Using a bacterial pathogen–induced acute inflammation model in the skin, we defined the roles of local lymphatic vessels and draining lymph nodes (DLNs) in antigen clearance and inflammation resolution. At the peak day of inflammation, robust expansion of lymphatic vessels and profound infiltration of CD11b+/Gr-1+ macrophages into the inflamed skin and DLN were observed. Moreover, lymph flow and inflammatory cell migration from the inflamed skin to DLNs were enhanced. Concomitantly, the expression of lymphangiogenic growth factors such as vascular endothelial growth factor C (VEGF-C), VEGF-D, and VEGF-A were significantly up-regulated in the inflamed skin, DLNs, and particularly in enriched CD11b+ macrophages from the DLNs. Depletion of macrophages, or blockade of VEGF-C/D or VEGF-A, largely attenuated these phenomena, and produced notably delayed antigen clearance and inflammation resolution. Conversely, keratin 14 (K14)–VEGF-C transgenic mice, which have dense and enlarged lymphatic vessels in the skin dermis, exhibited accelerated migration of inflammatory cells from the inflamed skin to the DLNs and faster antigen clearance and inflammation resolution. Taken together, these results indicate that VEGF-C, -D, and -A derived from the CD11b+/Gr-1+ macrophages and local inflamed tissues play a critical role in promoting antigen clearance and inflammation resolution.
Introduction
Peripheral tissues, such as skin and mucosa, are exposed to external environments and are constantly challenged by antigens. Some antigens, such as pathogen molecules derived from bacteria and viruses, evoke inflammatory responses in these tissues through a consecutive set of processes: activation of residential macrophages and mast cells leading to secretion bioactive amines, cytokines, chemokines, and lipid mediators; recruitment of leukocytes including neutrophils, monocytes, and T lymphocytes from circulation; activation of macrophages and dendritic cells (DCs) to engulf dead leukocytes, pathogens, and other inflammatory debris; and drainage of these cells into draining lymph nodes (DLNs) via local lymphatic vessels.1,–3 Throughout these processes, residential and recruited monocyte-derived macrophages are critical for evoking and resolving the acute inflammation caused by pathogen entry.1,,,–5 The pathophysiologic, molecular, and cellular mechanisms governing initiation and promotion of inflammatory processes have been extensively characterized; however, it is yet to be clarified how acute inflammation resolves, rather than leads to, chronic inflammation. Particularly, the roles of local lymphatic vessels and DLNs in antigen clearance and inflammation resolution are poorly understood.
During inflammation, macrophages actively participate in inducing the formation of new lymphatic vessels.6,,,,,,–13 In adult animals, new lymphatic vessels are formed mainly through lymphangiogenesis, which is a sequence of processes that include sprouting, migration, proliferation, and tubule formation by preexisting lymphatic endothelial cells (LECs).14,–16 Additional LECs may also be derived through transdifferentiation or incorporation of circulating bone marrow–derived cells and some macrophages.9,11 The discovery of specific lymphangiogenic growth factors (VEGF-C and VEGF-D), receptors (VEGF receptor-3 [VEGFR3]), a transcription factor (Prox-1), and markers (lymphatic vessel endothelial hyaluronan receptor-1 [LYVE-1], podoplanin) for LECs has led to a renewed interest in the roles of lymphatic vessels and lymphangiogenesis in tissue fluid homeostasis, tumor metastasis, wound healing, antigen presentation, and digested lipid absorption.14,,,,–19 Among the roles of lymphatic vessels and lymphangiogenesis, the functional importance of lymphatic vessels in inflammation was initially observed a long time ago.20 Lymph nodes, in concert with the lymphatic vessels, play a pivotal role in initiating adaptive immune responses.21 Notably, local tissue inflammation causes DLNs to undergo a transient but profound remodeling, with volume expansion, lymphoid hyperplasia, and markedly increased lymphatic vessel densities.22,23 Lymphatic vessels in DLNs act as essential conduits for antigen-presenting cells such as epidermal Langerhans cells and dermal DCs to T lymphocytes.21 Up-regulated induction of inflammatory adhesion molecules in the lymphatic vessels within inflamed DLNs enhances DC mobilization to the T lymphocytes.24,–26 The main mediators and factors governing inflammation-induced lymphangiogenesis (IIL) are yet to be defined. Intriguingly, our pilot experiment revealed that in bacterial pathogen–induced inflamed skin and DLNs, CD11b+ macrophages were profoundly infiltrated around expanded lymphatic vessels. Based on this observation, we have speculated that macrophages, including CD11b+ macrophages, play a pivotal role in the expansion of lymphatic vessels in the inflamed skin and DLNs by secreting lymphangiogenic growth factors. Moreover, we have sought to determine whether macrophage-induced lymphangiogenesis may be actively involved in antigen clearance, in addition to initiating the adaptive immune response.
In this study, we investigated the role of IIL in antigen clearance and inflammation resolution. To this end, we generated an inflammation model by the introduction of representative etiologic agents capable of inducing septic shock. These agents are lipopolysaccharide (LPS) from Gram-negative bacteria and a mixture of lipoteichoic acid (LTA) from Gram-positive bacteria plus muramyl dipeptide (MDP),27,28 which were injected into the dorsal side of ear dermis of mice. At the peak day of acute inflammation, we analyzed changes in lymphatic vessels, macrophage infiltration, lymph flow, inflammatory cell migration, antigen clearance, and inflammation resolution from the inflamed skin and DLNs in normal adult mice and in skin-specific VEGF-C–overexpressing transgenic mice.29 To determine the role of macrophages and lymphangiogenic growth factors in these processes, specific agents that block macrophage and lymphangiogenic growth factors were applied. Our results indicate that macrophages, including CD11b+/Gr-1+ macrophages, play a critical role in antigen clearance and inflammation resolution, and VEGF-C, -D, and -A derived from the macrophages, local inflamed tissue, and DLNs contribute to accelerate antigen clearance and inflammation resolution.
Methods
Mice
C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). GFP+ mice (C57BL/6J genetic background) were a gift from Dr Masaru Okabe (Osaka University, Osaka, Japan).30 K14-VEGF-C transgenic mice (FVB/N genetic background) were generated and maintained as previously described,29 and transferred to KAIST. Mice were bred in our pathogen-free animal facility, and male mice aged 7 to 8 weeks were used. Animal care and experimental procedures were performed under approval from the Animal Care Committee of KAIST.
Ear skin inflammation model
LPS (from Escherichia coli 0111:B4) and MDP were purchased from Sigma-Aldrich (St Louis, MO). Highly purified and structurally intact LTA was prepared from Staphylococcus aureus (ATCC, Manassas, VA) as previous described.28 Mice were anesthetized by intramuscular injection of a combination of anesthetics (80 mg/kg ketamine and 12 mg/kg xylazine), then a single intradermal injection of LPS (10 μg in 10 μL PBS) or LTA + MDP (10:1 ratio, 10 μg LTA + 1 μg MDP in 10 μL PBS)27 was given into the dorsal side of the ear using a 31-gauge syringe.
Depletion of macrophages and blockades of VEGF ligands
For systemic depletion of macrophages, mice were given an intravenous injection of clodronate liposome (CDL, 25 mg/kg)31 through the tail vein at 1 day before and after the intradermal injection of LPS or LTA. To block VEGF-C/D, mice were treated with a single intravenous injection of 109 pfu Ad-sVEGFR332 at 12 hours before the intradermal injection of LPS or LTA. To block VEGF-A, mice were given a subcutaneous injection of VEGF-Trap (25 mg/kg)33,34 or anti–VEGF-A blocking antibody (4 mg/kg; R&D Systems, Minneapolis, MN) under the back skin at 1 day before and after the intradermal injection of LPS or LTA.
