Abstract
Among 1614 children with acute lymphoblastic leukemia (ALL) treated with the Nordic Society for Paediatric Haematology and Oncology (NOPHO) ALL-92 protocol, 20 patients developed a second malignant neoplasm (SMN) with a cumulative risk of 1.6% at 12 years from the diagnosis of ALL. Nine of the 16 acute myeloid leukemias or myelodysplastic syndromes had monosomy 7 (n = 7) or 7q deletions (n = 2). In Cox multivariate analysis, longer duration of oral 6-mercaptopurine (6MP)/methotrexate (MTX) maintenance therapy (P = .02; longest for standard-risk patients) and presence of high hyperdiploidy (P = .07) were related to increased risk of SMN. Thiopurine methyltransferase (TPMT) methylates 6MP and its metabolites, and thus reduces cellular levels of cytotoxic 6-thioguanine nucleotides. Of 524 patients who had erythrocyte TPMT activity measured, the median TPMT activity in 9 patients developing an SMN was significantly lower than in the 515 that did not develop an SMN (median, 12.1 vs 18.1 IU/mL; P = .02). Among 427 TPMT wild-type patients for whom the 6MP dose was registered, those who developed SMN received higher average 6MP doses than the remaining patients (69.7 vs 60.4 mg/m2; P = .03). This study indicates that the duration and intensity of 6MP/MTX maintenance therapy of childhood ALL may influence the risk of SMNs in childhood ALL.
Introduction
A few percent of children with acute lymphoblastic leukemia (ALL) will develop a second malignant neoplasm (SMN), and this complication has a poor prognosis.1-5 Treatment-related risk factor analyses have focused primarily on irradiation, alkylating agents, and topoisomerase II inhibitors such as anthracyclines and epipodophyllotoxins that can induce DNA damage, whereas less attention has been paid to mechanisms that could interfere with DNA control, including drugs that may modify DNA repair.5
Previously, children with higher risk ALL had a higher risk of SMNs because of their more intensive chemotherapy, radiotherapy, or both.6 However, a few studies have indicated that the less intensive 6-mercaptopurine (6MP)/methotrexate (MTX) maintenance therapy also enhances the risk of SMNs. The Nordic Society for Paediatric Haematology and Oncology (NOPHO) ALL-92 6MP/MTX maintenance therapy trial reported 3 cases of acute myeloid leukemia or myelodysplastic syndrome (t-AML/MDS) among 55 children, who had thiopurine methyltransferase (TPMT) activity compatible with TPMT low-activity polymorphisms (cumulative risk, 9%), compared with only 2 cases among 384 patients (cumulative risk, 1%) with normal TPMT activity.7 Similarly, St Jude Children's Research Hospital has reported that children with ALL who were heterozygous for TPMT low-activity polymorphisms had an excessive risk of SMNs if exposed to cranial irradiation.8 Low-activity TPMT polymorphisms lead to higher intracellular levels of 6-thioguanine nucleotides (6TGN), which are the cytotoxic 6MP metabolites that are incorporated into DNA.9,10 In addition, some of the methylated 6MP metabolites formed by TPMT are strong inhibitors of purine de novo synthesis,11 and, because their levels increase with higher doses of 6MP,12 such 6MP dose increments in TPMT high-activity patients may lead to enhanced DNA-6TGN incorporation even in patients without TPMT low-activity polymorphisms.
To explore further the possible effect of 6MP/MTX maintenance therapy on the risk of SMNs, we reviewed the cumulative incidence of SMNs among the total cohort of 1614 patients treated according to the NOPHO ALL-92 protocol and report that the risk of SMNs in patients treated on this protocol correlates with TPMT activity or the dose intensity of oral 6MP/MTX maintenance therapy or both.
