Abstract
Although adoptive transfer of regulatory T cells (Foxp3+ Tregs) has proven to be efficacious in the prevention and treatment of autoimmune diseases and graft-versus-host disease in rodents, a major obstacle for the use of Treg immunotherapy in humans is the difficulty of obtaining a highly purified preparation after ex vivo expansion. We have identified latency-associated peptide (LAP) and IL-1 receptor type I and II (CD121a/CD121b) as unique cell-surface markers that distinguish activated Tregs from activated FOXP3− and FOXP3+ non-Tregs. We show that it is feasible to sort expanded FOXP3+ Tregs from non-Tregs with the use of techniques for magnetic bead cell separation based on expression of these 3 markers. After separation, the final product contains greater than 90% fully functional FOXP3+ Tregs. This novel protocol should facilitate the purification of Tregs for both cell-based therapies as well as detailed studies of human Treg function in health and disease.
Introduction
Regulatory T cells (CD4+FOXP3+, Tregs) are central to the maintenance of self-tolerance and the control of immune homeostasis.1 A diminished frequency or dysfunction of Tregs has been reported in many human diseases, including systemic lupus erythematosus (SLE),2 type 1 diabetes,3 multiple sclerosis,4 aplastic anemia,5 idiopathic thrombocytopenic purpura,6 graft-versus-host disease (GVHD)7 and transplant rejection.8 A detailed cellular and molecular understanding of the mechanisms of action of Tregs should provide a strong foundation for pharmacologic and therapeutic manipulation of their functions. Although numerous mechanisms have been proposed to explain the suppressive function of Tregs, none appears to be unifying, and the development of immunomodulating pharmacotherapies has been limited.9 In mouse models, adoptive immunotherapy with Tregs has been shown to be effective in the prevention of experimental autoimmune encephalomyelitis,10 type 1 diabetes,11 SLE,12 autoimmune gastritis,13 inflammatory bowel disease,14 aplastic anemia,15 graft rejection,16 and GVHD.17 It is likely that CD4+FOXP3+ Tregs represent a mixture of thymic-derived and FOXP3+ Tregs that are generated at peripheral sites.18 Human Tregs can be expanded ex vivo,19,20 and their use for the cell-based tolerogenic therapy of autoimmune diseases, graft rejection, or GVHD has been advocated.21,22
The major obstacle for the use of human Tregs in cell-based therapy is the difficulty of obtaining a highly pure population after ex vivo expansion. A CD4+FOXP3+ population of greater than 90% purity can be isolated by fluorescence-activated cell sorting (FACS) of the top 2% to 4% of CD4+ T cells with high CD25 expression (CD25hi) from peripheral blood, but frequently the percentage of FOXP3+ T cells decreases to 75% after 1 week and to 50% after 2 weeks of expansion by stimulation with anti-CD3/CD28 and IL-2.23 A more complex problem is the validity of FOXP3 as a bona fide marker of human Tregs. We have recently shown that expression of FOXP3 can be induced by T-cell receptor (TCR) stimulation of human CD4+CD25−FOXP3− T cells in the presence of TGFβ, but the induced cells lack all the functional properties of Tregs.24 Because TGFβ is present in the serum used for cultures, a similar induction of FOXP3 expression in contaminating FOXP3− T cells may occur during expansion cultures of partially purified Tregs. Although the expanded population might appear to be highly enriched in FOXP3+ cells, many of these cells may be induced FOXP3+ cells that lack Treg functions.
We have identified 3 unique cell-surface markers, latency-associated peptide (LAP) and IL-1 receptor type I (CD121a) and II (CD121b) that are selectively expressed on activated Tregs, but not on activated CD4+FOXP3− or induced FOXP3+ cells. We have used these cell-surface markers to design a protocol that allows for purification of FOXP3+ Tregs from ex vivo expansion cultures, starting with leukapheresis preparations and using only magnetic bead targeting reagents. The final Treg product is composed of greater than 90% FOXP3+ cells that is highly anergic and suppressive in vitro. This method provides an important advance for the preparation of Tregs for cell-based immunotherapy to treat or prevent autoimmunity and transplantation-related complications. Moreover, given the limitation of human blood samples, particularly in the pediatric population, and the importance of obtaining a highly purified Treg population for functional and genomic analyses, we show that with this technique it is possible to expand and purify Tregs from 5 to 10 mL of blood volume to achieve high numbers and purity for further studies.
Methods
Cell purification
Leukapheresis products containing approximately 5 × 109 cells were obtained from healthy adult donors by the Department of Transfusion Medicine at the National Institutes of Health (NIH). The acquisition of blood products was approved according to the policies of the NIH in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were obtained from 5 to 10 mL blood from patients with primary Sjögren syndrome or SLE who participated in Institutional Review Board-approved protocols at the National Institute of Dental and Craniofacial Research and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (Bethesda, MD). Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. PBMCs were prepared over Ficoll-Paque Plus gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom). For FACS, CD4+ cells were enriched over the AutoMACS Pro Separator by positive selection with human CD4 microbeads (Miltenyi Biotec, Auburn, CA). The cells were labeled with CD4 FITC, CD25 PE, CD45RA PE-Cy5.5 (all Invitrogen, Carlsbad, CA) and CD127 Alexa Fluor 647 (BD Biosciences, San Jose, CA). The FACSVantage DiVa or FACSAria flow cytometer was used to sort Tregs by gating on the top 2% CD25hi and non-Tregs by gating on CD4+CD25−CD127+CD45RA+ cells. For magnetic bead purification based on CD25, the Miltenyi CD4+CD25+ Regulatory T Cell Isolation Kit was used with a modified manufacturer's protocol. In brief, all non-CD4+ cells were depleted over the AutoMACS Pro with a cocktail of biotin-conjugated mAbs against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ, and CD235a. The unlabeled CD4+ T cells were incubated with CD25 microbeads (5 μL/107 CD4+) and positively selected over the AutoMACS Pro with Posseld2 program. For magnetic bead purification based on CD25 and CD127, the Miltenyi CD4+CD25+CD127dim/− Regulatory T Cell Isolation Kit was used with a similar protocol. For the one-step method of CD25+ cell purification, total PBMCs were incubated with CD25 microbeads (2 μL/107 cells) for 20 minutes at 4°C and positively selected over the AutoMACS Pro with Posseld2 program. For magnetic bead sorting of in vitro–expanded LAP+, CD121a+, or CD121b+ cells, the cells were stained with either anti-LAP PE or anti-CD121a PE followed by anti-PE microbeads or anti-CD121b biotin followed by antibiotin microbeads (both Miltenyi Biotec) then positively selected over the AutoMACS Pro with Possels then repeat with Possel program.
