and CD28 (our unpublished data). Although the detailed mechanism of how CD200R3 regulates T-cell function remains a key question for future studies, the engagement of the possible ligand expressed on CD4+ T cells by CD200R3 might trigger the impaired intracellular signaling events leading to the induction of the anergic state. Further investigation regarding the identification of the CD200R3 ligand is being conducted in our laboratories.
CD49b+CD200R3+ cells showed similarities in phenotype and function to BM-DCregs, which formally distinguishes them from other leukocytes, suggesting that they are the natural counterpart of BM-DCregs. In addition, CD49b+CD200R3+ cells could more efficiently convert CD4+CD25−Foxp3− T cells into CD4+CD25+Foxp3+ iTregs than CD11c+ DCs. Unlike CD11c+ DCs, which required TGF-β for the generation of CD4+CD25+Foxp3+ iTregs,26,27 CD49b+CD200R3+ cells as well as BM-DCregs13 induced the conversion of CD4+CD25−Foxp3− T cells to CD4+CD25+Foxp3+ iTregs in a TGF-β-independent manner, whereas anti-CD200R3 mAb partly suppressed this conversion. Therefore, the generation of CD4+CD25+Foxp3+ iTregs by CD49b+CD200R3+ cells could involve MHC class II and CD200R3, and the additional molecule(s) might be required for this conversion. CD200R3 contains the positively charged amino acid lysine in the transmembrane region, which has the potential to associate with the immunoreceptor tyrosine-activation motif-bearing adaptor molecule DAP12, suggesting that the CD200R3/DAP12 complex can transduce activating signals on basophils and mast cells.15,18 Indeed, we observed the association of CD200R3 with DAP12 in BM-DCregs (data not shown). However, the treatment with anti-CD200R3 mAb did not enhance the expression of CD80 and CD86 on CD49b+CD200R3+ cells and BM-DCregs (data not shown). These phenomena might rule out the possibility that the ligation of CD200R3 expressed on CD49b+CD200R3+ cells by anti-CD200R3 mAb could transduce activating signals, resulting in the abrogation of other molecular events involving the generation of CD4+CD25+Foxp3+ iTregs.
The crucial role of either donor- or host-derived APCs in the activation of donor-derived CD4+ T cells has been reported in murine model of cGVHD.4,5 Similar to the recipient-type BM-mDCs,13 treatment with the recipient-type CD11c+ DCs after alloBMT slightly promoted the progression of cutaneous cGVHD. In addition, these recipient mice exhibited the enhanced secretion of serum proinflammatory cytokines and alloreactive response of donor-derived CD4+ T cells. These phenomena imply that host residual CD11c+ DCs could be activated under inflammatory conditions to expand the pathogenic donor-derived CD4+ T cells, which lead to promotion of the pathogenesis of cutaneous cGVHD.
We showed that the recipient-type CD49b+CD200R3+ cells and BM-DCregs as well as CD200R3ext-huIgFc suppressed the progression of cutaneous cGVHD, and the protective effect was correlated with the reduced production of serum proinflammatory cytokines and the impaired alloreactive response of donor-derived CD4+ T cells. Furthermore, the protected recipient mice showed the enhanced production of donor-derived CD4+CD25+Foxp3+ Tregs, which exerted a more potent regulatory function against the allogeneic activation of donor-type CD4+CD25− T cells than donor-type CD4+CD25+Foxp3+ Tregs. On the other hand, the depletion of CD200R3+cells by anti-CD200R3 mAb before alloBMT resulted in the reduction of donor-derived CD4+CD25+Foxp3+ T cells as well as the enhanced progression of cutaneous cGVHD. Collectively, host residual CD49b+CD200R3+ cells could serve as sentinels, damping donor-derived alloreactive CD4+ T cells mediated through the generation of donor-derived alloreactive CD4+CD25+Foxp3+ iTregs, resulting in protection against cutaneous cGVHD.
In conclusion, the findings reported here highlight a functional identification of naturally occurring CD49b+CD200R3+ DCregs acting to regulate cutaneous cGVHD in an MHC-compatible and miHAg-incompatible murine allogeneic BMT. The functional identification of naturally occurring human DCregs and their modulation in vivo as well as the availability of human DCregs generated in vitro28 may provide an advantageous means of Ag-specific intervention for cGVHD in alloBMT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs S. Ishido and J. Shinga for valuable advice on making the vector constructs and all members of the Central Facility at the RIKEN Research Center for Allergy and Immunology for technical help in cell sorting.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (c 17790334 and 19590505; Kat. Sato).
Authorship
Contribution: Kao. Sato designed the research project, analyzed data, and wrote the paper; Kat. Sato, K.E., T.F., S.F., Y.S., H.T., and M.Y. performed the experiments; and N.Y., A.H., H.K., O.O., S.Y., and T.S. contributed vital new reagents or analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katsuaki Sato, Laboratory for Dendritic Cell Immunobiology, Research Center for Allergy and Immunology, RIKEN Yokohama Institute, Suehiro-cho 1-7-22, Tsurumi, Yokohama, Kanagawa 230-0045 Japan; e-mail: katsuaki@rcai.riken.jp.