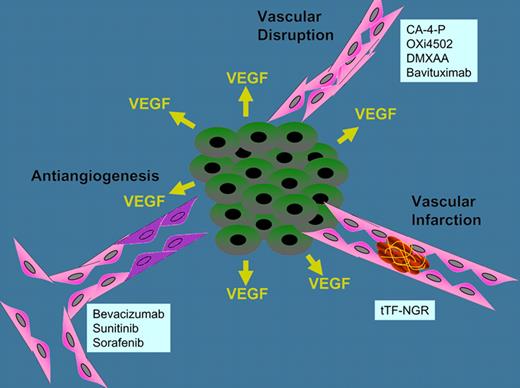
With the approval of several antiangiogenic agents including bevacizumab, sunitinb, and sorafenib, the usefulness of targeting tumor vasculature with inhibitors has been clinically established. Antiangiogenic agents may be described as drugs that block angiogenic signaling either by neutralizing secreted proangiogenic factors or by inhibiting intracellular signaling by their receptors resulting in regression of neovasculature in the tumor.1 A second approach to targeting the tumor vasculature therapeutically is vasculature disrupting agents. Vasculature disrupting agents are designed to produce rapid vascular collapse or shutdown in tumors.2,3 The resulting ischemia results in tumor cell killing, which causes extensive tumor necrosis. Although no vascular disrupting agents are approved as yet, several including combretastatin derivatives (CA4P and OXi4503), the dimethylxanthenone DMXAA, and the antiphosphatidylserine bavituximab are progressing through clinical trial. In preclinical in vivo models, decreased tumor blood flow can be detected in a few minutes after administration of these agents, and nearly complete vascular shutdown can occur in less than 1 hour.
Three mechanisms for therapeutically targeting tumor vasculature, antiangiogenesis, vascular disruption, and vascular infarction, and examples of agents.
Three mechanisms for therapeutically targeting tumor vasculature, antiangiogenesis, vascular disruption, and vascular infarction, and examples of agents.
While the recognition that tumor growth is angiogenesis-dependent and that angiogenesis could be an important target for cancer therapeutics is attributed to Folkman in 1971, and the recognition that tumors can be described as wounds that do not heal is attributed to Dvorak in 1986, the observation that tumors are hypercoagulative occurred much earlier, being attributed to Trousseau in 1865. The association of thrombosis in the extremities (thrombophlebitis) with a diagnosis of pancreatic or gastric cancer has historically been termed Trousseau syndrome. Growing tumors often develop a fibrin “capsule.” The fibrin clots attract host cells including platelets, monocytes/macrophages, and infiltrating lymphocytes, leading to continued matrix formation and formation of new blood vessels. These events mimic the early stages of wound healing which, in malignant diseases, becomes a continuous nonresolving process. Many malignant tissues express tissue factor, a pro-coagulant and key activator of the coagulation cascade. Disseminated intravascular coagulation is the most frequent coagulopathy associated with cancer and results from generalized activation of coagulation pathways. Cancer is the third most common cause of disseminated intravascular coagulation.4
Modern technologies have been applied to understanding the connection between cancer and wound healing. In 2004, Chang et al developed a wound-response gene signature including genes involved in matrix remodeling, cell motility, and angiogenesis.5 Expression of the wound-response gene signature predicted poor overall survival and increased risk of metastasis in a subset of patients with breast, lung, and gastric cancers. Later, the wound-response gene signature's prognostic value was validated in a data set of nearly 300 breast cancer patients.
In the current issue, Bieker et al take advantage of the coagulation cascade and offer a third approach to therapeutically targeting the tumor vascular supply by selectively focusing thrombosis in the tumor blood vessels.6 These investigators fused the extracellular domain of (truncated) tissue factor (tTF) with the peptide GNGRAHA, which was identified through phage display to bind to aminopeptidase N (CD13) and integrin αvβ3 (CD51/CD61) which are cell surface protein up-regulated on tumor vascular endothelial cells. The fusion protein therapeutic-designated tTF-NGR produces thrombosis in the tumor vasculature, resulting in infarction of tumor vessels.6 Previous studies have tried other tumor-targeting carrier molecules, such as the ED-B domain of fibronectin, PSA, VCAM-1 and integrins, to deliver tissue factor to tumor vasculature.7 Bieker et al found that the NGR peptide resulted in rapid homing to tumor vessel and rapid thrombosis. Studies in 3 human tumor xenograft tumors confirmed the efficacy of tTF-NGR. Furthermore, clinical first-in-man treatment of 5 patients with low doses of systemically administered tTF-NGR showed decreased tumor perfusion by MRI.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■