Reagents
Antibodies conjugated to PE were as follows: CD73, CD120b, CD127, CD134, CD137, CD278 (from BD Biosciences); CD39, CD101 (from eBioscience, San Diego, CA); and GITR (from Miltenyi Biotec). For staining of CD121a, CD121b, and LAP, anti-LAP PE or anti-CD121a PE (both R&D Systems, Minneapolis, MN) and anti-CD121b biotin followed by secondary staining with streptavidin PE or APC (BD Biosciences) were used. For intracellular staining of FOXP3, the cells were fixed and permeabilized with a Fixation/Permeabilization kit and stained with anti-FOXP3 mAb Alexa Fluor 488 or 647 clone 236A/E7 (eBioscience). All cells were cultured in complete media consisting of RPMI 1640 supplemented with 5% heat-inactivated autologous serum, penicillin (100 U/mL), streptomycin (100 μg/mL), 2 mM l-glutamine, 10 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (all BioSource International, Camarillo, CA) and 50 μM 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO).
In vitro expansion
Bead-purified CD4+CD25+ T cells (10 × 106) were stimulated with anti-CD3/CD28–conjugated Dynabeads Human Treg Expander (Invitrogen) at 4:1 ratio of cell-to-bead in complete media supplemented with 100 U/mL IL-2 (PeproTech, Rocky Hill, NJ) in the absence or presence of 25 nM rapamycin (Sigma-Aldrich). The cells were stimulated in 12-well culture plates (Corning, Corning, NY) at 2 × 106 cells/well in 2 mL media. On day 5, the cells were transferred to 75-cm2 culture flasks (Nalge Nunc International, Rochester, NY) with additional fresh complete media and 100 U/mL IL-2. No additional rapamycin was added to the rapamycin cultures. The cultures were maintained at cell concentration of 106/mL and split every 3 days with additional fresh IL-2 media. On day 12, the cells were washed and given fresh IL-2 media before restimulation with additional Dynabeads at 4:1 cell-to-bead ratio. After 48-hour stimulation, the cells were analyzed and purified based on the expression of LAP, CD121a, or CD121b.
For de novo induction of FOXP3, CD4+CD25−CD127+CD45RA+ or CD45RO+ T cells sorted by FACS were stimulated with anti-CD3/CD28–conjugated Dynabeads at a 4:1 cell-to-bead ratio in complete media with 100 U/mL IL-2 and 5 ng/mL TGFβ1 (PeproTech). On day 3, the Dynabeads were removed, and the cells were rested in IL-2 media. On day 7, the cells were restimulated with Dynabeads.
In vitro suppression assay
Fresh allogeneic CD4+CD25− T cells (50 000) sorted by FACS were stimulated with 50 000 irradiated (40 Gy [4000 rad]) autologous CD3-depleted PBMCs and 0.25 μg/mL OKT3 (Ortho Biotech Products, Bridgewater, NJ) alone or with various numbers of suppressor cells. The cells were cultured for 3 days in 96-well flat-bottom plates (Corning) and pulsed with 3H-TdR (1 μCi [0.037 MBq]/well) for the last 6 to 8 hours. In similar experiments, fresh allogeneic CD4+CD25− T cells were labeled with 2 μM CFSE (Invitrogen) and stimulated with CD3-depleted PBMCs and 0.5 μg/mL OKT3 alone or with 4:1 and 8:1 responder to suppressor cells. The cells were cultured for 3 days, and CFSE dilution was analyzed by FACS.
Flow cytometric analysis
FACSCalibur was used for data acquisition, and the data were analyzed with FlowJo software (TreeStar, Ashland, OR). For analysis of intracellular cytokine production, the cells were stimulated for 5 hours with 50 ng/mL PMA and 1 μg/mL ionomycin (Sigma-Aldrich) along with 3 μg/mL brefeldin A (eBioscience). Afterward, the cells were fixed and permeabilized with eBioscience kit and stained with anti-FOXP3 Alexa Fluor 647, anti-IFNγ Alexa Fluor 488, and anti–IL-2 PE (Invitrogen).
Statistical analysis
All group results are expressed as mean plus or minus SD, if not stated otherwise. The paired Student t test was used for the comparison of group values and discriminatory parameters, where appropriate. P values less than .05 were considered significant.
Results
Magnetic bead purification of human CD4+CD25+CD127lowFOXP3+ Tregs
Although expression of CD25 and Foxp3 are highly correlated in mouse CD4+ T cells, in humans only those CD4+ T cells expressing high levels of CD25 are uniformly FOXP3+ (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Stringent FACS of the top 2% to 4% CD4+CD25hi cells remains the best method of obtaining a greater than 90% FOXP3+ population of Tregs, but results in a yield of only 20% to 40% of the total FOXP3+ Tregs as the remainder are hidden within the population expressing intermediate level of CD25 (CD25int) consisting mainly of recently activated and memory FOXP3− T cells. We have tested a large panel of mAbs that have been claimed to specifically recognize Tregs for their reactivity with freshly explanted human PBMCs to determine whether any correlation could be detected between FOXP3 expression and their target antigens (Figure S1A). As has been previously reported,25,26 only expression of low levels of CD127 appeared to correlate with FOXP3 expression. Expression of CD39, CD73, CD101, GITR, CD134 (OX40), CD137 (4-1BB), CD278 (ICOS), or CD120b (TNF RII) failed to consistently correlate with FOXP3 expression only.
