Abstract
We conducted a double-blind, randomized multicenter trial to determine whether the addition of mycophenolate mofetil (MMF) improves the efficacy of initial systemic treatment of chronic graft-versus-host disease (GVHD). The primary endpoint was resolution of chronic GVHD and withdrawal of all systemic treatment within 2 years, without secondary treatment. Enrollment of 230 patients was planned, providing 90% power to observe a 20% difference in success rates between the 2 arms. The study was closed after 4 years because the interim estimated cumulative incidence of success for the primary endpoint was 23% among 74 patients in the MMF arm and 18% among 77 patients in the control arm, indicating a low probability of positive results for the primary endpoint after completing the study as originally planned. Analysis of secondary endpoints showed no evidence of benefit from adding MMF to the systemic regimen first used for treatment of chronic GVHD. The estimated hazard ratio of death was 1.99 (95% confidence interval, 0.9-4.3) among patients in the MMF arm compared with the control arm. MMF should not be added to the initial systemic treatment regimen for chronic GVHD. This trial was registered at www.clinicaltrials.gov as #NCT00089141 on August 4, 2004.
Introduction
Chronic graft-versus-host disease (GVHD) causes considerable morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT).1 High-dose glucocorticoids and continued administration of a calcineurin inhibitor have long served as the mainstay of treatment for chronic GVHD.2 In most patients, systemic treatment must be continued for at least 2 years.3 Long-term glucocorticoid treatment causes numerous complications, including infections, myopathy, avascular necrosis, osteoporosis, glucose intolerance, hypertension, growth retardation in children, weight gain, changes in body habitus, cutaneous atrophy and striae, cataracts, emotional lability, and interference with sleep.4 Development of less toxic treatments that could reduce the dose or duration of glucocorticoid administration or improve disease control would be of enormous benefit for patients with chronic GVHD.
Mycophenolate mofetil (MMF) is the 2-(4-morpholino) ethyl ester of mycophenolic acid (MPA).5 After oral administration, MMF is rapidly absorbed and hydrolyzed to MPA, which selectively and reversibly inhibits inosine monophosphate dehydrogenase, thereby blocking the de novo pathway of purine synthesis in lymphocytes and depleting the intracellular pool of guanosine triphosphate. Case-series reports and phase 2 studies have suggested that MMF might be effective for treatment of steroid-refractory chronic GVHD both in adults and in children.6-15 In 2 large surveys, respondents endorsed MMF more frequently than other agents for treatment of high-risk or steroid-refractory chronic GVHD.16,17
These results suggested that outcomes among patients with chronic GVHD might be improved by adding MMF to the initial systemic treatment regimen. The principal objective of the current clinical trial was to determine whether the addition of MMF improves the efficacy of initial systemic treatment for chronic GVHD. In addition to increasing the response rate and allowing earlier discontinuation of immunosuppressive therapy, more effective primary treatment for chronic GVHD would be expected to decrease the incidence of complications related to glucocorticoid treatment, reduce the probability of secondary therapy, and decrease the risk of death from causes other than recurrent malignancy.
Methods
Eligibility and enrollment
Patients were enrolled within 14 days after beginning systemic immunosuppressive treatment for chronic GVHD with at least one symptom or sign that is not characteristic of acute GVHD.1 Exclusion criteria included uncontrolled fungal, cytomegalovirus, or varicella zoster virus infection; inability to tolerate oral administration of medications; known hypersensitivity to MMF; melena, frank gastrointestinal hemorrhage, or ulceration; absolute neutrophil count less than 1500/μL; administration of immunosuppressive medications other than steroids, cyclosporine, tacrolimus, or sirolimus; onset of chronic GVHD during treatment with more than 1.0 mg/kg per day prednisone or equivalent; any prior systemic immunosuppressive treatment for chronic GVHD; bronchiolitis obliterans as a manifestation of chronic GVHD; any evidence at the time of enrollment indicating a high probability of subsequent recurrent or progressive malignancy; pregnancy or breastfeeding; or hospitalization for reasons other than rehabilitation at the time of enrollment. Women of childbearing potential were required to use effective contraception during administration of the study drug. Informed consent was documented with the use of forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center and the respective participating transplantation centers, in accordance with the Declaration of Helsinki.