Methods
Patients
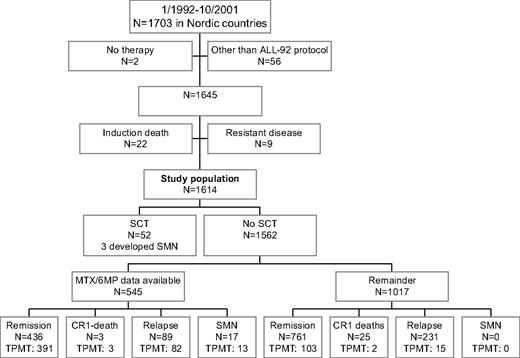
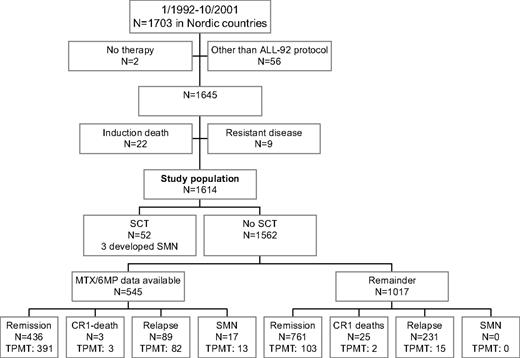
From January 1992 until October 2001, 1703 children 1.0 to 14.9 years of age were diagnosed with B-cell precursor or T-cell ALL in the Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden; Figure 1). Two patients with Down syndrome received no antileukemic therapy, 10 patients were treated according to non-NOPHO ALL protocols, 1 patient was treated according to the previous NOPHO ALL-86 protocol, and 45 patients were treated according to the NOPHO ALL-2000 protocol, before it was officially opened. None of these 58 patients have developed an SMN during their first complete remission (CR1). Of the remaining 1645 patients who started therapy according to the NOPHO ALL-92 protocol, 22 patients died during induction therapy, and 9 patients had resistant disease and were excluded from further analysis. The remaining 875 boys and 739 girls were included in the present study. Twenty-nine of these 1614 patients had Down syndrome. The data used in this publication were retrieved from the NOPHO Leukemia Registry. This registration has been approved by the relevant ethical committees and the data protection agencies in the involved countries. Informed consent was obtained in accordance with the Declaration of Helsinki.
Risk grouping
Treatment
For 55 of the 1614 patients, the assigned treatment did not completely match the risk group criteria. Thirty-five patients with morphologically suspected, but uncertain, M3 bone marrow on day 15 or M2 bone marrow on day 29 or both had standard risk (SR)–ALL (n = 20) or intermediate risk (IR)–ALL (n = 15) by all other criteria and were treated according to the SR or IR treatment arms, respectively. Two patients who fulfilled the protocol criteria for SR-ALL or IR-ALL were by decision of the treating physician assigned to the VHR-arm because of a t(1;19)(q23;p13) translocation (n = 1) or hypodiploidy (n = 1; modal number 35-36). Three patients who fulfilled the VHR-ALL criteria were by decision of the treating physician assigned to the high-risk (HR) arm. Furthermore, 9 patients who fulfilled the SR-ALL criteria were by decision of the treating physician assigned to the IR arm, and 6 patients who fulfilled the IR-ALL criteria were likewise assigned to the SR arm. None of those 55 patients have developed an SMN, and all were analyzed according to the therapy they actually received.
Induction therapy and consolidation therapy.
These therapies have previously been published in detail.13 In short, all patients received prednisolone (60 mg/m2 per day on days 1-36, then tapered), weekly vincristine (VCR; 2.0 mg/m2 times 6), doxorubicin (40 mg/m2 times 3 [SR and IR] or 4 [HR and VHR]), Erwinia asparaginase (30.000 IU/m2 daily on days 37-46), and intrathecal MTX on 4 occasions. Subsequently and before MT, SR-ALL patients received 3 courses of high-dose MTX (HD-MTX; 5 g/m2 every 24 hours with intrathecal MTX and leucovorin rescue), whereas patients with IR-, HR-, and VHR-ALL received alternating combination therapy blocks.
CNS irradiation and stem cell transplantation.
Because of CNS disease at diagnosis or allocation to the VHR protocol arm, 123 patients who did not receive a transplant (7.6% of the total study population) received cranial irradiation in CR1. Thirty-one boys and 21 girls with higher-risk criteria received a stem cell transplant (SCT) in CR1, but no uniform NOPHO indications for SC transplantation or for conditioning therapy existed during the protocol period.14
Oral 6MP/MTX maintenance therapy.
