Human dermal fibroblasts obtained by skin biopsy can be reprogrammed directly to pluripotency by the ectopic expression of defined transcription factors. Here, we describe the derivation of induced pluripotent stem cells from CD34+ mobilized human peripheral blood cells using retroviral transduction of OCT4/SOX2/KLF4/MYC. Blood-derived human induced pluripotent stem cells are indistinguishable from human embryonic stem cells with respect to morphology, expression of surface antigens, and pluripotency-associated transcription factors, DNA methylation status at pluripotent cell-specific genes, and the capacity to differentiate in vitro and in teratomas. The ability to reprogram cells from human blood will allow the generation of patient-specific stem cells for diseases in which the disease-causing somatic mutations are restricted to cells of the hematopoietic lineage.
Introduction
Pluripotency can be induced in mouse and human somatic cells by the forced expression of OCT4 and SOX2 with either the combinations of KLF4 and MYC or NANOG and LIN28.1,,–4 In humans, induced pluripotent stem (iPS) cells are commonly generated from dermal fibroblasts. However, the requirement for skin biopsies and the need to expand fibroblast cells for several passages in vitro make it a cumbersome source for generating patient-specific stem cells. A recent study revealed that human keratinocytes isolated from plucked hair can be reprogrammed to iPS cells.5 However, it remains unclear whether hair cells will be a faithful source for reprogramming because the growth and quality of the hair follicles are dependent on the age, genotype, and the medical conditions of the human donors.6,7
The report of reprogramming of murine B cells to pluripotency establishes that somatic cells of the hematopoietic lineage are receptive to reprogramming into iPS cells.8 However, in the study, “secondary” iPS cells were derived from primary B cells of adult spleen, bone marrow, lymph nodes, or embryonic liver of mice engineered to carry doxycycline-inducible Oct4, Sox2, Klf4, and Myc retroviruses in every tissue. Thus, we set out to determine whether iPS cells could be derived from primary, nontransgenic human hematopoietic cells. In the present study, we show that mobilized human CD34+ peripheral blood cells are amenable to direct reprogramming. The CD34+-derived iPS cells are molecularly and functionally indistinguishable from human embryonic stem (ES) cells. Reprogramming from human blood cells represents a novel way of establishing iPS cells from donor cells that require little manipulation time in culture. The ability to reprogram cells from the human blood will facilitate the development of a reliable method to generate patient-specific stem cells.
Methods
Detailed methods are included in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Briefly, mobilized peripheral blood cells obtained from Allcells (Emeryville, CA) were collected from a 26-year-old male donor, and CD34+ cells (mPB014F) were isolated. Generation of CD34 iPS cells was performed using a protocol modified from previously published methods.9
Results and discussion
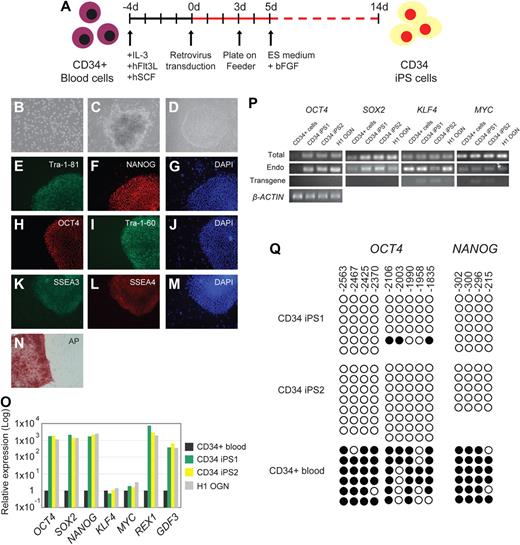
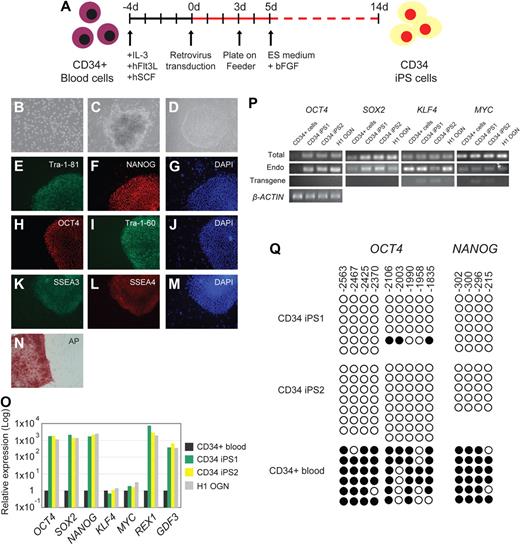
A recent attempt to reprogram mouse B cells reported that terminally differentiated B cells were more refractory to reprogramming than progenitor B cells.8 We therefore chose to reprogram human blood progenitor cells. To avoid risks associated with bone marrow harvest,10 we obtained mobilized CD34+ hematopoietic stem/progenitor cells isolated from peripheral blood.11 Because the integration and expression of retroviral constructs require mitotic division of the target cells, we first cultured CD34+ cells in vitro with a combination of hSCF, hFlt3L, and IL-3 cytokines,12 which resulted in proliferation and expansion of CD34+ cells by several orders