Low levels of expression of CD127 alone are not helpful for isolating Tregs, but selection of cells expressing low levels of CD127 in combination with CD25 does result in a higher yield of FOXP3+ T cells because it captures some of the CD4+CD25intFOXP3+ Tregs (Figure S1B). Although CD127 depletion followed by CD25 bead purification resulted in a higher percentage of FOXP3+ T cells (mean, 81%; range, 70%-90%; n = 10) than purification with CD25 beads alone (mean, 76%; range, 63%-90%; n = 10), the purity achieved by FACS of CD4+CD25hi cells (mean, 94%; range, 90%-98%; n = 20) was always significantly greater (Figure S1C,D).
In vitro expansion of CD127lowCD25+ Tregs results in the outgrowth of CD4+FOXP3− T cells and CD4+FOXP3+ non-Tregs
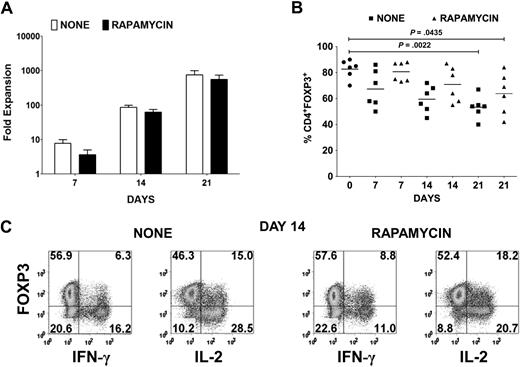
Cardinal features of FOXP3+ Tregs are their nonresponsiveness to TCR stimulation and their requirement for exogenous cytokines, in particular IL-2, to proliferate. Therefore, in vitro expansion of Tregs necessitates strong CD3/CD28 stimulation in the presence of high concentration of IL-2.19 Under these conditions, the suppressive effects of the Tregs are masked, and any contaminating CD4+CD25+FOXP3− cells are likely to overgrow the culture. Using a leukapheresis product containing approximately 5 × 109 cells from healthy donors followed by Ficoll gradient separation of mononuclear cells, we initially depleted all non-CD4+ and CD127+ cells with magnetic beads and then enriched for CD25+ cells to achieve a final yield of 20 to 60 × 106 CD4+CD25+ T cells. Several reports have shown that FOXP3− T cells are more susceptible to rapamycin-induced apoptosis, whereas Tregs are more resistant because of their constitutive expression of pim 2, a serine/threonine kinase with antiapoptotic effects.27,28 Therefore, 10 × 106 of the CD25+ population was stimulated with anti-CD3/CD28–conjugated beads and IL-2 in the absence or presence of rapamycin. Although there was a reduction of up to 50% in expansion of the CD25+ T cells in the presence of rapamycin for the first week, on subsequent restimulation in the absence of rapamycin, the expansion was only 20% to 30% less (Figure 1A). However, there was a progressive reduction in FOXP3 purity in the expansion cultures from an average of 82% (range, 70%-90%) FOXP3+ cells in the starting population to a mean of 67% (range, 50%-86%) on day 7, 60% (range, 45%-72%) on day 14, and 53% (range, 40%-67%) on day 21 (Figure 1B). In the presence of rapamycin, there was an enhancement in FOXP3 purity with an average of 81% (range, 73%-88%) on day 7, 71% (range, 55%-87%) on day 14, and 64% (range, 42%-84%) on day 21. Because we have previously shown that not all activated FOXP3+ T cells are Tregs based on their lack of anergic properties, we evaluated whether FOXP3+ non-Tregs were present in the expansion cultures. Indeed, a significant percentage of IFN-γ– and IL-2–producing cells were present in both expansion cultures after 14 days (Figure 1C). Most of these cells expressed lower levels of FOXP3 than did the cytokine nonproducers.
Treg expansion cultures contain cytokine-producing FOXP3− and FOXP3+ non-Tregs. (A) Fold expansion of magnetic bead–purified CD4+CD127lowCD25+ T cells from 6 donors after stimulation with anti-CD3/CD28 and IL-2 in the absence (none) or presence of rapamycin. Error bars represent SEM. (B) Percentage of CD4+FOXP3+ T cells in the starting population and after expansion of CD4+CD127lowCD25+ T cells as described in panel A. Horizontal lines represent the mean of each group. (C) Day 14 expansion cultures generated in the absence (none) or presence of rapamycin were restimulated for 5 hours with PMA/ionomycin. IFN-γ and IL-2 production was evaluated by intracellular staining. Data are representative of 6 independent experiments. The number in each quadrant represents the percentage of total population.
Treg expansion cultures contain cytokine-producing FOXP3− and FOXP3+ non-Tregs. (A) Fold expansion of magnetic bead–purified CD4+CD127lowCD25+ T cells from 6 donors after stimulation with anti-CD3/CD28 and IL-2 in the absence (none) or presence of rapamycin. Error bars represent SEM. (B) Percentage of CD4+FOXP3+ T cells in the starting population and after expansion of CD4+CD127lowCD25+ T cells as described in panel A. Horizontal lines represent the mean of each group. (C) Day 14 expansion cultures generated in the absence (none) or presence of rapamycin were restimulated for 5 hours with PMA/ionomycin. IFN-γ and IL-2 production was evaluated by intracellular staining. Data are representative of 6 independent experiments. The number in each quadrant represents the percentage of total population.