Histologic and morphometric analyses
Mice were anesthetized by intramuscular injection of anesthetics, as described in “Ear skin inflammation model,” at the indicated times after the intradermal injection of LPS or LTA. The histologic and morphologic analyses in the inflamed ear and DLNs were performed as described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Flow cytometric analysis of CD11b+ macrophages from ear skin and DLNs
After anesthesia, the ears and DLNs were harvested and dissected into small pieces by a microscissor, and the pieces were incubated with 2 mL Hank balanced salt solution (Sigma-Aldrich) containing 0.2% collagenase type-II (Worthington, Lakewood, NJ) for 1 hour at 37°C. Flow cytometric analysis of CD11b+ macrophages from ear skin and DLNs was performed as described in Document S1.
Enrichment of CD11b+ cells from DLNs by MACS
CD11b+ macrophages in the DLN were enriched using anti–mouse CD11b antibody-coupled MicroBeads (Miltenyi Biotec, Auburn, CA) and a magnetic cell sorter (magnetic-activated cell sorting [MACS]; Miltenyi Biotec) according to the manufacturer's instructions as described in Document S1.
Assessment of inflammation in the ear skin
RT-PCR
The semiquantitative and quantitative real-time reverse-transcription–polymerase chain reaction (RT-PCR) of total RNA isolated from ear skin, whole DLNs, and MACS-enriched CD11b+ cells from the DLNs was performed with appropriate primers (Table S3) as described in Document S1.
Monitoring lymph flow
Monitoring lymph flow was performed using 2 intravital imaging methods as described in Document S1.
Assay for inflammatory cell migration from the inflammation site to DLNs
GFP+ mice were intraperitoneally injected with 1 mL 3.0% thioglycolate (Sigma-Aldrich) in saline, and inflammatory cells were collected 3 days later by peritoneal washing with ice-cold DMEM culture medium. Approximately, 106 GFP+ inflammatory cells in 10 μL PBS were adaptively transferred by injection at the inflamed site of the ear. Twelve hours later, DLNs were collected and sectioned for immunohistology, and a cell suspension was made by collagenase digestion for the flow cytometric analysis.
Monitoring antigen clearance
The monitoring antigen clearance was performed as described in Document S1.
Statistics
Values presented are means plus or minus standard deviation (SD). Significant differences between means were determined by Student t test or analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. Statistical significance was set at a P value less than .05.
Results
Depletion of macrophages markedly reduces lymphangiogenesis in the inflamed skin and DLNs, and delays inflammation resolution
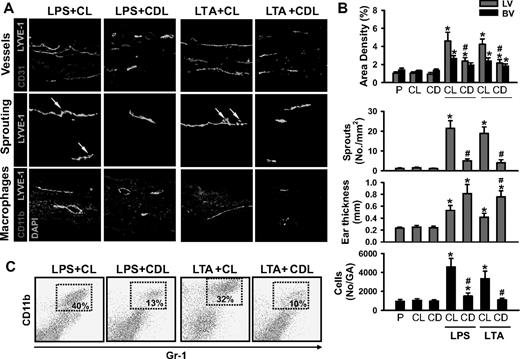
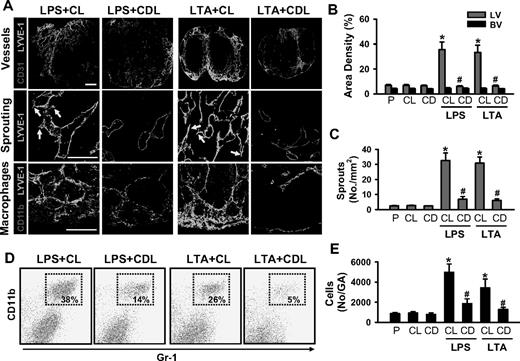
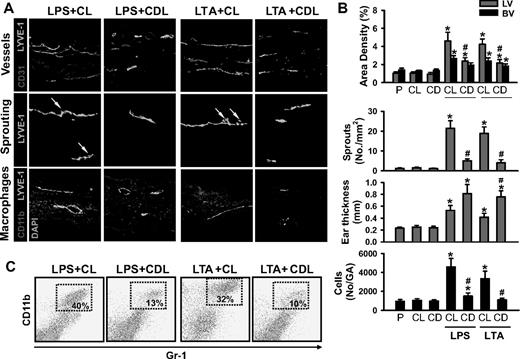
Acute skin inflammation induced by intradermal injection of LPS (10 μg in 10 μL PBS; hereafter referred to as LPS) or LTA (10:1 ratio, 10 μg LTA plus 1 μg MDP in 10 μL PBS; hereafter referred to as LTA) evokes robust lymphangiogenesis and marked recruitment of CD11b+/Gr-1+ macrophages in the inflamed skin and DLNs (Figures S1Figure S2. Intradermal LPS or LTA increase lymphatic and blood vessel densities, lymphatic sprouting, thickness, and recruitment of CD11b+/Gr-1+ macrophages (JPG, 112 KB)Figure S3. Intradermal LPS or LTA increase lymphatic vessel densities and sprouts, and recruitment of CD11b+/Gr-1+/F4/80+ macrophages in the DLN (JPG, 133 KB)–S4). These results led us to explore the involvement of macrophages, including CD11b+/Gr-1+ macrophages, in lymphangiogenesis in the inflamed skin and DLNs by depletion of whole macrophages by intraperitoneal treatment with clodronate liposome (CDL).31 The CDL treatment, but not control liposome (CL) treatment, efficiently depletes macrophages, including CD11b+/Gr-1+ macrophages, in all organs, including inflamed skin and DLNs (Figures 1A and 2A, and data not shown) by inducing apoptosis of macrophages.31 In this situation, the LPS- or LTA-induced increased lymphatic vessel densities and sprouting and infiltration of CD11b+/Gr-1+ macrophages were largely attenuated, not only in the inflamed skin but also in the DLNs (Figures 1,2). However, unexpectedly, the skin thickness was increased to a greater extent in LPS + CDL and LTA + CDL than in LPS + CL and LTA + CL (Figure 1A,B). In comparison, the inflamed skin and DLNs treated with LPS + CL or LTA + CL showed no significant differences in any parameters examined compared with the skin and DLNs treated with only LPS or LTA. In addition, the inflamed skin and DLNs treated with the CL or CDL showed no significant differences compared with the skin and DLNs treated with PBS (Figures 1,2). To determine the role of macrophages in inflammation resolution, we further examined LPS- or LTA-induced inflammatory reactions in the ear skin after treatment with CL or CDL. Compared with the inflamed skin treated with LPS + CL and LTA + CL, the inflamed skin treated with LPS + CDL and LTA + CDL exhibited exaggerated inflammatory reactions including severe swelling, erythema formation, and corresponding histologic changes (Figure S5). Local depletion of macrophages by a localized injection of CDL, but not CL, into the inflamed skin produced similar findings (data not shown). These data suggest that LPS- or LTA-induced recruitment of macrophages, including CD11b+/Gr-1+ macrophages, plays a critical role not only in lymphangiogenesis in the inflamed skin and DLNs, but also in inflammation resolution in the inflamed skin.