of magnitude (Figures 1A,B, S1A). Analysis by flow cytometry over the course of the 6-day culture period revealed a progressive decrease in the percentage of CD34+/CD38− hematopoietic progenitor cells and a simultaneous increase in the percentage of cells with differentiated phenotypes (CD14, CD15; Figure S1B,C). The presence of a high proportion of differentiated or mature cells has a strong suppressive effect on the expansion capacity of hematopoietic progenitors.13 Therefore, we performed viral transduction of the CD34+ cells on day 4 of culture, when the majority of the cells were still expressing CD34 and were actively proliferating (Figure S1). Three days after transduction, cells were harvested and plated onto feeder MEF cells (Figure 1A). Human ES (hES) cell medium supplemented with 10 ng/mL basic fibroblast growth factor was added on day 5 (Figure 1A). We first detected colonies approximately 14 days after transduction; most developed into granulated cell clusters that did not have hES cell properties (Figure 1C), whereas others exhibited distinct flat and compact morphology with clear-cut round edges characteristics of hES cells (Figure 1D). From 5 × 104 CD34+ cells, we routinely observed approximately 5 to 10 hES cell-like colonies (data from 3 independent experiments). Given that the retroviral transgenes contain a Gfp marker, transduced cells were initially Gfp+, but as described previously,4 we observed silencing of Gfp in the successfully reprogrammed colonies (Figure S2). In total, we picked and expanded 8 independent Gfp− colonies and fully characterized 2 lines, CD34 iPS1 and CD34 iPS2.
Reprogramming of human blood CD34+ cells to pluripotent iPS cells. (A) Schematic drawing representing the strategy used in this study for reprogramming human CD34+ cells from the mobilized peripheral blood. (B) Morphology of the CD34+ blood cells. (C) Image of a non-ES cell-like colony. (D) Image of a hES cell-like colony. All images were acquired with a standard microscope (Nikon, Tokyo, Japan) with a 10 × objective. (E-N) Immunohistochemistry of human blood-derived iPS cell colonies expressing markers for Tra-1-81 (E), NANOG (F), OCT4 (H), Tra-1-60 (I), SSEA3 (K), SSEA4 (L), and alkaline phosphatase (AP) (N). 4,6-Diamidino-2-phenylindole (DAPI) staining indicates the total cell content per field (G,J,M). Fibroblasts surrounding human iPS colonies serve as internal negative controls for immunohistochemistry staining. Images were acquired with a standard microscope (Nikon) with a 20× objective. (O) Quantitative RT-PCR analyses for the expression of ES cell-marker genes OCT4, SOX2, NANOG, KLF4, MYC, REX1, and GDF3 in human CD34 iPS and the parental CD34+ cells. Individual PCR reactions were normalized against β-ACTIN and plotted relative to the expression level in the parental CD34+ cell. (P) Repression of the exogenously introduced transgenes as shown by quantitative RT-PCR analyses of OCT4, SOX2, MYC, and KLF4 expression. Specific primers were designed to probe for either the coding regions (Total) to measure the expression of both the endogenous gene and the transgene, 3′ untranslated region (Endo), which measure the expression of the endogenous gene only, or primers specific (Transgene) to the region of the viral transgenes. β-ACTIN is shown at the bottom as a loading control for each sample. (Q) Bisulfite genomic sequencing of the OCT4 and NANOG promoters reveals demethylation in the iPS cell lines. Each horizontal row of circles represents an individual sequencing reaction for a given amplicon. Open and filled circles represent unmethylated and methylated CpGs dinucleotides, respectively. The cell lines (CD34+ and its derivatives CD34 iPS1 and CD34 iPS2) are indicated to the left of each cluster. The values above each column indicate the CpG position analyzed relative to the downstream transcriptional start site.