LAP, CD121a, and CD121b are expressed on activated FOXP3+ Tregs but not on FOXP3− and FOXP3+ non-Tregs
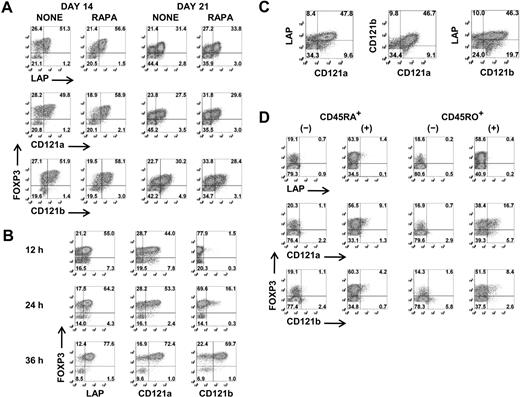
Although a combination of high levels of expression of CD25 and low levels of expression of CD127 facilitates the identification and isolation of freshly explanted Tregs, activation of CD4+CD25−/intFOXP3− T cells results in up-regulation of CD25 and down-regulation of CD127. It is therefore impossible to separate activated Tregs from activated non-Tregs based on differential expression of these surface markers. We therefore tested a panel of antibodies with purported specificity for FOXP3+ Tregs for their ability to distinguish expanded FOXP3+ T cells from activated FOXP3− T cells. None of the mAbs consistently differentiated FOXP3+ T cells during the expansion cultures before or after restimulation (Figure S2). In some studies, CD278 (ICOS) and CD137 (4-1BB) appeared to be selectively expressed on activated Tregs, but this result was highly variable and was observed approximately 50% of the time. However, when we restimulated the day 12– and day 19–expanded cells for 48 hours and repeated the staining, anti-LAP, anti-CD121a, and anti-CD121b specifically and consistently reacted with FOXP3+ T cells and not the FOXP3− T cells (Figure 2A). Similar to expanded Tregs, LAP and CD121a can be detected within 12 hours and CD121b after 24 hours of stimulation on freshly isolated Tregs, and all 3 surface markers are expressed optimally between 24 and 48 hours (Figure 2B) and disappear within 48 to 72 hours after stimulation (data not shown). LAP, CD121a, and CD121b were coexpressed on the activated Tregs, although approximately 10% to 20% remained negative (Figure 2C). LAP has been previously reported to be a Treg-specific marker,29,30 although there was no direct correlation to FOXP3. Our previous studies had identified LAP on FOXP3+ Tregs that had been previously activated via their TCRs.31 CD121a and CD121b was selected because our preliminary microarray studies had suggested that they were differentially expressed on activated Tregs but not TGFβ-induced FOXP3+ T cells. Interestingly, the TGFβ-induced FOXP3+ non-Tregs fail to express LAP, CD121a, or CD121b after primary stimulation and with subsequent restimulation (Figure 2D). Therefore, our result indicates that LAP, CD121a, and CD121b are uniquely expressed on activated FOXP3+ Tregs and might represent thymic-derived and not peripheral-converted Tregs.
Selective expression of LAP, CD121a, and CD121b on activated Tregs. (A) Flow cytometric analysis of surface LAP, CD121a, and CD121b and intracellular FOXP3 expression on day 14 and day 21 Treg expansion cultures after restimulation for 48 hours with anti-CD3/CD28 (data are from 1 representative donor of 6). (B) Kinetics of LAP, CD121a, and CD121b expression on fresh Tregs stimulated with anti-CD3/CD28 and 100 U/mL IL-2. (C) Costaining of LAP, CD121a, and CD121b on 48-hour restimulated day 14 cultures (data are from 1 donor representative of 6). (D) Expression of LAP, CD121a, and CD121b on 48-hour restimulated day 14 CD4+CD25−CD127+CD45RA+ and CD45RO+ T cells previously stimulated on day 0 with anti-CD3/CD28 and IL-2 for 5 days in the absence (−) or presence (+) of TGFβ1 and rested in IL-2 until day 12. Data are representative of 3 independent experiments. Number in each quadrant represents the percentage of total population.
Selective expression of LAP, CD121a, and CD121b on activated Tregs. (A) Flow cytometric analysis of surface LAP, CD121a, and CD121b and intracellular FOXP3 expression on day 14 and day 21 Treg expansion cultures after restimulation for 48 hours with anti-CD3/CD28 (data are from 1 representative donor of 6). (B) Kinetics of LAP, CD121a, and CD121b expression on fresh Tregs stimulated with anti-CD3/CD28 and 100 U/mL IL-2. (C) Costaining of LAP, CD121a, and CD121b on 48-hour restimulated day 14 cultures (data are from 1 donor representative of 6). (D) Expression of LAP, CD121a, and CD121b on 48-hour restimulated day 14 CD4+CD25−CD127+CD45RA+ and CD45RO+ T cells previously stimulated on day 0 with anti-CD3/CD28 and IL-2 for 5 days in the absence (−) or presence (+) of TGFβ1 and rested in IL-2 until day 12. Data are representative of 3 independent experiments. Number in each quadrant represents the percentage of total population.