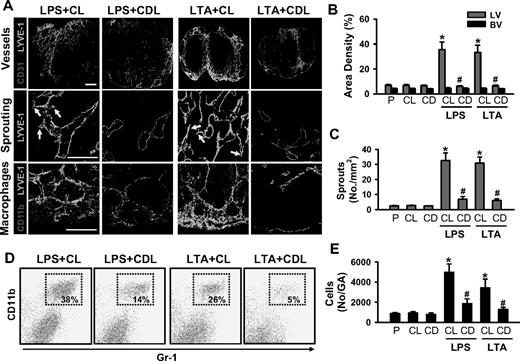
Depletion of macrophages including CD11b+/Gr-1+ macrophages markedly reduces LPS- or LTA-induced lymphangiogenesis in the skin. CDL (25 mg/kg, CD) was given intravenously to deplete macrophages at 1 day before and after the intradermal injection of LPS (LPS + CDL) or LTA (LTA + CDL). For controls, CL (25 mg/kg) was given in the same manner (LPS + CL, LTA + CL). As an alternative control, PBS (P), CL, or CDL-only was given in the same manner. At day 3 after the injection of LPS or LTA, the inflamed ears were excised and sectioned for histologic analysis or digested for flow cytometry. (A) Tissue sections were coimmunostained for LYVE-1, CD31, or CD11b and merged. ↓ indicates lymphatic sprouts. Scale bars represent 50 μm. (B) Quantification analyses of lymphatic (LV) and blood vessel (BV) densities (%), number of lymphatic sprouts, thickness of the ear skins, and number of CD11b+/Gr-1+ cells in the gated area (No/GA) are shown. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus CL; #P < .05 versus LPS + CL or LTA + CL. (C) Flow cytometric analysis of CD11b+/Gr-1+ macrophages in the ear skins.
Depletion of macrophages including CD11b+/Gr-1+ macrophages markedly reduces LPS- or LTA-induced lymphangiogenesis in the skin. CDL (25 mg/kg, CD) was given intravenously to deplete macrophages at 1 day before and after the intradermal injection of LPS (LPS + CDL) or LTA (LTA + CDL). For controls, CL (25 mg/kg) was given in the same manner (LPS + CL, LTA + CL). As an alternative control, PBS (P), CL, or CDL-only was given in the same manner. At day 3 after the injection of LPS or LTA, the inflamed ears were excised and sectioned for histologic analysis or digested for flow cytometry. (A) Tissue sections were coimmunostained for LYVE-1, CD31, or CD11b and merged. ↓ indicates lymphatic sprouts. Scale bars represent 50 μm. (B) Quantification analyses of lymphatic (LV) and blood vessel (BV) densities (%), number of lymphatic sprouts, thickness of the ear skins, and number of CD11b+/Gr-1+ cells in the gated area (No/GA) are shown. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus CL; #P < .05 versus LPS + CL or LTA + CL. (C) Flow cytometric analysis of CD11b+/Gr-1+ macrophages in the ear skins.
Depletion of macrophages markedly reduces LPS- or LTA-induced lymphangiogenesis in the DLNs. CDL (25 mg/kg, CD) was given intravenously to deplete macrophages at 1 day before and after the intradermal injection of LPS (LPS + CDL) or LTA (LTA + CDL). As a control, CL (25 mg/kg) was given in the same manner (LPS + CL, LTA + CL). As alternative controls, PBS (P), CL, or CDL-only was given in the same manner. At day 3 after the injection of LPS or LTA, the DLNs were sampled and sectioned for histologic analysis or digested for flow cytometry. (A) Tissue sections were coimmunostained for LYVE-1, CD31, or CD11b and merged. ↓ indicates lymphatic sprouts. Scale bars represent 50 μm. Quantification of lymphatic (LV) and blood vessel (BV) densities (%; B), number of lymphatic sprouts (C), and number of CD11b+/Gr-1+ cells in the gated region (No/GA; E) are shown. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus CL; #P < .05 versus LPS + CL or LTA + CL. (D) Flow cytometric analysis of CD11b+/Gr-1+ macrophages in the DLNs.
Depletion of macrophages markedly reduces LPS- or LTA-induced lymphangiogenesis in the DLNs. CDL (25 mg/kg, CD) was given intravenously to deplete macrophages at 1 day before and after the intradermal injection of LPS (LPS + CDL) or LTA (LTA + CDL). As a control, CL (25 mg/kg) was given in the same manner (LPS + CL, LTA + CL). As alternative controls, PBS (P), CL, or CDL-only was given in the same manner. At day 3 after the injection of LPS or LTA, the DLNs were sampled and sectioned for histologic analysis or digested for flow cytometry. (A) Tissue sections were coimmunostained for LYVE-1, CD31, or CD11b and merged. ↓ indicates lymphatic sprouts. Scale bars represent 50 μm. Quantification of lymphatic (LV) and blood vessel (BV) densities (%; B), number of lymphatic sprouts (C), and number of CD11b+/Gr-1+ cells in the gated region (No/GA; E) are shown. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus CL; #P < .05 versus LPS + CL or LTA + CL. (D) Flow cytometric analysis of CD11b+/Gr-1+ macrophages in the DLNs.
Depletion of macrophages markedly reduces lymphatic flow and mobilization of inflammatory cells from the inflamed ear skin to the DLNs
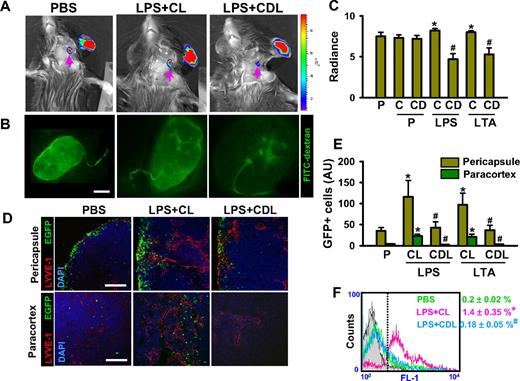
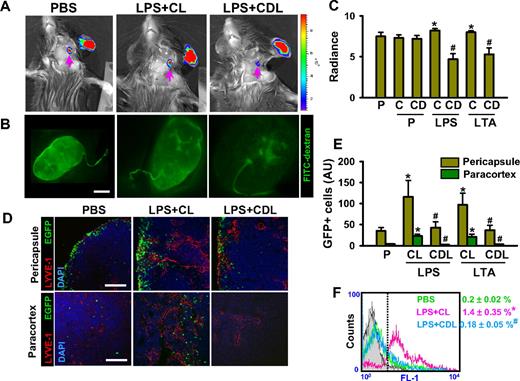
To elucidate the underlying mechanism of how macrophages play a role in inflammation resolution, we first monitored lymph flow from the inflamed ear skin to DLNs. As a monitoring system, we obtained simultaneous vital images for 30 minutes using an in vivo imaging system (IVIS) imaging system and a fluorescence stereomicroscope to track the movement of a lymphatic tracking fluorescent dye, fluorescein isothiocyanate (FITC)–dextran, at the injection site on day 3 after the LPS or LTA injection. Fluorescence intensities in the DLNs were similar in PBS, PBS + CL, and PBS + CDL, whereas they were slightly but significantly higher in LPS + CL and LTA + CL (Figure 3A-C), indicating greater lymphatic flow during LPS- and LTA-induced inflammation. However, fluorescence intensities in the DLNs were lower in LPS + CDL and LTA + CDL than in LPS + CL, LTA + CL, and PBS (Figure 3A-C), indicating that lymphatic flows were decreased by depletion of macrophages, even during LPS- and LTA-induced inflammation. Second, we determined the mobilization rate of inflammatory cells from the inflamed ear skin to DLNs by the intradermal injection of GFP+ inflammatory cells (∼ 106 cells). Twelve hours after the injection of green fluorescent protein–positive (GFP+) inflammatory cells, the mobilized inflammatory cells were localized mainly in the pericapsular region of DLNs and rarely detectable in the paracortical region of DLNs in PBS (Figure 3D). Compared with PBS, the number of the mobilized inflammatory cells substantially increased both in the pericapsular region (∼ 3.3-fold and ∼ 2.8-fold by LPS + CL and LTA + CL, respectively) and paracortical region (∼ 6.1-fold and ∼ 5.5-fold by LPS + CL and LTA + CL, respectively; Figure 3D,E). However, compared with LPS + CL and LTA + CL, the numbers of the mobilized inflammatory cells were markedly lower in both the pericapsular region (∼ 63% and ∼ 62% by LPS + CDL and LTA + CDL, respectively) and the paracortical regions (∼ 90% and ∼ 85% by LPS + CDL and LTA + CDL, respectively; Figure 3D,E). Flow cytometric analyses on the total DLNs exhibited similar findings (Figure 3F). These data suggest that macrophages play a substantial role in increases of lymph flow and inflammatory cell mobilization from the inflammatory site to the DLNs for antigen clearance and inflammatory resolution.