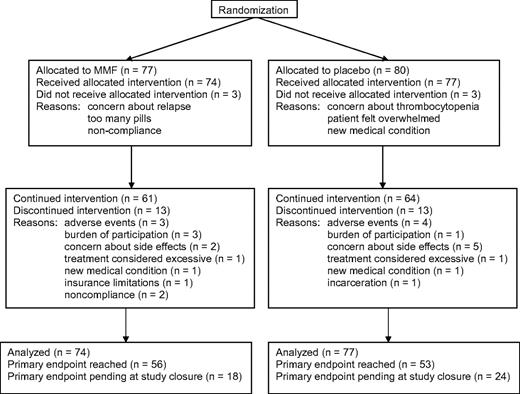
From May 6, 2004 to June 11, 2008, 157 patients from 15 transplantation centers enrolled in the study. Six patients (3 in the MMF arm and 3 in the placebo arm) withdrew after randomization but before taking any study drug (Figure 1). Follow-up information could not be obtained for these patients. Results are reported for the remaining 151 patients.
Flow diagram summarizing results of the randomization, administration of the study drug, and analysis of results at the end of the study.
Flow diagram summarizing results of the randomization, administration of the study drug, and analysis of results at the end of the study.
Treatment plan
Patients continued treatment with a calcineurin inhibitor or sirolimus, according to the regimen administered when the diagnosis of chronic GVHD was made. Most patients were also treated with prednisone, initially at a dose of 1.0 mg/kg per day, at the discretion of the physician. The study drug was provided by Roche Laboratories (Nutley, NJ) and was dispensed under investigator-initiated US IND 64390 and Canadian CTA 115111. Participants were given capsules containing either MMF or placebo according to randomization by an independent registrar. Identity of the study drug was not disclosed to participants, physicians, or study staff until after the study was closed.
In kidney transplantation recipients, concurrent treatment with cyclosporine decreases the area under the curve (AUC) of plasma MPA concentrations across time between doses of MMF.18-22 This effect is proportional to the concentration of cyclosporine in the blood23 and does not occur with tacrolimus21 or sirolimus.24 The MPA AUC after a 750-mg dose of MMF in the absence of cyclosporine approximates the MPA AUC after a 1000-mg dose of MMF in the presence of cyclosporine at therapeutic concentrations in the blood.20,21 Because similar drug interactions were expected in patients with chronic GVHD, the study drug was administered at 1000 mg orally twice daily among patients with trough cyclosporine concentrations more than or equal to 100 ng/mL in the blood, and at 750 mg orally twice daily for all other patients. Doses were reduced or withheld temporarily in patients who had neutropenia or gastrointestinal side effects.
Topical therapy, including glucocorticoid creams, topical tacrolimus, oral beclomethasone, topical azathioprine, and ophthalmic glucocorticoids, and other supportive measures were managed at the discretion of the physician.25,26 Medications to prevent Pneumocystis pneumonia and infection with cytomegalovirus, herpes simplex virus, varicella zoster virus, encapsulated bacteria, and fungal organisms were administered according to institutional practice.26
Decisions regarding the administration of immunosuppressive medications were made by the treating physician. The protocol provided guidelines for tapering the doses of immunosuppressive medications, together with recommendations for order in which immunosuppressive medications should be withdrawn. The recommended sequence was withdrawal of prednisone, followed by withdrawal of the calcineurin inhibitor or sirolimus, followed by withdrawal of the study drug, as allowed by resolution of chronic GVHD.
Plasma trough concentrations of MPA
Consenting patients had a blood sample drawn to measure the trough concentrations of MPA in the plasma. Testing was done at 3 months after enrollment because the MPA AUC increases during the first 3 months after beginning treatment with MMF.27-29 Total MPA and free MPA (ie, not bound to protein) concentrations were measured in the laboratory of Dr Pamala Jacobson at the University of Minnesota, using methods described previously.30 Free MPA has biologic activity, whereas protein-bound MPA does not. Unblinded results were not disclosed until after the study was closed.
Definition of endpoints
Treatment success was defined as withdrawal of all systemic treatment, including the study drug, after resolution of all reversible manifestations of chronic GVHD with no secondary systemic therapy. Ocular and oral sicca and joint contractures, and any sequelae of scleroderma present at the onset of systemic treatment were not considered to be reversible for purposes of defining treatment success. Discontinuation of immunosuppressive medications for the purpose of improving donor chimerism after HCT with nonmyeloablative conditioning or for inducing an antitumor response after the development of recurrent or secondary malignancy was not considered as treatment success.
Treatment failure was defined as the initiation of secondary systemic therapy or as development of bronchiolitis obliterans, recurrent malignancy, or death from causes other than recurrent malignancy during primary treatment for chronic GVHD, each indicating that treatment success did not occur or could not be attained. Discontinuation of treatment with study drug because of toxicity was not considered as treatment failure.