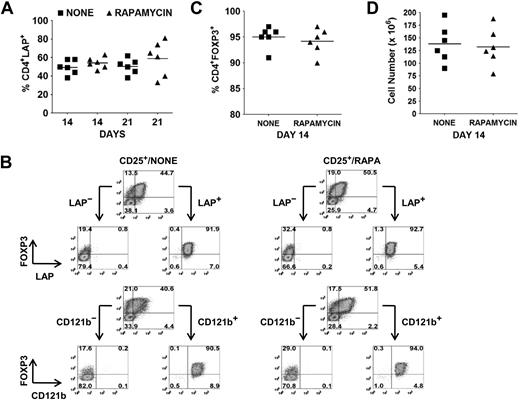
Selective expression of LAP, CD121a, or CD121b allows for separation of Tregs from non-Tregs in ex vivo expansion cultures
The identification of LAP, CD121a, and CD121b as specific markers of activated Tregs provides an opportunity to separate activated FOXP3+ and FOXP3− cytokine-producing non-Tregs from activated FOXP3+ non–cytokine-producing Tregs by FACS or magnetic bead purification. As previously shown, the average percentages of FOXP3+ Tregs in the absence or presence of rapamycin on day 14 and 21 after 48 hours of restimulation were 60% versus 71% and 53% versus 64%, respectively. At the same time, the average percentages of LAP+ cells in the absence or presence of rapamycin on day 14 and 21 were 50% (range, 38%-58%) versus 54% (range, 46%-63%) and 51% (range, 38%-62%) versus 59% (range, 33%-81%), respectively (Figure 3A). Using magnetic bead selection for LAP on day 14–expanded cultures, we were able to purify the Tregs to a level of greater than 90% FOXP3+ from both populations (Figure 3B,C). The average cell yield was 138 × 106 in the absence and 132 × 106 Tregs in the presence of rapamycin, starting with 10 × 106 CD25+ cells (mean, 82% FOXP3+; range 70%-90%) on day 0 (Figure 3D). Similar results were obtained with CD121a and CD121b bead separation (data not shown).
Selective expression of LAP and CD121b allows for separation of Tregs from non-Tregs in ex vivo expansion cultures. (A) Percentage of CD4+LAP+ cells after 48 hours of restimulation of day 14 and 21 expansion cultures. (B) Day 14 expansion cultures generated in the absence (CD25+/NONE) or presence (CD25+/RAPA) of rapamycin were restimulated for 48 hours with anti-CD3/CD28. LAP+/LAP− and CD121b+/CD121b− fractions were then purified with magnetic beads and analyzed by flow cytometry for FOXP3 expression. Number in each quadrant represents the percentage of total population. (C) Percentage of CD4+FOXP3+ and (D) cell yield after purification of LAP+ cells from restimulated 14-day expansion cultures. Horizontal lines in panels A, C, and D represent the mean of each group.
Selective expression of LAP and CD121b allows for separation of Tregs from non-Tregs in ex vivo expansion cultures. (A) Percentage of CD4+LAP+ cells after 48 hours of restimulation of day 14 and 21 expansion cultures. (B) Day 14 expansion cultures generated in the absence (CD25+/NONE) or presence (CD25+/RAPA) of rapamycin were restimulated for 48 hours with anti-CD3/CD28. LAP+/LAP− and CD121b+/CD121b− fractions were then purified with magnetic beads and analyzed by flow cytometry for FOXP3 expression. Number in each quadrant represents the percentage of total population. (C) Percentage of CD4+FOXP3+ and (D) cell yield after purification of LAP+ cells from restimulated 14-day expansion cultures. Horizontal lines in panels A, C, and D represent the mean of each group.
We have presented the CD127 depletion, CD25 bead selection method to show that even using the current “gold standard” method of purifying a starting population of FOXP3+ Tregs, the outgrowth of FOXP3− T cells is a major issue after the expansion cultures. However, we also have used a previously published protocol involving a one-step method of purifying Tregs with just CD25 magnetic beads.32 With this method, the starting population contains an average of 62% CD4+FOXP3+ T cells (range, 47%-86%) with the remainder being B cells and non-CD4/CD8 cells. During the 21-day expansion, there was not a dramatic decrease in FOXP3 purity because of the disappearance of these contaminating non-T cells and an expansion of CD4+FOXP3− T cells (Figure S3A). We then can purify the Tregs from the expanded cultures with anti-LAP to obtain results similar to what we achieve with the multiple antibody approach (Figure S3C-E). This procedure would be more practical and feasible, because it would require the development of only one new GMP reagent: anti-LAP, anti-CD121a, or anti-CD121b.
LAP+, CD121a+, and CD121b+ FOXP3+ T cells are fully functional Tregs
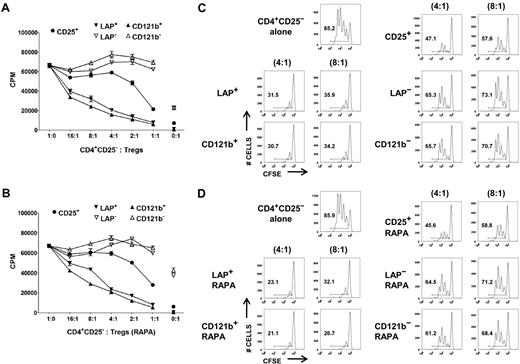
Although it is possible to obtain a highly purified FOXP3+ population based on selection of LAP+, CD121a+, or CD121b+ cells after restimulation of the expanded CD25+ cells or CD127lowCD25+ T cells, it is critical to show that the purified population is functional and represents a superior potential product for immunotherapy than the unseparated population. We evaluated the capacity of LAP+, CD121a+, and CD121b+ Tregs purified from day 14 and 21 expansion cultures to suppress the proliferative responses of CD4+CD25− T cells in both 3H-TdR uptake and CFSE dilution assays. LAP+ and CD121b+ Tregs purified from CD127lowCD25+ cells expanded in the absence or presence of rapamycin were more potent suppressors than was the unseparated expanded Treg population in both assays (Figure 4). The LAP− and CD121b− populations showed minimal suppressive activity. Similarly, LAP+, CD121a+, and CD121b+ Tregs purified from single-step CD25+ cells on day 21 of expansion were highly suppressive and more superior to the unseparated population (Figure 5A,D). Because LAP represents the surface membrane TGFβ complex, we assessed whether the LAP+ Tregs mediate their suppression via TGFβ. We were unable to show that suppression was abrogated in the presence of high concentrations of neutralizing anti-TGFβ mAb (50 μg/mL) or recombinant LAP (5 μg/mL) or with siRNA knockdown of TGFβ1 that prevented LAP expression (data not shown).