Depletion of macrophages markedly reduces lymphatic flow and mobilization of inflammatory cells from the inflammation site of ear skin to DLNs. CDL (25 mg/kg, CD) was given intravenously to deplete macrophages at 1 day before and after the intradermal injection of PBS (PBS + CDL), LPS (LPS + CDL), or LTA (LTA + CDL). As a control, CL (25 mg/kg, C; PBS + CL, LPS + CL, LTA + CL) or PBS (P) only was given in the same manner. (A-C) Three days later, 3 μL FITC-conjugated dextran was intradermally injected into the same sites. Fluorescence intensities were determined in DLNs at 30 minutes after the injection (A), quantified, and presented as relative radiance (photons/sec per cm2/steradian; panel C). Panel A right bar: the color scale indicates fluorescence intensity. Upper red color indicates maximum fluorescence intensity, whereas lower blue color indicates minimum fluorescence intensity. (B) In parallel, at 30 minutes after the FITC-dextran injection, the DLNs are imaged by a fluorescence stereomicroscope. (D-F) At day 3 after intradermal injection of LPS or LTA, GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sampled and sectioned for histologic analysis or digested for flow cytometry. (D,E) Pericapsular and paracortical regions of DLN sections were coimmunostained for LYVE-1 and DAPI, and merged. Scale bars represent 50 μm. GFP+ inflammatory cells in the section are quantified and presented as AU. (F) Flow cytometric analysis of GFP+ inflammatory cells in the DLNs after collagenase digestion is shown. All bars and numbers represent mean ± SD from 4 to 5 mice. *P < .05 versus P; #P < .05 versus LPS + CL or LTA + CL.
Depletion of macrophages markedly reduces lymphatic flow and mobilization of inflammatory cells from the inflammation site of ear skin to DLNs. CDL (25 mg/kg, CD) was given intravenously to deplete macrophages at 1 day before and after the intradermal injection of PBS (PBS + CDL), LPS (LPS + CDL), or LTA (LTA + CDL). As a control, CL (25 mg/kg, C; PBS + CL, LPS + CL, LTA + CL) or PBS (P) only was given in the same manner. (A-C) Three days later, 3 μL FITC-conjugated dextran was intradermally injected into the same sites. Fluorescence intensities were determined in DLNs at 30 minutes after the injection (A), quantified, and presented as relative radiance (photons/sec per cm2/steradian; panel C). Panel A right bar: the color scale indicates fluorescence intensity. Upper red color indicates maximum fluorescence intensity, whereas lower blue color indicates minimum fluorescence intensity. (B) In parallel, at 30 minutes after the FITC-dextran injection, the DLNs are imaged by a fluorescence stereomicroscope. (D-F) At day 3 after intradermal injection of LPS or LTA, GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sampled and sectioned for histologic analysis or digested for flow cytometry. (D,E) Pericapsular and paracortical regions of DLN sections were coimmunostained for LYVE-1 and DAPI, and merged. Scale bars represent 50 μm. GFP+ inflammatory cells in the section are quantified and presented as AU. (F) Flow cytometric analysis of GFP+ inflammatory cells in the DLNs after collagenase digestion is shown. All bars and numbers represent mean ± SD from 4 to 5 mice. *P < .05 versus P; #P < .05 versus LPS + CL or LTA + CL.
Role of paracrine factors associated with CD11b+/Gr-1+ macrophages in LPS- and LTA-induced dermal and DLN lymphangiogenesis
To investigate the source and expression of lymphangiogenic factors that drive LPS- or LTA-induced lymphangiogenesis, we performed a series of reverse-transcription–polymerase chain reaction (RT-PCR) assays in the skins and their DLNs with and without macrophage depletion by CDL treatment, and in the enriched CD11b+ macrophages from DLNs. Compared with PBS, LPS + CL and LTA + CL increased VEGF-A164 (2.3- and 2.2-fold, respectively), VEGF-A120 (2.6- and 1.9-fold, respectively), VEGF-C (2.1- and 1.9-fold, respectively), and VEGF-D (1.5- and 1.4-fold, respectively) in the ear skins (Figure S6A,C). Compared with PBS, LPS + CL and LTA + CL increased VEGF-A164 (2.4- and 2.3-fold, respectively), VEGF-A120 (3.1- and 3.2-fold, respectively), VEGF-C (2.6- and 2.5-fold), respectively, and VEGF-D (1.3- and 1.3-fold, respectively) in the DLNs (Figure S6A,C). CDL treatment markedly reduced LPS + CL- and LTA + CL-induced increases in expression of VEGF ligands in the ear skin and DLNs, and the relative levels were similar to those of PBS. In CD11b+ macrophages enriched by magnetic-activated cell sorting (MACS), LPS and LTA increased VEGF-A164 (4.7- and 4.2-fold, respectively), VEGF-A120 (3.2- and 3.0-fold, respectively), VEGF-C (4.8- and 3.4-fold, respectively), and VEGF-D (3.6- and 3.1-fold, respectively), compared with PBS (Figure S6B,D). Consistently, quantitative real-time RT-PCR analyses revealed that LPS and LTA increased VEGF-A, VEGF-C, and VEGF-D mRNA levels, whereas they did not change the placental growth factor (PlGF) mRNA level in the skin, DLNs, and enriched CD11+ macrophages (Figure S7). Thus, macrophages, including CD11b+ macrophages, could be the main mediators or sources of lymphangiogenic VEGF ligands in the skin and DLNs during acute IIL.