Secondary systemic therapy included any intervention intended to control chronic GVHD through any systemic treatment that was not included in the primary treatment regimen. Administration of systemic glucocorticoids to patients who were not treated initially with glucocorticoids was considered as secondary systemic therapy. Topical therapy was not considered as secondary systemic therapy. An increase in the dose of prednisone and any resumption of treatment with prednisone or study drug after previous discontinuation for any reason was not considered as secondary systemic therapy. Any increase in the dose of cyclosporine or tacrolimus or resumption of treatment with cyclosporine or tacrolimus after previous discontinuation for any reason was not considered as secondary systemic treatment if the drug in question was included as part of the primary treatment regimen. A change in treatment from cyclosporine to tacrolimus or vice versa resulting from drug toxicity was not considered as secondary treatment, but any such change made because of uncontrolled chronic GVHD was considered as secondary treatment.
Recurrent malignancy was defined as clinical or histopathologic evidence demonstrating the reappearance or progression of any malignancy considered as an indication for the transplantation. Recurrent malignancy was also defined as any posttransplantation intervention not routinely used to prevent the development of overt recurrence, prompted by any evidence of persisting malignant cells.
Stratification and statistical analysis
Randomization was stratified according to involvement of a single organ versus multiple organs and by the use of a myeloablative or nonmyeloablative pretransplantation conditioning regimen. Randomization was also stratified by transplantation center for purposes of ensuring balance between the arms but not for analysis.
Based on historical experience of patients comparable with those who were eligible for this study, the 2-year success rate for the placebo arm was expected to be approximately 22%. The study was designed to test whether treatment with MMF could increase the success rate from 22% to 42%. The original plan was to enroll a total of 230 patients assigned to the MMF and placebo arms at a 1:1 ratio to have 90% power to observe a statistically significant difference with a 2-sided type 1 error of 0.05. The primary endpoint was to have been analyzed as a binomial outcome by comparing the proportion of treatment successes at 2 years between arms, according to randomized assignment, using a χ2 test. Because the study was stopped before this endpoint could be determined in all patients, the current analysis is based on the estimated cumulative incidence at 2 years.31 Stratified Cox regression was used for hazard ratio analysis, with P values based on associated likelihood ratio statistics. All P values are 2-sided. Adverse event data were analyzed as the proportion of patients in each arm who developed a given complication during administration of the study drug or within 30 days after discontinuation, using χ2 tests and Fisher exact tests, as appropriate.
Data and safety monitoring
A data and safety monitoring board (DSMB) reviewed interim study progress and safety data at 3-month intervals according to a written charter. No interim analyses of efficacy were planned when the study was designed. Follow-up for up to 2 years was required to ascertain the primary endpoint, and it was anticipated that enrollment could be completed within 2 years. The DSMB reviewed the interim efficacy results after 151 patients had been enrolled during a period of 4 years, when it became apparent that the rationale for not reviewing interim efficacy results was no longer valid. On June 11, 2008, the DSMB recommended closure of the study because the estimated conditional probability of positive results after enrollment of all 230 patients was 0.27 under the most optimistic assumptions.
Results
Patient characteristics
Demographic and transplantation characteristics were well balanced between the 2 arms (Table 1). GVHD-associated risk factors,1 including patient age, use of female donors for male patients, use of mobilized blood cells, donor-recipient relationship, and human leukocyte antigen matching, showed no differences that would be expected to bias outcomes of the study. The disease categories associated with risk of recurrent malignancy32 and the proportions of patients who had nonmyeloablative conditioning regimens were well balanced between the 2 arms (Table 1 and Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). GVHD characteristics at baseline (Table 2)33 were also well balanced between the 2 arms. Other risk factors potentially associated with prolonged duration of immunosuppression or increased risk of nonrelapse mortality,1,3 including progressive onset from acute GVHD, multiple sites involved with chronic GVHD, and hyperbilirubinemia or thrombocytopenia at the onset of chronic GVHD, showed no differences that would be expected to bias outcomes of the study.
Study drug administration and trough plasma concentrations of MPA
Ten patients (14%) in the MMF arm and 9 (12%) in the control arm stopped taking the study drug earlier than prescribed by the protocol for reasons other than adverse events, as summarized in Figure 1. The median interval from registration in the study to premature discontinuation of study drug administration was 4.6 months (range, 0.3-19 months) among patients in the MMF arm, compared with 4.0 months (range, 0.9-16 months) in the control arm. Follow-up information was obtained for all but one of the patients who stopped taking the study drug prematurely. Follow-up information could not be obtained in one case because the patient was incarcerated. Based on counts of study drug capsules, patients in the MMF arm took a median 98% (90% range, 82.0%-100%) of the prescribed amount of study drug, compared with 95.7% (90% range, 74.7%-100%) in the control arm.