Reprogramming of human blood CD34+ cells to pluripotent iPS cells. (A) Schematic drawing representing the strategy used in this study for reprogramming human CD34+ cells from the mobilized peripheral blood. (B) Morphology of the CD34+ blood cells. (C) Image of a non-ES cell-like colony. (D) Image of a hES cell-like colony. All images were acquired with a standard microscope (Nikon, Tokyo, Japan) with a 10 × objective. (E-N) Immunohistochemistry of human blood-derived iPS cell colonies expressing markers for Tra-1-81 (E), NANOG (F), OCT4 (H), Tra-1-60 (I), SSEA3 (K), SSEA4 (L), and alkaline phosphatase (AP) (N). 4,6-Diamidino-2-phenylindole (DAPI) staining indicates the total cell content per field (G,J,M). Fibroblasts surrounding human iPS colonies serve as internal negative controls for immunohistochemistry staining. Images were acquired with a standard microscope (Nikon) with a 20× objective. (O) Quantitative RT-PCR analyses for the expression of ES cell-marker genes OCT4, SOX2, NANOG, KLF4, MYC, REX1, and GDF3 in human CD34 iPS and the parental CD34+ cells. Individual PCR reactions were normalized against β-ACTIN and plotted relative to the expression level in the parental CD34+ cell. (P) Repression of the exogenously introduced transgenes as shown by quantitative RT-PCR analyses of OCT4, SOX2, MYC, and KLF4 expression. Specific primers were designed to probe for either the coding regions (Total) to measure the expression of both the endogenous gene and the transgene, 3′ untranslated region (Endo), which measure the expression of the endogenous gene only, or primers specific (Transgene) to the region of the viral transgenes. β-ACTIN is shown at the bottom as a loading control for each sample. (Q) Bisulfite genomic sequencing of the OCT4 and NANOG promoters reveals demethylation in the iPS cell lines. Each horizontal row of circles represents an individual sequencing reaction for a given amplicon. Open and filled circles represent unmethylated and methylated CpGs dinucleotides, respectively. The cell lines (CD34+ and its derivatives CD34 iPS1 and CD34 iPS2) are indicated to the left of each cluster. The values above each column indicate the CpG position analyzed relative to the downstream transcriptional start site.
Using immunohistochemistry, we analyzed the CD34 iPS cell lines for expression of markers shared with ES cells. Consistent with their hES cell-like morphology, the iPS cells were positive for Tra-1-81, NANOG, OCT4, Tra-1-60, SSEA3, SSEA4, and alkaline phosphatase staining (Figure 1E-N). Using quantitative reverse-transcriptase–polymerase chain reaction (RT-PCR) analysis, we found that the expression of the pluripotency markers OCT4, SOX2, NANOG, REX1, and GDF3 was markedly increased in the CD34 iPS cell lines compared with the parental CD34+ cell population and was similar to the expression in H1-OGN human ES cells (Figure 1O). The expression of KLF4 and MYC did not vary significantly between the CD34 iPS and the parental cells, consistent with prior observations that the 2 genes are already expressed in multiple cell types.4 Efficient transgene silencing is essential for the derivation of pluripotent iPS cell lines.14 Quantitative RT-PCR using primers specific for retroviral transcripts confirmed that OCT4 and SOX2 transgenes were efficiently silenced in the CD34 iPS cells (Figure 1P). Additional analysis using real-time PCR also indicates silencing of the KLF4 and MYC transgenes in most CD34 iPS clones (Figure S2E). Notably, expression from the OCT4, SOX2, MYC, and KLF4 endogenous loci was restored to levels comparable with human ES cells (Figure 1P). Consistent with the activation of endogenous pluripotency-associated gene expression, reprogramming of the CD34+ cells is accompanied by the demethylation of CpG dinucleotides at the OCT4 and NANOG promoters (Figure 1Q).