LAP+ and CD121b+ Tregs are anergic and manifest potent T-suppressor activity. CD4+CD25− T cells were stimulated with anti-CD3 and APCs alone (■) or in the presence of various numbers of unseparated CD25+ or purified LAP+, LAP−, CD121b+, and CD121b− cells from 48 hours of restimulated day 14 cultures generated in the absence (A) or presence (B) of rapamycin. Error bars represent SEM. 3H-TdR incorporation was determined after 72 hours of stimulation. CFSE-labeled CD4+CD25− T cells were stimulated with anti-CD3 and APCs alone or in the presence of unseparated CD25+ or purified LAP+, LAP−, CD121b+, and CD121b− cells from 48-hour restimulated day 14 cultures generated in the absence (C) or presence (D) of rapamycin. Cocultures were performed at responder to suppressor ratios of 4:1 and 8:1. CFSE dilution was measured by FACS analysis after 72 hours of culture. Number in each quadrant represents percentage of dividing cells from the total population. Data are from 1 donor representative of 6.
LAP+ and CD121b+ Tregs are anergic and manifest potent T-suppressor activity. CD4+CD25− T cells were stimulated with anti-CD3 and APCs alone (■) or in the presence of various numbers of unseparated CD25+ or purified LAP+, LAP−, CD121b+, and CD121b− cells from 48 hours of restimulated day 14 cultures generated in the absence (A) or presence (B) of rapamycin. Error bars represent SEM. 3H-TdR incorporation was determined after 72 hours of stimulation. CFSE-labeled CD4+CD25− T cells were stimulated with anti-CD3 and APCs alone or in the presence of unseparated CD25+ or purified LAP+, LAP−, CD121b+, and CD121b− cells from 48-hour restimulated day 14 cultures generated in the absence (C) or presence (D) of rapamycin. Cocultures were performed at responder to suppressor ratios of 4:1 and 8:1. CFSE dilution was measured by FACS analysis after 72 hours of culture. Number in each quadrant represents percentage of dividing cells from the total population. Data are from 1 donor representative of 6.
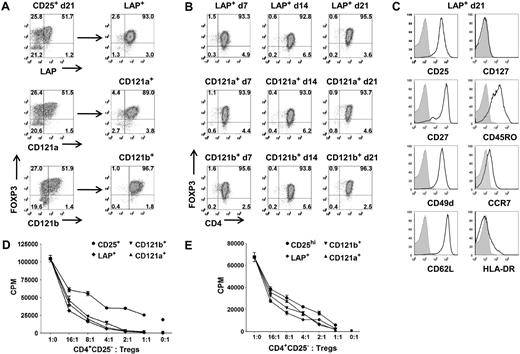
LAP+, CD121a+, and CD121b+ Tregs maintain purity, suppressive function, and phenotype after expansion. (A) Expression and purification based on LAP, CD121a, and CD121b on 48-hour restimulated CD25+ cells initially obtained with one-step CD25 selection method and expanded for 21 days. (B) Analysis of FOXP3 during an additional 21 more days of expansion for LAP+, CD121a+, and CD121b+ Tregs with anti-CD3/CD28 Dynabeads and IL-2 and (C) their typical Treg surface markers at the end of the 21-day expansion (42 total days in culture). Data are representative of CD121a+ and CD121b+ Tregs as well. Numbers in quadrants in panels A and B represent percentage of total population. (D) In vitro suppression assay of day 21 unseparated CD25+ cells (CD25+) and postpurified LAP+, CD121a+, and CD121b+ Tregs from panel A. Error bars represent SEM. (E) In vitro suppression assay of fresh Tregs (CD25hi) and 21-day expanded LAP+, CD121a+, and CD121b+ Tregs from panel B. Error bars represent SEM.
LAP+, CD121a+, and CD121b+ Tregs maintain purity, suppressive function, and phenotype after expansion. (A) Expression and purification based on LAP, CD121a, and CD121b on 48-hour restimulated CD25+ cells initially obtained with one-step CD25 selection method and expanded for 21 days. (B) Analysis of FOXP3 during an additional 21 more days of expansion for LAP+, CD121a+, and CD121b+ Tregs with anti-CD3/CD28 Dynabeads and IL-2 and (C) their typical Treg surface markers at the end of the 21-day expansion (42 total days in culture). Data are representative of CD121a+ and CD121b+ Tregs as well. Numbers in quadrants in panels A and B represent percentage of total population. (D) In vitro suppression assay of day 21 unseparated CD25+ cells (CD25+) and postpurified LAP+, CD121a+, and CD121b+ Tregs from panel A. Error bars represent SEM. (E) In vitro suppression assay of fresh Tregs (CD25hi) and 21-day expanded LAP+, CD121a+, and CD121b+ Tregs from panel B. Error bars represent SEM.
Even starting with a Treg population of greater than 90% FOXP3 positivity after FACS on CD4+CD127−CD25hi, there is typically a progressive loss of FOXP3 purity in the expansion cultures either secondary to the outgrowth of the few contaminating non-Tregs or a down-regulation of FOXP3. It is conceivable that there exists some peripherally converted, noncommitted FOXP3+ T cells that would lose their FOXP3 and revert back to effector T cells during the expansion cultures. Therefore, we evaluated whether we can continue expanding the purified LAP+, CD121a+, and CD121b+ Tregs to increase their cell numbers while maintaining their purity and phenotype. The Tregs purified from the expansion cultures after 14 or 21 days could be further expanded to increase their cell yield while maintaining their FOXP3 purity (Figure 5A,B; Figure S4A). The expanded Tregs continue to retain their surface phenotype (CD25+, CD127−, CD27+, CD62L+) and suppressive functions (Figure 5C,E; Figure S4C).