Six patients taking MMF at 1000 mg twice daily and 35 patients taking MMF at 750 mg twice daily had plasma samples drawn 3 months after enrolling in the study. One patient in each group had no MPA detectable in the sample. The median total MPA trough concentration was 2.18 μg/mL (range, 0.79-4.11 μg/mL) among the 5 remaining patients taking MMF at 1000 mg twice daily, compared with 1.12 μg/mL (range, 0.08-10.1 μg/mL) among the 34 remaining patients taking MMF at 750 mg twice daily (P = .09, Wilcoxon rank-sum test). The median free MPA trough concentration was 31.2 μg/mL (range, 16.1-73.9 ng/mL) among patients taking MMF at 1000 mg twice daily, compared with 14.3 μg/mL (range, 0-249 ng/mL) among those taking MMF at 750 mg twice daily (P = .02, Wilcoxon rank-sum test). Two of the 34 patients taking MMF at 750 mg twice daily had MPA detectable in whole plasma but not in the protein-free fraction.
Outcomes related to treatment of chronic GVHD
In the MMF arm, 11 of the 74 patients (15%) had treatment success, compared with 13 of the 77 patients (17%) in the control arm (Table 3), although 3 of the successes in the control arm occurred after the 2-year limit used to define the primary endpoint. The estimated cumulative incidence of treatment success at 2 years was 22.6% in the MMF arm, compared with 18.3% in the control arm. The estimated difference in the cumulative incidence of success between the 2 arms was 4.3% (95% confidence interval [CI], −11.4%-20.0%) in favor of the MMF arm, clearly failing to reject the null hypothesis and inconsistent with the alternative hypothesis. When the study was closed, 20 patients (27%) in the MMF arm were continuing primary therapy, compared with 26 (34%) in the control arm. In the MMF arm, 43 patients (58%) had treatment failure, compared with 38 (49%) in the control arm. Recurrent malignancy accounted for most of the difference in treatment failures between the 2 arms.
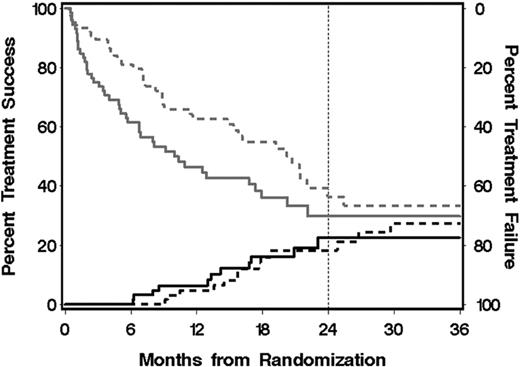
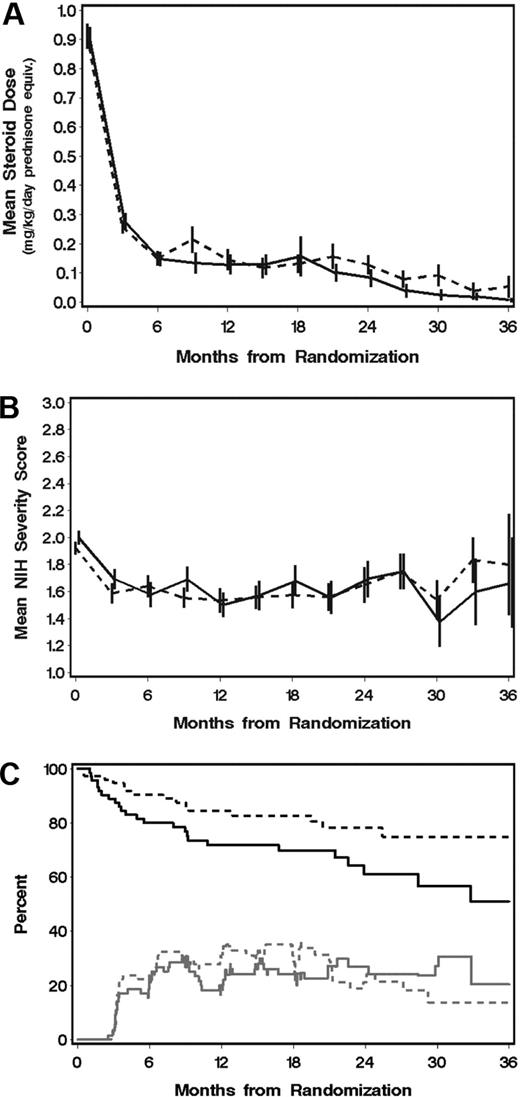
As shown in Figure 2, the cumulative incidence of treatment failure appeared to be higher in the MMF arm than in the placebo arm, with little apparent difference in the cumulative incidence of treatment success between the 2 arms. A hazard ratio analysis suggests that a possibly higher underlying rate of treatment success in the MMF arm was negated by an increased rate of treatment failure (Table 4). The hazards of secondary systemic treatment, development of bronchiolitis obliterans, withdrawal of prednisone, and withdrawal of all systemic treatment after resolution of GVHD showed no statistically significant differences between the 2 arms. Further analysis showed that prednisone doses and the prevalence of complete response were similar across time in the 2 arms (Figure 3).