Maintenance therapy was initiated for a total of 1317 patients at treatment week 13 (SR), 32 (IR), or 63 (HR) and scheduled to continue until 2 years (IR and HR) or 2.5 years (SR) after diagnosis. However, for 32 patients the total duration of therapy (and thus also the maintenance therapy phase) was by decision of the treating physician prolonged for more than 12 weeks beyond that of the protocol (median, 15 weeks). For a total of 85 patients, the precise dates for the initiation (n = 60) or cessation (n = 17) of MTX/6MP maintenance therapy or for both (n = 8) were missing from the NOPHO leukemia registry. For those 85 patients, the date for start of MTX/6MP maintenance therapy was estimated from the median number of days from diagnosis for the patients in that risk group for whom the dates were available (SR, 103 days; IR, 245 days; HR, 489 days), whereas the date for cessation of maintenance therapy was set to be 2.0 years (IR- and HR-ALL) or 2.5 years (SR-ALL) from diagnosis. None of those 85 patients developed SMN during CR1. The starting doses of 6MP and MTX were 75 mg/m2 per day and 20 mg/m2 per week, respectively. During the first year of maintenance therapy, patients with SR- or IR-ALL received alternate pulses at 4-week intervals of (1) VCR (2.0 mg/m2 once) and prednisolone (60 mg/m2 per day for 1 week) and (2) HD-MTX (5 g/m2 every 24 hours with intrathecal MTX and leucovorin rescue) until 5 courses of HD-MTX had been given.15 Every 8 weeks throughout maintenance therapy, HR patients received reinductions of VCR (1.5 mg/m2 once) and prednisolone (40 mg/m2 per day for 5 days) with intrathecal MTX. Between 1992 and 1996, 538 patients with SR-, IR-, or HR-ALL were included in the randomized ALL-92 maintenance therapy trial.16 It explored the prognostic effect of dose adjustments of oral 6MP/MTX maintenance therapy by erythrocyte levels of 6MP/MTX metabolites. TPMT genotype, phenotype, or both were not included in the dose adjustments of that study. As part of that study nearly 30 000 datasets on MTX and 6MP doses as well as parameters for myelotoxicity and hepatotoxicity were registered and used in the present study for comparisons between patients who did or did not develop SMNs. For the purpose of the present study, we also collected data on MTX and 6MP dosing and on leukocyte and absolute neutrophil counts during maintenance therapy for the patients with SMNs who were not included in the randomized study.
LSA2L2 maintenance therapy.
On the basis of the unsatisfactory results of the NOPHO ALL-86 protocol for patients with higher risk ALL,13,17 NOPHO tested in a nonrandomized study the efficacy of multidrug, cyclic LSA2L2 remission maintenance therapy to VHR-patients (all Nordic countries) and to all Finnish patients with HR-ALL. LSA2L2 maintenance therapy was scheduled to be given from protocol weeks 48 to 95.13 However, antileukemic treatment was discontinued at week 104, even if less than 6 LSA2L2 cycles had been due to treatment delays. If a patient were able to complete 6 LSA2L2 maintenance therapy cycles before treatment week 104, up to 9 weeks of oral MTX/6MP maintenance therapy was given until week 104. Each LSA2L2 block consisted of 4 courses at 2-week intervals with alternating combinations of (1) 6-thiogunanine, intravenous cyclophosphamide, and intrathecal MTX, (2) hydroxyurea and daunorubicin (substituted with prednisolone/VCR after the first 4 hydroxyurea/daunorubicin cycles), (3) oral MTX and BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea), and (4) intravenous cytarabine and VCR.13,18-20
TPMT.
The TPMT genotype (low-activity polymorphisms G460A and A719G) or erythrocyte TPMT activity or both were available for 609 patients.21 For 484 of those patients only the erythrocyte TPMT activity was available, and it was measured 1 to 5 times during maintenance therapy as previously described.22 For patients with more than one TPMT activity measurement, an arithmetic mean TPMT activity was calculated. All TPMT phenotype assays were performed at least 8 weeks after the most recent blood transfusion. The antimode of the TPMT activity distribution was 14 IU/mL. The 62 patients below that level, and for whom no TPMT genotype was available, were classified as TPMT presumed heterozygous (n = 60; median TPMT-activity, 10.4 IU/mL; range, 6.2-13.9 IU/mL) or TPMT-deficient (n = 2; activity < 1.0 IU/mL). Among the 40 patients for whom both TPMT genotyping and phenotyping were available, all 9 patients who were TPMT heterozygous by genotyping had TPMT activity less than 14.0 IU/mL. However, also 5 (16%) of 31 patients who had a wild-type genotype had TPMT activity less than 14 IU/mL (range, 9.0-13.4 IU/mL), but they were classified by their genotype results as TPMT wild-type. In total, 533 patients were classified as TPMT wild-type, 74 as heterozygous, and 2 as TPMT deficient. In the following analyses the latter 2 subsets were grouped together as TPMT low activity, which included the presumed heterozygous patients (TPMT activity < 14 IU/mL; no genotype available), and the remainder as TPMT wild-type. The TPMT phenotype and genotype were not revealed to the physicians while the patients were on therapy.
Karyotype.