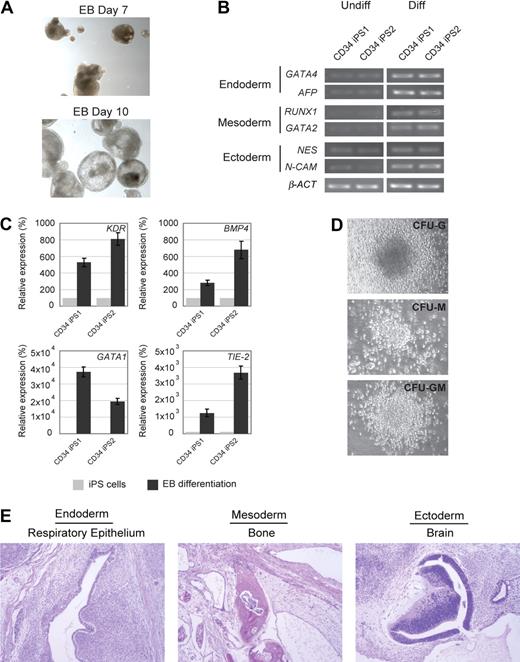
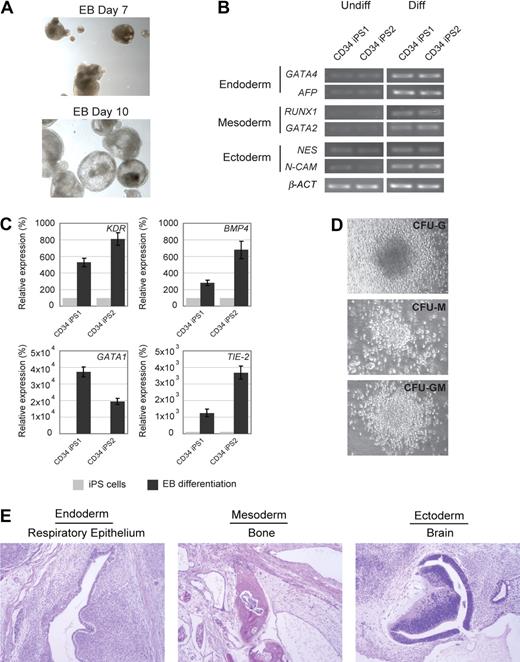
Next, we evaluated the differentiation potential of the CD34 iPS cell lines by in vitro embryoid body formation, hematopoietic differentiation assay, and in vivo teratoma induction. The CD34 iPS cells readily differentiated in vitro into embryoid bodies (Figure 2A), and quantitative RT-PCR of the differentiated cells showed marker gene expression for all 3 embryonic germ layers (Figure 2B). Hematopoietic differentiation of CD34 iPS cell lines resulted in up-regulation of blood lineage markers and produced myeloid and granulocyte colony types (Figures 2C,D, S3). The most rigorous test for pluripotency of human ES cells is the formation of teratomas in immunodeficient murine hosts.15 On subcutaneous injection into immunodeficient Rag2−/−γC−/− mice, our iPS cell lines generated well-differentiated cystic teratomas representing all 3 embryonic germ layers (Figure 2E). Cytogenetic analysis showed normal karyotypes (Figure S4), and DNA fingerprinting analysis verified that these cells were indeed derived from the parental CD34+ cells and not a result of contamination from existing hES cell lines (Table S1). The iPS clones have been propagated for at least 18 passages as of this submission. Collectively, our analyses indicate the successful reprogramming of mobilized peripheral blood CD34+ cells into pluripotent iPS cells.
In vitro and in vivo differentiation potential of the CD34 iPS cells. (A) Embryoid body-mediated differentiation of CD34 iPS cells. Differentiation of embryoid bodies (EB) consisting of tight clusters of differentiating cells was observed by day 7 and will cavitate, becoming cystic, by day 10. Images were acquired with a standard microscope (Nikon) with a 10× objective. (B) In vitro-differentiated human CD34 iPS cells demonstrate gene expression from all 3 embryonic germ layers. Semiquantitative RT-PCR performed on undifferentiated (U) and embryoid body-differentiated (D) iPS cells shows up-regulated expression of lineage markers from the 3 embryonic germ layers (endoderm, GATA4 and AFP; mesoderm, RUNX1 and GATA2; and ectoderm, NESTIN and N-CAM). β-ACTIN is shown as a positive amplification and loading control. (C) In vitro–differentiated human CD34 iPS cells demonstrate gene expression of hematopoietic lineage markers. Semiquantitative RT-PCR performed on undifferentiated iPS cells and embryoid bodies differentiated in hematopoietic inducing medium shows up-regulated expression of KDR, BMP4, GATA1, and TIE-2. (D) Embryoid bodies derived from CD34 iPS cells yield myeloid colonies in semisolid methylcellulose media: colony-forming unit-granulocyte (CFU-G), colony-forming unit-macrophage (CFU-M), and colony-forming unit-granulocyte macrophage (CFU-GM). Images were acquired with a standard microscope (Nikon) with a 20× objective. (E) Hematoxylin and eosin staining of teratomas derived from immunodeficient mice injected with human CD34 iPS cells shows tissues representing all 3 embryonic germ layers, including respiratory epithelium (endoderm), bone (mesoderm), and immature neural tissue (ectoderm).