In addition to their suppressive function, FOXP3+ Tregs are characterized by their nonresponsiveness to TCR stimulation in vitro. The LAP+, CD121a+, and CD121b+ populations were also completely nonresponsive when cultured alone, whereas the LAP−, CD121a−, and CD121b− populations showed substantial proliferative responses. A more quantitative assay for the anergic state of Tregs is the measurement of cytokine production on a per cell basis by intracellular staining. After 14 days of expansion, the cultures were stimulated for 5 hours with PMA and ionomycin. Low percentages of IL-2–, TNF-α–, IFN-γ–, and IL-17–producing cells were detected in the FOXP3+ population, and higher percentages were found in the FOXP3− population, but no significant IL-4 or IL-10 producers were detected (Figure S5A). After purification with anti-LAP, the majority of the IL-2–, IFN-γ–, and IL-17–producing cells could be readily detected in both the FOXP3lowLAP− and FOXP3−LAP− populations, and few could be detected in the LAP+ population (Figure S5B,C). We have analyzed the cytokine profiles at other time points, including day 7 and day 21 of the expansion cultures, with similar results (data not shown). Taken together, these functional studies confirm that, compared with the unseparated population of expanded CD4+CD25+CD127low or CD25+ cells, the purified LAP+, CD121a+, and CD121b+ FOXP3+ populations are a relatively pure population of Tregs. All 3 populations appear to be similar in phenotype and function so that there does not seem to be an advantage in any of the 3 markers for purification.
Application of method to obtain sufficient Treg number and purity from small blood samples of patients
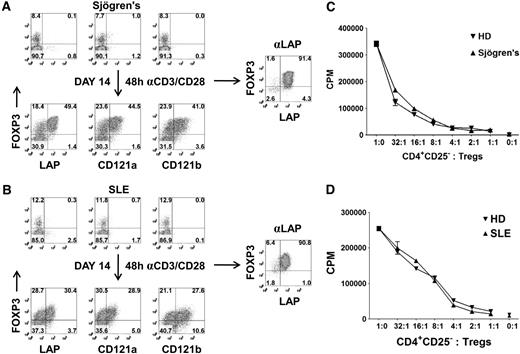
A major obstacle in studying Tregs from patients, particularly the pediatric population, is the difficulty of obtaining sufficient Treg numbers and purity for functional and genomic analysis. For these reasons, most human studies evaluating Tregs are restricted to surface phenotyping. To overcome these limitations, we have evaluated whether our expansion and repurification method can be used to isolate sufficient numbers of Tregs at high purity when starting with a sample of 5 to 10 mL blood from adults. CD25+ cells were isolated with magnetic beads from patients with Sjögren syndrome or SLE and expanded in vitro for 14 days. We typically obtain approximately 1 to 2 × 106 CD25+ cells from 5 to 10 mL blood and achieve a 25- to 50-fold expansion after 14 days of culture. Consistent with normal healthy donors, there were selective expression of LAP, CD121a, and CD121b on the FOXP3+ cells after 48 hours of restimulation of the expanded populations (Figure 6A,B). Using anti-LAP magnetic bead purification, we were able to reisolate the Tregs from the expansion cultures with greater than 90% FOXP3 purity and a cell yield ranging from 10 to 25 × 106 cells. LAP+ Tregs from patients with Sjögren syndrome or SLE were anergic and suppressive (Figure 6C,D). The application of this technique should facilitate the detailed analysis of Tregs from patients with diseases in which Treg function may be abnormal.
Expansion and purification of Tregs from patients with Sjögren syndrome and SLE with 5 to 10 mL peripheral blood. Expression of LAP, CD121a, CD121b, and FOXP3 on CD4+ T cells from a patient with (A) Sjögren syndrome or (B) SLE (top). Correlation of LAP, CD121a, and CD121b with FOXP3 on 48-hour restimulated CD25+ cells from one-step CD25 selection method expanded for 14 days (bottom). Numbers in each quadrant represent percentage of total population. Right panel represents the FOXP3 purity after reisolation with anti-LAP from day 14–expanded CD25+ cells of patients with Sjögren syndrome and SLE. In vitro suppression assay of LAP+ Tregs from patient with (C) Sjögren syndrome or (D) SLE, comparing with LAP+ Tregs from healthy control donors (HD). Data represent 1 of 3 patients with Sjögren syndrome and SLE. Error bars represent SEM.
Expansion and purification of Tregs from patients with Sjögren syndrome and SLE with 5 to 10 mL peripheral blood. Expression of LAP, CD121a, CD121b, and FOXP3 on CD4+ T cells from a patient with (A) Sjögren syndrome or (B) SLE (top). Correlation of LAP, CD121a, and CD121b with FOXP3 on 48-hour restimulated CD25+ cells from one-step CD25 selection method expanded for 14 days (bottom). Numbers in each quadrant represent percentage of total population. Right panel represents the FOXP3 purity after reisolation with anti-LAP from day 14–expanded CD25+ cells of patients with Sjögren syndrome and SLE. In vitro suppression assay of LAP+ Tregs from patient with (C) Sjögren syndrome or (D) SLE, comparing with LAP+ Tregs from healthy control donors (HD). Data represent 1 of 3 patients with Sjögren syndrome and SLE. Error bars represent SEM.