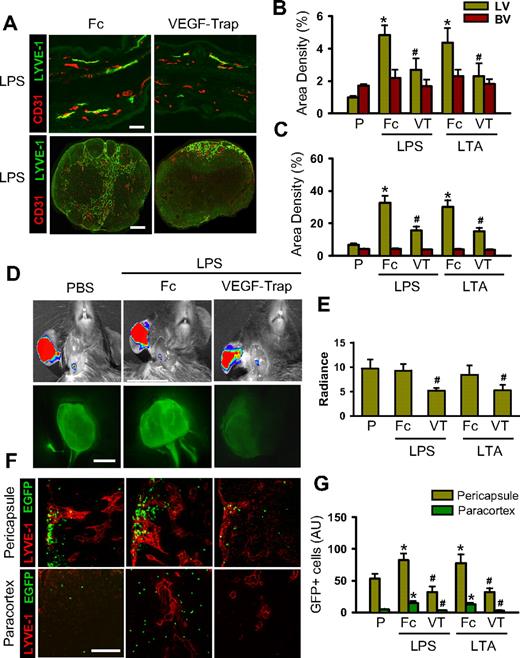
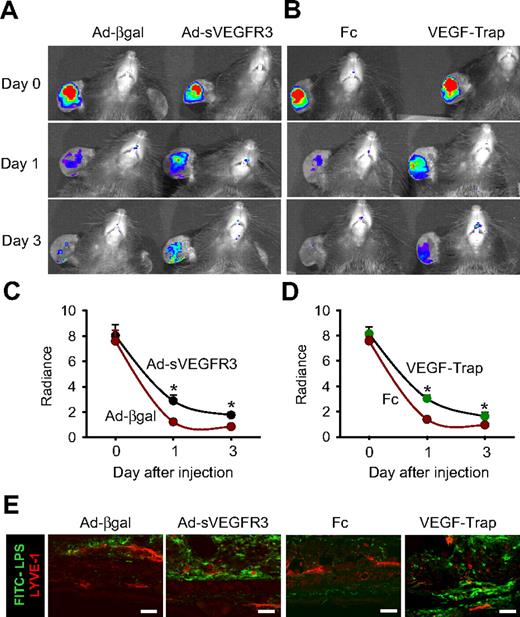
Blockade of VEGF-C/D or VEGF-A reduces the LPS- and LTA-induced dermal and DLN lymphangiogenesis, lymph flow, inflammatory cell migration, and inflammation resolution
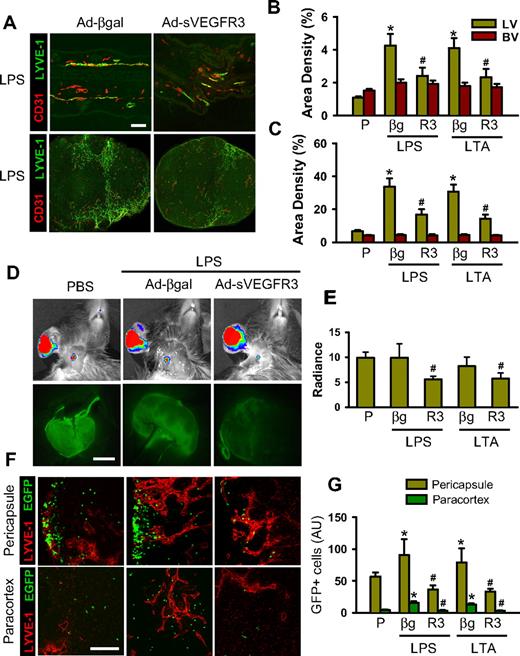
The pacarine role of macrophages as a source of VEGFs led us to explore the role of VEGF ligands on LPS- or LTA-driven lymphangiogenesis, lymph flow, inflammatory cell migration, and inflammation resolution. To block VEGF-C and VEGF-D (VEGF-C/D), an adenovirus encoding the soluble extracellular domain of VEGFR-3 (Ad-sVEGFR3) was used.32 Compared with the control, which was treated with 109 pfu adenovirus encoding β-galactosidase (Ad-βgal), treatment with 109 pfu Ad-sVEGFR3 markedly attenuated the LPA- and LTA-increased lymphatic density (∼ 47% and ∼ 48%, respectively, in the skin and ∼ 50% and ∼ 52%, respectively, in the DLNs), lymph flow (∼ 44% and ∼ 30%, respectively), and inflammatory cell migration to DLNs (∼ 59% and ∼ 58%, respectively, in the pericapsular region and ∼ 78% and ∼ 75%, respectively, in the paracortical region), and delayed inflammation resolution (Figures 4,S8). The mice treated with the Ad-βgal showed no significant differences from the mice treated with PBS. Compared with the controls, which were treated with dimeric-Fc protein, mice receiving a blockade of VEGF-A with either VEGF-Trap33,34 or anti–VEGF-A antibody also had significant attenuation of the LPA- and LTA-increased lymphatic density in the skin and DLNs, increased lymph flow and inflammatory cell migration from the inflamed skin to DLNs (pericapsular and paracortical regions), and delayed inflammation resolution (Figures 5,S9,S10). The mice treated with the dimeric-Fc protein showed no significant differences from the mice treated with PBS. In addition, flow cytometric analysis of the DLNs revealed that infiltration of CD11b+/Gr-1+ macrophages to the DLNs was markedly decreased by blockade of VEGF-C/D or VEGF-A (Figure S11). These results indicate that VEGF-C, -D, and -A derived from inflamed skin and DLNs make a major contribution to the induction of dermal and DLN lymphangiogenesis by LPS and LTA, as well as to the enhancement of lymph flow and inflammatory cell migration, and to the resolution of inflammation.
Blockade of VEGF-C/D profoundly attenuates the LPS- and LTA-induced lymphangiogenesis in the inflamed ear skin and DLNs, and lymphatic flow and mobilization of inflammatory cells from the inflamed skin to DLNs. AdsVEGFR-3 (R3, 109 pfu), Adβ-gal (βg, 109 pfu), or none was injected 12 hours before the intradermal injection of PBS (P), LPS, or LTA. (A) Three days later, the inflamed ears and DLNs were sampled and sectioned for histology. Tissue sections were coimmunostained for LYVE-1 and CD31. Scale bars represent 50 μm. (B,C) Lymphatic (LV) and blood vessel (BV) densities in the inflamed ear skins (B) and DLNs (C) are quantified and presented as percentages. (D,E) Three days later, 3 μL FITC-conjugated dextran was intradermally injected into the inflamed skin. Thirty minutes later, fluorescence intensities in DLNs were determined with the IVIS (top panels) and fluorescence stereomicroscope (bottom panels), and quantified and presented as relative radiance (photons/sec per cm2/steradian). (F) At day 3 after intradermal injection of LPS or LTA, GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sectioned and immunostained for LYVE-1. Scale bars represent 50 μm. (G) The GFP+ inflammatory cells in the section of DLNs are quantified and presented as AU. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus P; #P < .05 versus LPS+βg or LTA+βg.
Blockade of VEGF-C/D profoundly attenuates the LPS- and LTA-induced lymphangiogenesis in the inflamed ear skin and DLNs, and lymphatic flow and mobilization of inflammatory cells from the inflamed skin to DLNs. AdsVEGFR-3 (R3, 109 pfu), Adβ-gal (βg, 109 pfu), or none was injected 12 hours before the intradermal injection of PBS (P), LPS, or LTA. (A) Three days later, the inflamed ears and DLNs were sampled and sectioned for histology. Tissue sections were coimmunostained for LYVE-1 and CD31. Scale bars represent 50 μm. (B,C) Lymphatic (LV) and blood vessel (BV) densities in the inflamed ear skins (B) and DLNs (C) are quantified and presented as percentages. (D,E) Three days later, 3 μL FITC-conjugated dextran was intradermally injected into the inflamed skin. Thirty minutes later, fluorescence intensities in DLNs were determined with the IVIS (top panels) and fluorescence stereomicroscope (bottom panels), and quantified and presented as relative radiance (photons/sec per cm2/steradian). (F) At day 3 after intradermal injection of LPS or LTA, GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sectioned and immunostained for LYVE-1. Scale bars represent 50 μm. (G) The GFP+ inflammatory cells in the section of DLNs are quantified and presented as AU. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus P; #P < .05 versus LPS+βg or LTA+βg.