Cumulative incidence of treatment success and treatment failure. The lower black curves and left scale represent the cumulative incidence of discontinued systemic treatment for chronic GVHD without secondary therapy for chronic GVHD, development of bronchiolitis obliterans, recurrent malignancy during primary treatment for chronic GVHD, or death during primary treatment for chronic GVHD; upper gray curves and right scale, cumulative incidence of treatment failure, including secondary therapy for chronic GVHD, development of bronchiolitis obliterans, recurrent malignancy during primary treatment for chronic GVHD, or death during primary treatment for chronic GVHD. The gap between the lower and upper curves indicates the proportion of patients continuing primary treatment for chronic GVHD without recurrent malignancy or development of bronchiolitis obliterans. Vertical line at 2 years represents the prespecified interval time designated for assessment of the primary endpoint; —, MMF group; and ----, control group.
Cumulative incidence of treatment success and treatment failure. The lower black curves and left scale represent the cumulative incidence of discontinued systemic treatment for chronic GVHD without secondary therapy for chronic GVHD, development of bronchiolitis obliterans, recurrent malignancy during primary treatment for chronic GVHD, or death during primary treatment for chronic GVHD; upper gray curves and right scale, cumulative incidence of treatment failure, including secondary therapy for chronic GVHD, development of bronchiolitis obliterans, recurrent malignancy during primary treatment for chronic GVHD, or death during primary treatment for chronic GVHD. The gap between the lower and upper curves indicates the proportion of patients continuing primary treatment for chronic GVHD without recurrent malignancy or development of bronchiolitis obliterans. Vertical line at 2 years represents the prespecified interval time designated for assessment of the primary endpoint; —, MMF group; and ----, control group.
Mean steroid doses, mean GVHD severity scores, and percentages of patients with complete response after randomization show no benefit of MMF for initial treatment of chronic GVHD. (A) Prednisone doses. (B) Mean National Institutes of Health severity scores and (C) prevalence of complete response (CR) across time among surviving patients without recurrent malignancy. (A,B) Bars represent ± SD. (C) The upper black curves represent relapse-free survival for reference. Changes in prevalence of CR (gray lines) can occur either with the onset or end of CR or with death or recurrent malignancy in the presence or absence of CR. In all panels: —, MMF group; and ----, control group.
Mean steroid doses, mean GVHD severity scores, and percentages of patients with complete response after randomization show no benefit of MMF for initial treatment of chronic GVHD. (A) Prednisone doses. (B) Mean National Institutes of Health severity scores and (C) prevalence of complete response (CR) across time among surviving patients without recurrent malignancy. (A,B) Bars represent ± SD. (C) The upper black curves represent relapse-free survival for reference. Changes in prevalence of CR (gray lines) can occur either with the onset or end of CR or with death or recurrent malignancy in the presence or absence of CR. In all panels: —, MMF group; and ----, control group.
Adverse events
Three of the 74 patients (4%) in the MMF arm stopped taking the study drug permanently because of side effects: one resulting from gastrointestinal intolerance, one resulting from recurrent neutropenia, and one resulting from tinnitus and muscle cramping (Table 5). Four of the 77 patients (5%) in the control arm stopped taking the study drug because of side effects: one resulting from pruritis and urticaria and 3 resulting from recurrent neutropenia.
The proportion of patients who had infection associated with administration of the study drug was lower among patients in the MMF arm than in the control arm (P = .05), but the proportion of patients given antibiotics for empiric treatment of documented infection was possibly higher among patients in the MMF arm than in the control arm (Table 5). Among patients in the MMF arm, the median number of infections per quarterly observation interval was 0.38 (range, 0-2.5), compared with 0.38 (range, 0-2.25) in the control arm (P = .81, Wilcoxon rank-sum test).
Results showed no statistically significant differences in the proportions of patients who developed neutropenia in the 2 arms. The proportion of all patients with thrombocytopenia (platelet count < 100 000/μL) was possibly higher in the MMF arm than in the control arm. Among 58 patients with a platelet count more than 100 000/μL at enrollment in the MMF arm, 17 (29%) developed thrombocytopenia during administration of the study drug. Among 61 patients with a platelet count more than 100 000/μL at enrollment in the placebo arm, 7 (11%) developed thrombocytopenia during administration of the study drug (P = .015).