Only conventional G-band karyotyping was mandatory. In addition, many patients were explored with high-resolution comparative genomic hybridization, fluorescent in situ hybridization, or reverse transcriptase–polymerase chain reaction for selected translocations [eg, t(9;22)(q34;q11)].23 The karyotypes of the patients were described according to ISCN (International System for Human Cytogenetic Nomenclature) 1995.24 For 664 patients, information on karyotypes was missing, or only normal metaphases were obtained. Of the 950 aberrant karyotypes, 166 had t(12;21)(p13;q22), 362 patients were classified as high-hyperdiploid with a modal chromosome number of 51 to 60 chromosomes, 18 had t(1;19)(q23;p13), 27 had t(9;22)(q34;q11), 11 had MLL rearrangements, 19 had a hypodiploid karyotype (chromosome modal number < 45, including one case with t(9;22)[BCR/ABL]), and 348 cases had other chromosomal aberrations.
Statistics
All patients were analyzed according to the treatment they actually received. The duration of 6MP/MTX maintenance therapy was calculated as the number of weeks between the initiation of 6MP/MTX maintenance therapy and performance of a stem cell transplantation in CR1 (n = 1), the occurrence of an event, or the end of antileukemic therapy, whichever came first. The average doses of 6MP and MTX were calculated as the total prescribed doses divided by the duration of maintenance therapy. The risk of SMN was calculated from the time of diagnosis, and survival analyses were performed with a basic time scale defined by the date of diagnosis. Patients who died in CR1, received a SCT in CR1, or developed a relapse were censored at the time of these events in the analysis of SMN risk factors. Cox proportional hazard regression analyses were performed with the likelihood ratio test for differences in risk of SMN.25,26 Wilcoxon rank sum test was applied to compare the distribution of parameters between subgroups.27 The Kaplan-Meier method was applied to estimate remission duration and to generate survival curves.28 Subgroups were compared with the log-rank test.29 Two-sided P values less than .05 were regarded as significant.
Results
The median follow-up time for the 1225 patients who remain in first remission is 10.4 years (50% range, 8.0-12.6 years). At the end of the study, 34 patients had died in CR1, 335 patients had developed a relapse of ALL 0.2 to 12.0 years from diagnosis (median, 2.6 years), and 20 patients had developed an SMN (Figure 1). The overall projected 12-year event-free survival and overall survival were 75% (± 1%) and 86% (± 1%), respectively.
Flow chart of patient inclusion or exclusion. Patients (n = 545) participated in the randomized MTX/6MP maintenance therapy study16 or they developed SMNs and had their data on maintenance therapy 6MP/MTX dosage and blood counts retrieved. Data on both 6MP/MTX doses and blood counts were not available for all the 545 patients. TPMT phenotyping (n = 524) or genotyping or both were available for 609 patients.
Flow chart of patient inclusion or exclusion. Patients (n = 545) participated in the randomized MTX/6MP maintenance therapy study16 or they developed SMNs and had their data on maintenance therapy 6MP/MTX dosage and blood counts retrieved. Data on both 6MP/MTX doses and blood counts were not available for all the 545 patients. TPMT phenotyping (n = 524) or genotyping or both were available for 609 patients.
The 20 SMNs, including 3 that occurred after SC transplantation, were diagnosed at a median of 3.4 years (range, 0.8-11.6 years) from the initial diagnosis of ALL (Table 2). All SMNs in patients who did not receive a transplant occurred after cessation of maintenance therapy. None of the patients with Down syndrome developed an SMN. Sixteen of the 20 SMNs were AML (n = 8) or MDS (n = 8), all but 2 of which occurred within 5.5 years from diagnosis (median, 3.7 years). One of these AMLs occurred after SC transplantation. The last 4 patients included a primitive neuroectodermal CNS tumor, an oral carcinoma, and 2 post-SC transplantation cancers (a posttransplantation, lymphoproliferative disease and a papillary thyroid cancer). The 12-year cumulative risk of developing an SMN (pSMN12y) was 1.6% plus or minus 0.4%. Thirteen of the 16 patients with t-AML/MDS received a SCT. Twelve of the 20 patients died within 21 months from diagnosis of their SMN with a median time to death of 8.8 months and a projected survival fraction of 0.39 plus or minus 011 (Table 2).
