Radioimmunotherapy (RIT or RAIT) dates back more than 2 decades and has evolved to encompass a variety of tumor-seeking antibodies in various forms, with different radionuclides, different delivery methods, for different clinical indications (usually advanced disease).1 Although selective antibody targeting has been demonstrated, the radiation doses delivered have been insufficient to markedly improve outcomes in most solid tumors. However, these agents do show efficacy in the more radiation-sensitive lymphomas, thus resulting in approval of the first RIT products, 131I-tositumomab and 90Y- ibritumomab tiuxetan, for treatment of low-grade and transformed non-Hodgkin lymphoma (NHL).2 Despite results with anti-CD20 RIT being superior to rituximab therapy,2 hematologists usually prefer other therapies. But if pretargeted RIT shows superior efficacy with mitigated toxicities, these newer agents could constitute the next generation of RIT for use in combination with other therapeutic modalities, perhaps gaining greater adoption.
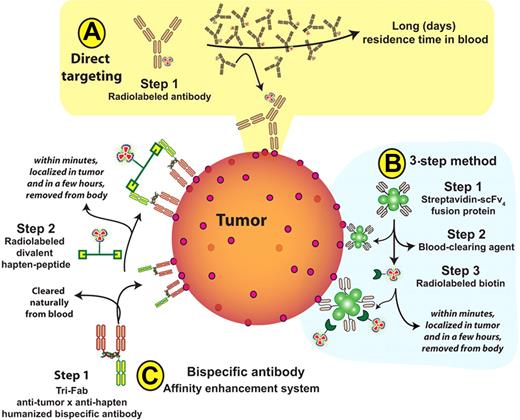
Principal methods for radioimmunotherapy. (A) Conventional directly-radiolabeled antibody. (B) Three-step pretargeted radioimmunotherapy (RIT) used by Pagel et al,4 comprising injection of streptavidin-antibody conjugate, followed by a clearing agent, and then administration of radiolabeled biotin. (C) Two-step bispecific, trivalent antibody (bsAb) pretargeted RIT (“affinity enhancement system”), whereby a bsAb is injected and binds to tumor with 2 of the 3 antibody arms. It is followed by injection of a radiolabeled hapten-peptide that binds to the third arm of the bsAb once the latter has cleared from the blood naturally.
Principal methods for radioimmunotherapy. (A) Conventional directly-radiolabeled antibody. (B) Three-step pretargeted radioimmunotherapy (RIT) used by Pagel et al,4 comprising injection of streptavidin-antibody conjugate, followed by a clearing agent, and then administration of radiolabeled biotin. (C) Two-step bispecific, trivalent antibody (bsAb) pretargeted RIT (“affinity enhancement system”), whereby a bsAb is injected and binds to tumor with 2 of the 3 antibody arms. It is followed by injection of a radiolabeled hapten-peptide that binds to the third arm of the bsAb once the latter has cleared from the blood naturally.
Pretargeted RIT separates tumor targeting with a nonradioactive antibody from a secondary radioactive effector (eg, radiolabeled biotin or a hapten-peptide) that binds to the antibody after it has been cleared from the blood and has localized on tumor cells.3 In this issue, Pagel et al use a streptavidin-antibody construct followed by a clearing agent that removes the complex from the blood, followed by administration of radiolabeled biotin that binds to streptavidin-antibody localized on tumor cells.4 While studying the therapeutic effects with 3 antibody systems in 3 human lymphoma xenografts models, they found that pretargeted RIT is more effective than direct RIT, but also showed that anti-CD20 RIT is not improved by combination therapy with anti–HLA-DR and/or anti-CD22 RIT, since RIT of the more highly expressed CD20 in these tumors was already very potent. Antibody cocktails, where different antibody specificities are used, would expect to have a higher accretion in tumor, possibly binding to more tumor cells, but are not an improvement if a dose reduction is required for each individual antibody.
In the study by Pagel et al, anti-CD22 RIT alone, either pretargeted or directly targeted, was less effective in these models than the other 2 antibodies, yet another radiolabeled anti-CD22 antibody, as cited by the authors, has shown efficacy in patients with NHL.5 So the first question is, how predictive are these models for translation to humans? The most obvious issue is that mice lack the antigen “sink” encountered in patients for each of these antigens, since human CD20 or human CD22 are not expressed on non-tumor cells in these models, and this factor may be critical in determining a therapeutic index of an experimental agent that only targets human antigens. A second observation is that antigen expression could predict which antibody performed best in vivo, suggesting that a similar evaluation could be predictive in clinical specimens. Unfortunately, prior imaging to determine targeting of a radiolabeled antibody to be used in RIT has not been predictive of clinical efficacy in anti-CD22 and anti-CD20 RIT,5,6 suggesting there are other factors controlling response. They also encountered difficulties when combining the antibodies in the pretargeted RIT experiments, apparently exceeding the capacity of the clearing agent, which may be a potential limitation of this pretargeting method in general or a problem of the murine model. Despite these concerns, Pagel et al add to a growing literature of evidence that pretargeted RIT can achieve a higher therapeutic index compared with conventional, 1-step, RIT,3 and therefore deserves clinical evaluation.
The real value may not depend on which pretargeted method is chosen, but how it is employed. Just as chemotherapy is delivered in repeated cycles, so too can RIT be more effective when given repeatedly or even in fractionated doses. For example, in direct RIT with yttrium Y90 epratuzumab tetraxetan (90Y-DOTA–anti-CD22 IgG), fractionation showed that much higher cumulative doses could be tolerated and appears to be more potent than prior studies with a single RIT dose,5 achieving 100% objective responses in a subgroup of 11 patients with relapsed follicular lymphoma given 2 weekly doses of 20 mCi/m2 (with 10/11 having CR/CRu).7 Assuming these results can also be achieved with pretargeted RIT, which method will provide more durable responses?
Since target antigens may alter their expression as tumors evolve or progress, RIT against different target antigens could be applied sequentially as suggested by Pagel et al, but also in combination with the same or different (unlabeled) antibodies used in immunotherapy.8 This is certainly not more complex than multidrug cycles combined with rituximab, which has become standard practice in NHL therapy. But how will this or the more complex pretargeted RIT be regarded following the challenges experienced by current RIT? Perhaps the difference will depend on the first step not involving radiation, but a targeting antibody that can be administered in an oncologist's office with the radiolabeled moiety provided in a nuclear medicine facility a day or more later, thus making pretargeting truly the province of the oncologist. Also, if the outcome is less myelotoxicity than direct RIT, it lends itself to combination modalities, which have become the mainstay of cancer therapy. Given these prospects, those of us who like their antibody therapy hot must persevere and remain optimistic.
Conflict-of-interest disclosure: The author is a director, officer, and shareholder of Immunomedics Inc, and IBC Pharmaceuticals Inc. ■