Results showed no statistically significant difference in the numbers of patients with diabetes between the 2 arms. No statistically significant differences were observed in the proportions of patients with avascular necrosis or osteoporosis between the 2 arms. Six patients (8%) in the MMF arm and 9 (12%) in the control arm developed skin cancer after enrollment in the study. In addition, 3 patients in the control arm developed other secondary malignancies (one adenocarcinoma of the lung, one renal cell carcinoma, and one prostate cancer).
Survival, nonrelapse mortality, recurrent malignancy, and survival without recurrent malignancy
At closure of the study, 19 of 74 patients (26%) in the MMF arm had died, compared with 10 of 77 (13%) in the control arm. Infections and recurrent malignancy appear to account for most of the excess deaths in the MMF arm (Table 6). The estimated hazard ratio of death was 1.99 (95% CI, 0.9-4.3) among patients in the MMF arm compared with the control arm (P = .10; Figure 4). The estimated hazard ratio of death without recurrent malignancy was 1.62 (95% CI, 0.5-5.0, P = .41). The estimated hazard ratio of recurrent malignancy was 1.72 (95% CI, 0.8-3.8, P = .18), and with adjustment for risk category, the hazard ratio was 1.74 (95% CI, 0.8-3.9, P = .17). With adjustment for risk category, the estimated hazard ratios of recurrent malignancy were 2.48 (95% CI, 0.9-7.0, P = .09) for patients taking MMF at 1000 mg twice daily compared with placebo, 1.49 (95% CI, 0.6-3.6, P = .38) for patients taking MMF at 750 mg twice daily compared with placebo, and 1.67 (95% CI, 0.6-4.7, P = .33) for patients taking MMF at 1000 mg twice daily compared with those taking MMF at 750 mg twice daily. The estimated hazard ratio of death or recurrent malignancy was 1.69 (95% CI, 0.9-3.2) among patients in the MMF arm compared with the control arm (P = .14). Inspection of outcomes within pretransplantation disease risk or disease categories did not identify subgroups that disproportionately accounted for the excess of deaths or recurrent malignancy in the MMF arm (Table S1). A time-dependent Cox proportional hazards analysis accounting for premature discontinuation of MMF administration and treatment with open-label MMF after administration of study drug was discontinued showed trends suggesting increased risks of overall mortality and recurrent malignancy associated with administration of MMF (Table S2).
Analysis of major outcomes shows no benefit of MMF for initial treatment of chronic GVHD. (A) Outcomes according to treatment arm. (B) Hazard ratio estimates and 95% confidence intervals for outcomes among patients in the MMF arm compared with those in the control arm. (A) — represent the MMF group; and ----, control group. (B) Hazard ratio estimates and confidence intervals were derived from Cox regression analysis stratified on the number of involved sites at onset (1 vs > 1) and conditioning regimen (myeloablative vs nonmyeloablative).
Analysis of major outcomes shows no benefit of MMF for initial treatment of chronic GVHD. (A) Outcomes according to treatment arm. (B) Hazard ratio estimates and 95% confidence intervals for outcomes among patients in the MMF arm compared with those in the control arm. (A) — represent the MMF group; and ----, control group. (B) Hazard ratio estimates and confidence intervals were derived from Cox regression analysis stratified on the number of involved sites at onset (1 vs > 1) and conditioning regimen (myeloablative vs nonmyeloablative).
Discussion
In this study, addition of MMF to the systemic immunosuppressive regimen first used for treatment of chronic GVHD did not increase the proportion of patients who had resolution of chronic GVHD and withdrawal of all systemic treatment within 2 years. Any possible increase in the rate of treatment success was offset by an increase in the rate of treatment failure. In this double-blind trial, addition of MMF to the immunosuppressive regimen had no effect on the amount of systemic glucocorticoid treatment used to control manifestations of chronic GVHD or the proportion of patients with complete responses across time after enrollment. Administration of MMF did not cause an increased risk of neutropenia or infection but was possibly associated with increased risks of thrombocytopenia and recurrent malignancy. The death rate among patients who received MMF was possibly higher than among controls. Most of the excess deaths in the MMF arm were caused by infection or recurrent malignancy.
Results of this study were unexpected because case series reports and phase 2 studies had suggested that MMF might have efficacy for treatment of steroid-refractory chronic GVHD.6-15 Moreover, the tolerability of MMF and ease of oral administration for prolonged periods of time made MMF an attractive alternative among other agents that could have been tested for efficacy in the initial treatment of chronic GVHD. Several hypothetical a priori explanations could be considered as possibly accounting for an inability of MMF to produce favorable results in the current study. First, the primary endpoint might have been too stringent as a test of the efficacy of MMF for treatment of chronic GVHD. Second, the dose of MMF might not have been optimal because inappropriately low doses would not be sufficient to have a beneficial effect, whereas inappropriately high doses could cause an inordinately increased risk of opportunistic infection or other possible adverse effects. Third, the dose of MMF might have been optimal, but the rate of steroid withdrawal in the MMF arm might have been too slow, thereby contributing to an increased risk of infections. Fourth, even at an optimal dose with an appropriate rate of steroid withdrawal, MMF might not provide any benefit as an added component in the initial treatment of chronic GVHD.