Blockade of VEGF-A profoundly attenuates the LPS- and LTA-induced lymphatic vessel densities in the inflamed ear skin and DLNs, and lymphatic flow and mobilization of inflammatory cells from the inflamed skin to DLNs. VEGF-Trap (25 mg/kg), dimeric-Fc (Fc, 25 mg/kg), or none was treated at 1 day before and after the intradermal injection of PBS (P), LPS, or LTA. (A) At day 3 after the injection, the inflamed ears and DLNs were sectioned for histologic analysis. Tissue sections were coimmunostained for LYVE-1 and CD31. Scale bars represent 50 μm. (B,C) Lymphatic (LV) and blood vessel (BV) densities in the inflamed skin (B) and DLNs (C) were quantified and presented as percentages. (D,E) Three days later, 3 μL FITC-conjugated dextran was intradermally injected into the inflamed skin. Thirty minutes later, fluorescence intensities in DLNs were determined with the IVIS (top panels) and fluorescence stereomicroscope (bottom panels), and quantified and presented as relative radiance (photons/sec per cm2/steradian). (F) At day 3 after intradermal injection of LPS or LTA into the ear, the GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sectioned and immunostained for LYVE-1. Scale bars represent 50 μm. (G) The GFP+ inflammatory cells in the section of DLNs are quantified and presented as AU. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus P; #P < .05 versus LPS + Fc or LTA + Fc.
Blockade of VEGF-A profoundly attenuates the LPS- and LTA-induced lymphatic vessel densities in the inflamed ear skin and DLNs, and lymphatic flow and mobilization of inflammatory cells from the inflamed skin to DLNs. VEGF-Trap (25 mg/kg), dimeric-Fc (Fc, 25 mg/kg), or none was treated at 1 day before and after the intradermal injection of PBS (P), LPS, or LTA. (A) At day 3 after the injection, the inflamed ears and DLNs were sectioned for histologic analysis. Tissue sections were coimmunostained for LYVE-1 and CD31. Scale bars represent 50 μm. (B,C) Lymphatic (LV) and blood vessel (BV) densities in the inflamed skin (B) and DLNs (C) were quantified and presented as percentages. (D,E) Three days later, 3 μL FITC-conjugated dextran was intradermally injected into the inflamed skin. Thirty minutes later, fluorescence intensities in DLNs were determined with the IVIS (top panels) and fluorescence stereomicroscope (bottom panels), and quantified and presented as relative radiance (photons/sec per cm2/steradian). (F) At day 3 after intradermal injection of LPS or LTA into the ear, the GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sectioned and immunostained for LYVE-1. Scale bars represent 50 μm. (G) The GFP+ inflammatory cells in the section of DLNs are quantified and presented as AU. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus P; #P < .05 versus LPS + Fc or LTA + Fc.
Blockade of VEGF-C/D or VEGF-A delays antigen clearance
To ascertain whether VEGF-C, -D, and -A are involved in antigen clearance through local lymphatic vessels and DLNs, a single intradermal injection of FITC-LPS (10 μg/ear in 10 μL PBS) into the ear skin was performed in the mice treated with the AdsVEGFR3, Ad-βgal, VEGF-Trap, and dimeric-Fc, and the clearance of FITC-LPS was monitored. Compared with treatment with Ad-βgal, treatment with Ad-sVEGFR3 attenuated the disappearance of FITC-LPS from the ear (∼ 19.8% at day 1 and ∼ 11.1% at day 3; Figure 6A,C). Compared with treatment with dimeric-Fc protein, treatment with VEGF-Trap attenuated FITC-LPS disappearance from the ear (∼ 18.9% at day 1 and ∼ 7.5% at day 3), and treatment with anti–VEGF-A antibody also attenuated FITC-LPS disappearance from the ear (∼ 25.2% at day 1 and ∼ 4.4% at day 3; Figures 6B,D, S12). Histologic analyses revealed that more FITC-LPS remained in the injected ear skin of mice treated with Ad-sVEGFR3, VEGF-Trap, or anti–VEGF-A antibody compared with the mice treated with Ad-βgal or dimeric-Fc (Figures 6E, S12), confirming that blocking VEGF-C/D or VEGF-A delays antigen clearance from the inflamed skin.
Blockade of VEGF-C/D or VEGF-A delays FITC-LPS from the inflammation site to DLNs in the inflamed skin. AdsVEGFR-3 (R3) or Ad-βgal (109 pfu) was injected 12 hours before the intradermal injection of FITC-LPS into ear skin. VEGF-Trap (25 mg/kg) or dimeric-Fc (Fc, 25 mg/kg) was given 1 day before and after FITC-LPS into ear skin. (A-D) At days 0, 1, and 3 after the injection of FITC-LPS, the remaining FITC-LPS was quantified with IVIS and presented as relative radiance (photons/sec per cm2/steradian). All dots represent mean ± SD from 4 to 5 mice. *P < .05 versus Ad-βgal or Fc. (E) At day 3 after the intradermal injection of FITC-LPS, the ears were sectioned and immunostained for LYVE-1, and merged. Scale bars represent 50 μm. Four independent experiments show similar results.
Blockade of VEGF-C/D or VEGF-A delays FITC-LPS from the inflammation site to DLNs in the inflamed skin. AdsVEGFR-3 (R3) or Ad-βgal (109 pfu) was injected 12 hours before the intradermal injection of FITC-LPS into ear skin. VEGF-Trap (25 mg/kg) or dimeric-Fc (Fc, 25 mg/kg) was given 1 day before and after FITC-LPS into ear skin. (A-D) At days 0, 1, and 3 after the injection of FITC-LPS, the remaining FITC-LPS was quantified with IVIS and presented as relative radiance (photons/sec per cm2/steradian). All dots represent mean ± SD from 4 to 5 mice. *P < .05 versus Ad-βgal or Fc. (E) At day 3 after the intradermal injection of FITC-LPS, the ears were sectioned and immunostained for LYVE-1, and merged. Scale bars represent 50 μm. Four independent experiments show similar results.