In vitro and in vivo differentiation potential of the CD34 iPS cells. (A) Embryoid body-mediated differentiation of CD34 iPS cells. Differentiation of embryoid bodies (EB) consisting of tight clusters of differentiating cells was observed by day 7 and will cavitate, becoming cystic, by day 10. Images were acquired with a standard microscope (Nikon) with a 10× objective. (B) In vitro-differentiated human CD34 iPS cells demonstrate gene expression from all 3 embryonic germ layers. Semiquantitative RT-PCR performed on undifferentiated (U) and embryoid body-differentiated (D) iPS cells shows up-regulated expression of lineage markers from the 3 embryonic germ layers (endoderm, GATA4 and AFP; mesoderm, RUNX1 and GATA2; and ectoderm, NESTIN and N-CAM). β-ACTIN is shown as a positive amplification and loading control. (C) In vitro–differentiated human CD34 iPS cells demonstrate gene expression of hematopoietic lineage markers. Semiquantitative RT-PCR performed on undifferentiated iPS cells and embryoid bodies differentiated in hematopoietic inducing medium shows up-regulated expression of KDR, BMP4, GATA1, and TIE-2. (D) Embryoid bodies derived from CD34 iPS cells yield myeloid colonies in semisolid methylcellulose media: colony-forming unit-granulocyte (CFU-G), colony-forming unit-macrophage (CFU-M), and colony-forming unit-granulocyte macrophage (CFU-GM). Images were acquired with a standard microscope (Nikon) with a 20× objective. (E) Hematoxylin and eosin staining of teratomas derived from immunodeficient mice injected with human CD34 iPS cells shows tissues representing all 3 embryonic germ layers, including respiratory epithelium (endoderm), bone (mesoderm), and immature neural tissue (ectoderm).
Mobilized peripheral blood is the primary source of cells for hematopoietic transplantation, immunotherapy, and gene therapy.10 Our study describes a novel application for mobilized peripheral blood in the establishment of iPS cells. Our findings also provide the first proof of principle that cells from the human blood lineage are amenable to reprogramming. Blood cells represent a source of cells that obviate the need for skin biopsies and require minimal maintenance in culture before reprogramming. Reprogramming of blood cells is therefore an important step toward the development of more efficient ways of generating patient-specific pluripotent stem cells. Moreover, our study provides a strategy for the generation of pluripotent stem cells for diseases caused by somatic genetic disorders specific to the hematopoietic system, which cannot be recapitulated by conventional fibroblast reprogramming.16,–18
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sabine Loewer, Rui Zhao, and Kelvin Wong for helpful discussions and critical comments on the manuscript.
This work was supported by grants from the National Institutes of Health (Bethesda, MD) and the NIH Director's Pioneer Award of the NIH Roadmap for Medical Research. Y.-H.L. is a recipient of the A*Star (Agency of Science, Technology and Research) International Fellowship and was funded by the A*Star Graduate Academy and the Institute of Medical Biology, Singapore. G.Q.D. is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (Research Triangle Park, NC).
Authorship
Contribution: Y.-H.L. designed and performed research, analyzed data, and wrote the paper; S.A., I.-H.P., A.U., and G.C.H. designed and conducted research and analyzed data; H.H., K.K., J.D.M., and K.N. designed and conducted research; and G.Q.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George Q. Daley, Howard Hughes Medical Institute, Children's Hospital Boston Karp Family Research Bldg 7214, 300 Longwood Ave, Boston, MA 02115; e-mail: george.daley@childrens.harvard.edu.