The primary endpoint in this study was selected for its unquestionable medical significance and robust ascertainment characteristics. The absence of validated, objective, and easily applied scales for measurement of GVHD manifestations has made it difficult to define criteria for partial response.34 Resolution of reversible manifestations of chronic GVHD unambiguously indicates complete response, although some disagreement might persist with respect to the manifestations that should be considered irreversible. Permanent withdrawal of all immunosuppressive treatment in the absence of recurrent malignancy indicates cure of chronic GVHD. In the current study, the validity of cure was confirmed by the absence of recurrent GVHD after withdrawal of systemic treatment. Physicians were allowed to use clinical judgment in deciding whether treatment should be ended or changed. The double-blind design ensured that these judgments and decisions were not influenced by awareness of whether MMF was administered or not.
The benchmark difference of 20% in the primary endpoint between the 2 arms was not based on results of prior phase 2 studies testing the efficacy of MMF for initial treatment of chronic GVHD. Instead, the study was based on results of previous reports suggesting the efficacy of MMF for treatment of steroid-refractory chronic GVHD,6-15 and the proposed effect size was based on the number of patients who could be enrolled in a multicenter study within a reasonable period of time. Even so, the negative results of this study did not simply reflect an overstringent primary endpoint or an unrealistic effect size because the much less stringent endpoint of complete response also did not show an advantage for the MMF arm.
MPA pharmacokinetics differ in patients with chronic GVHD compared with those receiving MMF to treat or prevent acute GVHD. With MMF administered orally at 1000 mg twice daily for treatment of GVHD, the median MPA AUC estimate for patients with chronic GVHD was almost 2-fold higher than for patients with acute GVHD.6 The MPA AUC estimates in patients with chronic GVHD6 were also higher than in patients who received MMF early after HCT to prevent acute GVHD30 but closely similar to MPA AUC estimates in patients who received MMF to prevent rejection after kidney transplantation.29
Although trough levels of MPA correlate poorly with the MPA AUC,30,35 total MPA trough concentrations of 1 to 4 μg/mL have been provisionally considered within the therapeutic range in kidney transplantation recipients.22,36,37 Total MPA trough concentrations among patients taking MMF at 1 g orally twice daily in the current study approximated this therapeutic range. The results of our study did not fit the expectation that total MPA trough concentrations would be similar among patients taking cyclosporine compared with those not taking cyclosporine despite the difference in MMF dose, suggesting that HCT or chronic GVHD might interfere with enterohepatic recirculation. On the other hand, the 14 ng/mL median trough concentrations of biologically active free MPA among patients taking MMF at 750 mg twice daily for 3 months were comparable with the 12 to 24 ng/mL concentrations reported for islet cell transplantation recipients who were not taking cyclosporine and who had MMF doses adjusted according to tolerance and side effects.38 Moreover, the trough concentrations of total and free MPA among patients taking MMF at 750 mg twice daily exceeded those reported for patients who took MMF at 1000 mg twice daily together with cyclosporine to prevent acute GVHD.30 Among patients taking oral MMF at 1000 mg 3 times daily or 1500 mg twice daily to prevent acute GVHD, median total MPA trough concentrations were approximately 1 μg/mL, and free MPA trough concentrations were 15 to 18 ng/mL,39 comparable with results observed among patients taking MMF at 750 mg twice daily in the current study. Higher doses of MMF in the current study would probably have increased the risk of thrombocytopenia and possibly have increased the risk of recurrent malignancy, whereas lower doses of MMF would probably have diminished any possible benefits. Although samples were not obtained for full pharmacokinetic analysis, the available data suggest that inappropriately high or low doses of MMF did not account for the failure of MMF to produce favorable results in this study.
The balance between a possibly increased rate of treatment success together with an increased hazard of treatment failure suggests that the rate of steroid withdrawal in the MMF arm was appropriate. The similar mean overall GVHD severity scores and the similar prevalence of complete response across time in the 2 arms also suggest that the rate of steroid withdrawal could not have been accelerated in the MMF arm. For these reasons, we surmise that the failure of MMF to produce favorable results did not result from inappropriately slow withdrawal of steroid withdrawal in the MMF arm.