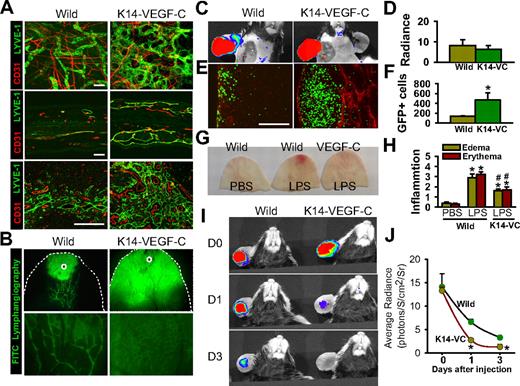
K14-VEGF-C transgenic mice display enhanced inflammatory cell migration, antigen clearance, and inflammation resolution
Compared with wild-type littermates, K14-VEGF-C transgenic mice displayed profoundly denser and larger lymphatic vessel networks in the ear skin and DLNs (Figure 7A). Moreover, vital fluorescence lymphangiography by injection of FITC-dextran revealed an immediate and diffuse distribution of the FITC-dextran from the injection site in the transgenic mice, compared with limited and selective distribution of the FITC-dextran from the injection site in normal littermates (Figure 7B). In the LPS-induced inflamed skin, no differences were seen in the rate of lymphatic drainage to the DLNs between the normal littermates and the transgenic mice (Figure 7C,D). However, inflammatory cell migration into the DLNs was higher (∼ 7.8-fold) in the K14-VEGF-C transgenic mice than in the normal littermates (Figure 7E,F). Furthermore, the inflammatory responses to LPS, including swelling and erythema, were significantly less in the transgenic mice than in the normal littermates (Figure 7G,H), suggesting that K14-VEGF-C transgenic mice have faster antigen clearance in the skin. In fact, the disappearance of FITC-LPS from the ear was significantly faster over time in the transgenic mice than in the normal littermates (Figure 7I,J), further indicating faster antigen clearance in the skin. To determine the role of macrophages in lymphatic function in the K14-VEGF-C mice, lymph flow and inflammatory cell migration were measured after treatment with CL or CDL. Depletion of macrophages by CDL did not affect lymph flow or inflammatory cell migration from the skin to DLNs in the K14-VEGF-C mice (Figure S13A-D). Furthermore, depletion of macrophages by CDL did not significantly exaggerate or alleviate the LPS-induced inflammatory reactions, including severe swelling and erythema formation (Figure S13E,F), indicating that the profoundly denser and larger lymphatic vessel networks in the ear skin and DLNs are sufficient to resolve inflammation and can compensate for the role of macrophage-induced lymphangiogenesis in the inflamed skin.
K14-VEGF-C transgenic mice display greater inflammatory cell migration and faster resolution of inflammation. (A) Coimmunostaining of LYVE-1 and CD31 in whole mounted (top panels) and sectioned (middle panels) ear skin and paracortical regions of sectioned DLNs (bottom panels) in K14-VEGF-C (K14-VC) and wild-type mice. Scale bars represent 50 μm. (B) FITC-conjugated dextran (3 μL) was intradermally injected into the ear skin (○), and 5 minutes later the distribution of FITC was visualized by a fluorescence stereomicroscope. Bottom panels show higher magnifications. (C,D) At day 3 after the injection of LPS, 3 μL FITC-conjugated dextran was intradermally injected into the inflamed skin. Fluorescence intensities were determined in DLNs at 30 minutes after the injection using IVIS, quantified, and presented as relative radiance (photon/sec per cm2/steradian). (E,F) At day 3 after intradermal injection of LPS, the GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sectioned and immunostained for LYVE-1. Scale bars represent 50 μm. The GFP+ inflammatory cells in the DLNs were quantified and presented as AU. (G,H) At day 6 after intradermal injection of LPS, ears were photographed, and the severities of erythema and swelling were scored. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus wild type. (I,J) At days 0, 1, and 3 after the injection of FITC-LPS, the remaining FITC-LPS was quantified using IVIS and presented as relative radiance (photons/sec per cm2/steradian). All dots represent mean ± SD from 4 to 5 mice. *P < .05 versus wild type; #P < .05 versus LPS in wild type.
K14-VEGF-C transgenic mice display greater inflammatory cell migration and faster resolution of inflammation. (A) Coimmunostaining of LYVE-1 and CD31 in whole mounted (top panels) and sectioned (middle panels) ear skin and paracortical regions of sectioned DLNs (bottom panels) in K14-VEGF-C (K14-VC) and wild-type mice. Scale bars represent 50 μm. (B) FITC-conjugated dextran (3 μL) was intradermally injected into the ear skin (○), and 5 minutes later the distribution of FITC was visualized by a fluorescence stereomicroscope. Bottom panels show higher magnifications. (C,D) At day 3 after the injection of LPS, 3 μL FITC-conjugated dextran was intradermally injected into the inflamed skin. Fluorescence intensities were determined in DLNs at 30 minutes after the injection using IVIS, quantified, and presented as relative radiance (photon/sec per cm2/steradian). (E,F) At day 3 after intradermal injection of LPS, the GFP+ inflammatory cells (∼ 106 cells) were injected intradermally into the inflamed skin. Twelve hours later, the DLNs were sectioned and immunostained for LYVE-1. Scale bars represent 50 μm. The GFP+ inflammatory cells in the DLNs were quantified and presented as AU. (G,H) At day 6 after intradermal injection of LPS, ears were photographed, and the severities of erythema and swelling were scored. All bars represent mean ± SD from 4 to 5 mice. *P < .05 versus wild type. (I,J) At days 0, 1, and 3 after the injection of FITC-LPS, the remaining FITC-LPS was quantified using IVIS and presented as relative radiance (photons/sec per cm2/steradian). All dots represent mean ± SD from 4 to 5 mice. *P < .05 versus wild type; #P < .05 versus LPS in wild type.
Discussion
As part of the innate immune response during skin inflammation, locally generated interstitial fluid containing activated immune cells is drained unidirectionally into the DLNs via afferent lymphatic vessels. The cellular and molecular mechanisms of DC migration from inflamed skin to DLNs with respect to antigen presentation have been well characterized.21,,,,–26 However, little is known about the underlying cellular and molecular mechanisms for clearance of pathogen molecules and the resolution of inflammation. In this study, we found that VEGF ligands secreted from infiltrated macrophages, including CD11b+/Gr-1+ macrophages, could be major mediators for pathogen clearance and inflammation resolution through profound expansion of lymphatic vessels, enhanced lymph flow, and mobilization of inflammatory cells from the inflamed skin to the DLNs (Figure S14).
Macrophages play diverse roles in IIL.6,,,,,,–13 For example, CD11b+ macrophages are actively involved in IIL in the cornea,10 and VEGF-A stimulates IIL mainly via macrophage recruitment.6 Accordingly, depletion of macrophages abrogates this IIL.10 Moreover, normal wound healing requires macrophage-induced lymphangiogenesis.12,13 In a diabetic mouse model, administration of macrophages promotes wound healing with enhanced lymphangiogenesis,13 demonstrating the critical role of macrophages in IIL in inflamed tissues. Our recent study in an ovarian cancer model indicated that VEGF ligands derived from CD11b+ macrophages induce profound but dysfunctional lymphangiogenesis in inflammatory carcinogenesis.34 The present results show that acute inflammation induced by intradermal administration of virulence factors, LPS and LTA, results in the abundant recruitment of CD11b+/Gr-1+ macrophages, and these macrophages appear to induce a temporary but profound lymphangiogenesis in the inflamed skin as well as in the DLNs. Furthermore, the subsequent experiments demonstrated that the CD11b+/Gr-1+ macrophages were mainly F4/80+ cells. These cells could have substantial phagocytic activity because they were depleted by CDL, and F4/80+ macrophages are known to have a relatively high phagocytic activity.31,35 Because of the phagocytic activity of these cells, they could actively participate in clearing dead leukocytes, pathogens, and cellular debris by phagocytosis. Then these cells are transmigrated into the lumen of local lymphatic vessels and finally drained into the DLNs.36 These phagocytic macrophages are also known to be the main cell type for secreting major cytokines, chemokines,1,,,–5 and lymphangiogenic growth factor.6,,,,,,–13 Indeed, upon phagocytosis of the apoptotic bodies, macrophages secrete abundant VEGF-A, which promotes endothelial cell growth.37 Our RT-PCR analyses revealed that activated macrophages, including CD11b+ macrophages, are the main producers of lymphangiogenic VEGF ligands in the inflamed skin and DLNs during acute IIL. Furthermore, depletion of macrophages largely attenuated IIL, indicating that macrophages could be the major source of lymphangiogenic growth factors. Thus macrophages, including CD11b+/Gr-1+/F4/80+ macrophages, help themselves by laying the groundwork for their own transportation to the DLN by secreting lymphangiogenic growth factors (Figure S14).