Discussion
Studies over the past 10 years have defined a critical role for Tregs in the control of all aspects of immune responses. Although several different types of Tregs have been described,33 Tregs that express the transcription factor FOXP3 have emerged as the most dominant.34 Adoptive transfer of both polyclonal and antigen-specific Foxp3+ Tregs has proven to be highly efficacious in animal models for the prevention and treatment of autoimmune diseases and in the prevention of GVHD. Adoptive Treg biotherapy has also been proposed for the treatment of autoimmune disease and prevention of GVHD in humans, but a major pitfall has been the ability to achieve a consistent, highly purified FOXP3+ Treg product after ex vivo expansion secondary to the outgrowth of contaminating non-Tregs. Recent modification of the methods used to select and expand human FOXP3+ Tregs, including the use of rapamycin, have resulted in some improvement in the yields and purity of the product, but a significant variation and percentage (∼ 25%) of FOXP3− and FOXP3+ non-Treg contaminants exist. It remains unclear whether this level of contamination would affect Treg immunotherapy in humans, but from a safety and efficacy standpoint a more pure Treg product would be desirable. It has been reported that a highly purified population of FOXP3+ human Tregs can be obtained by expansion of so-called naive CD4+CD45RA+CD25hi Tregs.23 However, it is difficult to obtain FOXP3+CD45RA+ T cells from adult peripheral blood, as the majority (> 80%) of FOXP3+ T cells are CD45RA− memory cells.35 Another concern is that any contaminating CD4+CD45RA+FOXP3− cells would be highly susceptible to conversion to FOXP3+ non-Tregs by TGFβ in the culture medium used for expansion.24 A recent publication has claimed that most Tregs are CD49d−; therefore, depletion of CD49d+ cells allows for purification of Tregs free of contaminating effector cells.36 In our healthy adult population, most Tregs are not CD49d−, and there is high variability because CD49d is up-regulated with activation. Even if the starting population is highly enriched in Tregs, their data indicate that a wide variability in Treg percentage can occur during expansion. Two recent publications showed that with the incorporation of CD127 in their Treg isolation strategies, they were able to achieve a Treg starting population of approximately 90% FOXP3 purity either by GMP-certified bead method37 or FACS.38 However, even with a starting population of greater than 90% FOXP3 purity, by day 14 of expansion there was a wide variability in FOXP3+ Tregs in the expansion cultures, ranging between 60% and 95% with an average of 75%. Regardless of what strategy is used to obtain the starting population, the novelty of our method is that it targets the expansion cultures to provide a second purification process resulting in a highly purified Treg product with low variability.
We have identified LAP, CD121a, and CD121b as unique cell- surface markers that distinguish activated Tregs from activated FOXP3− and FOXP3+ non-Tregs and show that it is feasible to sort expanded FOXP3+ Tregs from non-Tregs based on expression of these 3 markers. We obtained a postsort FOXP3 purity of greater than 90% that would be ideal for cellular biotherapy. Most importantly, the purified cell populations are completely free of FOXP3+ non-Tregs that produce IL-2, IL-17, and IFN-γ. In addition to their use in therapeutic protocols, the purified expanded Treg population should be ideal for further studies of the function and mechanism of action of human Tregs and for a more detailed analysis of potential defects in disease. We are currently investigating the potential use of these markers to identify and select for antigen-specific Tregs from in vitro cultures stimulated with antigens.
LAP, CD121a, and CD121b are atypical markers, because they are not expressed constitutively on resting or expanded FOXP3+ Tregs, but are rapidly induced and expressed only on FOXP3+ Tregs for a short period after TCR-mediated activation. CD121b is an IL-1 decoy receptor that does not transmit a signal, but may sequester IL-1 and provide Tregs with a suppressor mechanism at sites of inflammation. It also might function to limit the activity of IL-1 in the Tregs, because CD121a, the IL-1 receptor type I that is critical for binding and delivering the signal of IL-1, is expressed earlier than CD121b in the activated Tregs. These 3 markers might also be useful as a biomarker for the identification of recently activated Tregs at sites of inflammation. We have recently shown that the role of the LAP-TGFβ complex on mouse Tregs is to mediate infectious tolerance by converting Foxp3− T cells into functional Foxp3+ Tregs.31 Studies are in progress to determine whether LAP-TGFβ on human Tregs functions in a similar manner. LAP expression on the activated Tregs represents the latent TGFβ complex consisting of LAP and TGFβ. The LAP and TGFβ are produced by the Tregs, because stimulation of the Tregs with anti-CD3/CD28 and 100 U/mL IL-2 for 12 hours with monensin for the last 8 hours prevented the surface expression of LAP (data not shown). Moreover, treatment of Tregs with siRNA specific for TGFβ1 also inhibited the expression of LAP on activation.
The identification and characterization of FOXP3+ Tregs represent one of the major advances in the field of immunology over the past decade. The major issue for the future is how to translate the large body of data obtained with mouse Tregs to humans and to use Tregs as immunomodulatory agents in autoimmune diseases and transplantation. The identification of LAP, CD121a, and CD121b on activated Tregs as highly specific markers and their use in protocols for the isolation of large numbers of highly purified Tregs should facilitate the rapid advancement of the therapeutic application and functional analysis of Tregs in human disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the NIAID Flow Cytometry Section, particularly Carol Henry, Tom Moyer, and Calvin Eigsti, for all their help in sorting our cells. We also thank Cynthia Matthews in the Department of Transfusion Medicine for providing us with the leukapheresis products.
This work was supported by the Intramural Research Program of the NIAID, NIH (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: D.Q.T. proposed the project, conducted all the experiments, and wrote the manuscript; J.A. provided the supportive evidence of the selective expression of LAP in mouse Tregs; D.H. and L.B. coordinated the visits and blood samples for the patients with Sjögren syndrome or SLE; G.G.I. managed and cared for the patients with Sjögren syndrome or SLE; and E.M.S. supervised and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dat Q. Tran or Ethan M. Shevach, Laboratory of Immunology, NIAID, NIH, 10 Center Dr, Bldg 10, Rm 11N256 or 11N315, MSC 1892, Bethesda, MD 20892; e-mail: dtran@niaid.nih.gov or eshevach@niaid.nih.gov.