Even at an optimal dose of MMF and with an appropriate rate of steroid withdrawal, the results of this study indicate that MMF did not provide any overall benefit as a component in the initial treatment of chronic GVHD. In this respect, the results are similar to those of a previous randomized trial evaluating the addition of azathioprine to prednisone for initial treatment of chronic GVHD.40 Azathioprine and MMF induce cytotoxic effects on proliferating lymphocytes through different mechanisms,41 but neither trial showed an increase in the proportion of patients with complete response. Addition of azathioprine to the treatment regimen was associated with an increased risk of infection and decreased survival. The azathioprine study did not include a hazard ratio analysis of recurrent malignancy, but 10 of 63 patients (16%) in the azathioprine arm died with recurrent malignancy, compared with 14 of 63 patients (22%) in the control arm, suggesting that azathioprine did not adversely affect the risk of recurrent malignancy. In contrast, results of the current study provoke concern that prolonged treatment with MMF might be associated with an increased risk of recurrent malignancy, particularly among patients taking MMF at 1000 mg twice daily together with cyclosporine.
Our results raise some points for consideration by investigators interested in chronic GVHD. First, clinical trials should use randomized designs whenever possible. Case-series reports and uncontrolled phase 2 studies suggested encouraging results with the use of MMF for treatment of steroid-refractory chronic GVHD,6-15 but our results with the use of MMF for initial treatment of chronic GVHD offer no support for the use of MMF in the management of steroid-refractory chronic GVHD. Second, the field would benefit from a higher level of enthusiasm and support for enrolling patients in clinical trials. Despite concerted efforts to facilitate participation by centers, physicians, and patients, it took 4 years to enroll 157 patients in our study. Progress would be enhanced if physicians could more readily acknowledge the limitations of previous studies, thereby maintaining appropriate clinical equipoise with respect to unproven treatments. Enrollment might also be enhanced if the burden of participation for patients could be kept as low as possible so as to avoid “trial fatigue.” Third, clinical trials should involve collaborations between investigators and industry so that trials can use double-blind designs to produce robust results. Blinding in chronic GVHD trials is especially important because objective measures remain difficult to document and because clinical endpoints unavoidably involve subjective judgment. Finally, improved understanding of the pathophysiology of chronic GVHD will be needed to develop more effective approaches for treatment.42 A challenge for the future will be the discovery of approaches that can target the cause of chronic GVHD while permitting reconstitution of immune defenses against pathogens and preserving immunologic effects of donor cells against malignant cells in the recipient.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jeanne Maffit, MaryJoy Lopez, Sheri Shanabarger, Maggie Jackson, and Barbara Manion for coordinating the study; Jane Jocom for nursing support; Peggy Adams Myers for administrative assistance; Dr Katherine Guthrie for statistical support to the DSMB; Drs Donna Neuberg, Joachim Deeg, Andrew Gilman, John Zaia, and Steve Pavletic and Ms Susan Stewart for service as members of the DSMB; Caroline McKallor and Geoff Hirschi for assistance with data management; Sheree Miller and staff at the University of Washington and Daniel McMannis and staff at Costco for pharmacy services; the coordinating liaisons and study nurses at each of the participating centers for assistance with implementing the study at each participating site; and, especially, the patients who agreed to participate in the study.
Participating centers included the Fred Hutchinson Cancer Research Center, University of Minnesota, Hackensack University Medical Center, University of Florida, Baylor University Medical Center at Dallas, Stanford University, University of Nebraska, Texas Transplant Institute, Vanderbilt University, University of Chicago, City of Hope Medical Center, Oregon Health & Science University, M. D. Anderson Cancer Center, Princess Margaret Hospital, and the University of Michigan.
This work was supported by the National Cancer Institute (grant CA98906), Department of Health and Human Services, Roche Laboratories (represented by Dr Kristine Golebski), and the National Marrow Donor Program through funding from the Office of Naval Research. The double-blind design of this study would not have been feasible without the study drug generously supplied by Roche Laboratories.
National Institutes of Health
Authorship
Contribution: P.J.M. and B.E.S. designed the study, analyzed and interpreted data, and wrote the paper; S.D.R., M.E.D.F, S.J.L., P.A.C., J.R.W., P.J.S., M.P.D., M.J., J.W.F., K.v.B., V.G., C.K., L.J.J., R.T.M., M.A., and D.W. enrolled patients, interpreted data, and wrote the paper; and P.A.J. analyzed MPA levels, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: P.J.M., P.A.J., and D.W. have received research funding from Roche Laboratories. All other authors declare no competing financial interests.
Correspondence: Paul J. Martin, Fred Hutchinson Cancer Research Center, PO Box 19024, Seattle, WA 98109-1024; e-mail: pmartin@fhcrc.org.