Our results also show that profound increases of lymphatic vessel density in the inflamed skin and DLNs result from active proliferation and sprouting of LECs. The proliferation and sprouting of these cells are essential processes of lymphangiogenesis.14,–16 The increased lymphatic vessel densities obviously increase the surface area of the initial, collecting, and conduit lymphatic vessels, and these vessels would facilitate entry and transport of interstitial fluid and immune cells from the inflamed skin to the conducting lymphatic vessels and the DLNs. Johnson et al showed that the expression of the key leukocyte adhesion molecules, intercellular adhesion molecule, and vascular cell adhesion molecule, are up-regulated in LECs in inflamed skin, and these adhesion molecules facilitate DC transmigration.38 Furthermore, Baluk et al elegantly described the preferential entry of MHC-II+ DCs and macrophages and CD45+ leukocytes into the proximal half of initial lymphatic vessels having “buttonlike junctions” in tracheal mucosa during LPS-induced acute inflammation.39 Likewise, activated macrophages, including CD11b+/Gr-1+/F4/80+ macrophages, could actively transmigrate to inflamed lymphatic vessels by virtue of these up-regulated adhesion molecules, preferentially through the proximal half of the initial lymphatic vessels. The robust expansion of CD11b+/Gr-1+ macrophages observed in the DLNs during acute skin inflammation implies recruitment of these cells from the outside to the DLNs. Previous studies21,40,–42 indicate that recruitment of circulating monocyte-driven macrophages to the DLNs during skin inflammation is mediated through high endothelial venules (HEVs) by “remote control” of chemokines including “macrophage chemoattractant protein-1” and “monokine induced by gamma interferon” secreted from the inflamed skin tissues. However, our compelling evidence favors a model in which draining lymphatic vessels, rather than HEVs, are responsible for the monocyte-driven macrophage recruitment to the DLNs during acute inflammation. The evidence in favor of this model is (1) our immunohistologic analyses revealing that robust increases of CD11b+ macrophages are localized mainly around subcapsular afferent lymphatic vessels, which are directly connected to peripheral tissues, and (2) the adaptively transferred inflammatory cells are also localized mainly around subcapsular afferent lymphatic vessels, with only a few localized in the HEV-rich regions of the DLNs during the inflammation. Thus, the draining lymphatic vessels that lead from the inflamed tissue to the DLNs are the main routes for monocyte-driven macrophage recruitment to the DLNs during acute inflammation.
Recently, lymphangiogenesis in the DLNs has emerged as a significant factor in several pathophysiologic conditions. During inflammation, B cell–driven DLN lymphangiogenesis enhances DC mobilization,25 whereas tumor-induced DLN lymphangiogenesis enhances lymph flow and metastasis.43,44 These intriguing reports led us to examine whether inflammation-induced DLN lymphangiogenesis promotes inflammatory cell mobilization and lymph flow, and subsequently enhances antigen clearance and inflammation resolution. Indeed, our findings exhibited that DLN lymphangiogenesis is closely linked to accelerated mobilization of inflammatory cells and to a lesser extent, enhanced lymph flow, from inflamed tissue to DLNs. IIL at the inflamed tissue could be an additional factor in accelerating inflammatory cell mobilization and lymph flow. Interestingly, Halin et al45 found that DLN lymphangiogenesis during chronic inflammation is due to VEGF-A secreted from the inflamed tissue, but not from the DLNs. However, our results show a clear correlation between abundant macrophage infiltration, up-regulated expression of VEGF-C, -D, and -A, and increased lymphatic densities within the DLNs. Moreover, the enriched CD11b+ macrophages from the DLNs displayed markedly up-regulated expression of VEGF-C, -D, and -A. Furthermore, depletion of macrophages including CD11b+/Gr-1+, and blockade of VEGF-C/D or VEGF-A, largely attenuated DLN lymphangiogenesis. These data indicate that the lymphangiogenic growth factors secreted from robustly infiltrated macrophages in the DLNs could be the major driving force for DLN lymphangiogenesis. Therefore, we propose that ILL in DLNs could be caused not only by VEGF ligands from inflamed tissue, but also by VEGF ligands secreted from CD11b+/Gr-1 macrophages within DLNs in acute inflammation.
The most intriguing and novel finding in this study is that inflammation-induced up-regulation of VEGF-C, -D, and -A from the macrophages and local tissues plays an active role in antigen clearance and inflammation resolution by promoting IIL and lymphatic function. Accordingly, depletion of macrophages or blocking of VEGF-C/D or -A strikingly reduced inflammatory cell migration, lymph flow, antigen clearance, and inflammation resolution with attenuated DLN lymphangiogenesis. Thus, these components largely contribute to regulate inflammatory cell migration and lymph flow directly and indirectly with the expansion of lymphatic surface area through local and DLN lymphangiogenesis. (Figure S14). Accordingly, the K14-VEGF-C transgenic mice with abundant lymphatic vessels in skin and DLNs displayed higher inflammatory cell migration, and faster antigen clearance and inflammation resolution than the wild-type mice. In fact, VEGF-C is known not only to augment lymphatic growth, but also to maintain or enhance lymphatic functions including lymph drainage and flow from the interstitial compartment to the lymphatic lumen and DLNs,46 and VEGF-A is one of the most potent factors that promotes permeability from the blood vascular lumen to the interstitial compartment.47 Thus, because VEGF-C, -D, and -A may control and contribute to homeostasis between lymph drainage and flow and blood vascular permeability, disturbances in this homeostasis could be involved in delayed antigen clearance and inflammation resolution in the inflamed skin tissue. However, it should be noted that VEGF-Trap treatment attenuates the ability of transgenic VEGF-A to induce chronic inflammation in skin, corneal inflammation, and the release of inflammatory cytokines induced by polymicrobial sepsis.6,48,49 Therefore, the blockade of VEGF-A with VEGF-Trap can reduce or promote inflammation, depending on the type and location of the inflammation.
In conclusion, our findings shed light on an additional role of macrophages during acute inflammation. The lymphangiogenic growth factors secreted from infiltrated macrophages in inflamed tissue and DLNs appear to be critical in lymphatic vessel expansion, antigen clearance, and inflammation resolution through enhancement of lymphangiogenesis, lymph flow, and mobilization of inflammatory cells from inflamed tissue to the DLNs. A subset of CD11b+/Gr-1+/F4/80+ macrophages and the lymphangiogenic growth factors VEGF-C, -D, and -A could be the key components in promoting antigen clearance and inflammation resolution during bacterial pathogen–induced acute inflammation. Disruptions in these orchestrated cellular and molecular actions may disrupt early inflammation resolution, leading to uncontrolled chronic inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory Program (2004-02376, G.Y.K.) funded by the Ministry of Science and Technology and BioGreen 21 Program, Rural Development Administration (20080401034061, S.H.H.), Republic of Korea.
Authorship
Contribution: R.P.K., S.H.H., K.A., and G.Y.K. designed and organized the experiments; R.P.K., C.J., and G.Y.K. performed the animal studies, analyzed the data, generated the figures, and wrote the paper; K.J. and H.Y. performed histologic analyses; and R.A.S. and J.E.B. prepared materials for the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gou Young Koh, Department of Biological Sciences, KAIST, 373-1, Guseong-dong, Daejeon, 305-701, Republic of Korea; e-mail:gykoh@kaist.ac.kr.