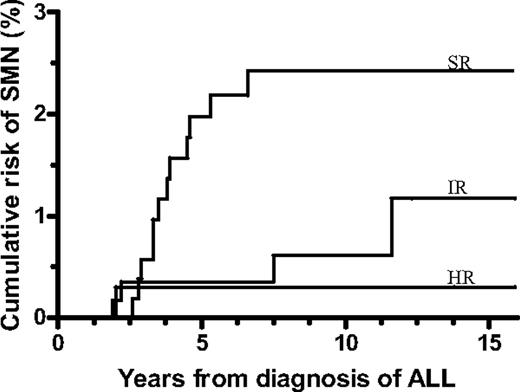
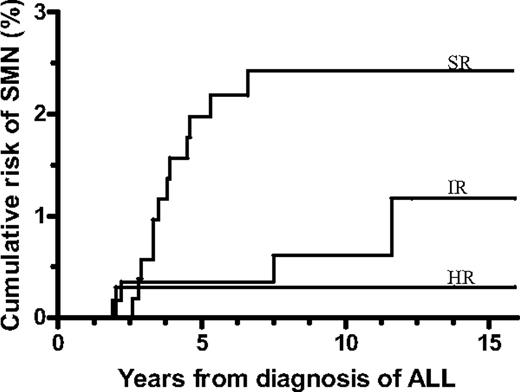
The projected risk of SMN among the patients who did not receive a transplant was significantly related to the risk group and was 2.4% (± 0.7%) for SR-ALL, 1.2% (± 0.7%) for IR-ALL, and 0.3% (± 0.3%) for higher risk ALL patients (P = .007; Figure 2).
Cumulative risk of a second malignant neoplasm (SMN) by risk group for patients who did not receive a transplant in first remission. Standard risk (SR) was 2.4% ± 0.7%, intermediate risk (IR) was 1.2% ± 0.7%, and higher risk (HR) was 0.3% ± 0.3% (P = .007). ALL indicates acute lymphoblastic leukemia.
Cumulative risk of a second malignant neoplasm (SMN) by risk group for patients who did not receive a transplant in first remission. Standard risk (SR) was 2.4% ± 0.7%, intermediate risk (IR) was 1.2% ± 0.7%, and higher risk (HR) was 0.3% ± 0.3% (P = .007). ALL indicates acute lymphoblastic leukemia.
Of the 16 SMNs (15 t-AML/MDS) that occurred among the SR/IR patients, 10 patients had an aberrant karyotype at the time of ALL diagnosis of which 8 were high-hyperdiploid compared with 301 high-hyperdiploid cases among the 684 SR/IR patients with an aberrant karyotype who did not develop an SMN (P = .03). This reaches borderline significance if all successful karyotypes are included as the denominator (P = .07). Among all 562 SR-ALL patients, the 161 patients with a high-hyperdiploid karyotype had a 4.8% (± 1.8%) risk of developing an SMN compared with a 1.5% (± 0.6%) risk for the remaining 401 SR patients (P = .02). No other subsets of genetic aberrations were significantly related to the risk of developing an SMN.
In all 16 cases of t-AML/MDS a karyotype was available and showed chromosomal aberrations in all, including one 11q23-aberration, monosomy 7 (n = 7), 7q deletion (n = 2), or chromosome 5 deletions or translocations (n = 5; Table 2). Cytogenetic aberrations involving chromosomes 5 or 7 occurred in 11 of 15 patients with t-AML/MDS who did not receive a transplant. The 9 -7/7q- t-AML/MDS cases occurred among patients with high-hyperdiploidy (n = 4), t(12;21)(p13;q22) (n = 1), or a normal/missing (n = 4) karyotype at diagnosis of ALL. The projected survival of the 9 -7/7q- t-AML/MDS did not differ significantly from that of the remaining 11 SMNs (Table 2).
The cumulative risk of developing an SMN was 1.7% (± 0.4%) for the 1316 patients who were in CR1 when they initiated MTX/6MP maintenance therapy compared with 0% for the 169 patients in CR1 at the start of LSA2L2 maintenance therapy.
To analyze the effect of MTX/6MP maintenance therapy on the risk of SMN, the 1562 patients who did not receive a SCT in CR1 were grouped according to whether they received MTX/6MP maintenance therapy and its duration in weeks. In backward, multivariate Cox regression analysis of the SR/IR/HR-ALL patients who had received MTX/6MP maintenance therapy only the duration of oral MTX/6MP maintenance therapy in weeks (regression coefficient, 0.02; P = .04) was related to an increased risk of an SMN, whereas neither sex, age, and white blood cell count at diagnosis came out statistically significant. If the analysis included the presence of a high-hyperdiploid karyotype, only the duration of MTX/6MP maintenance therapy (regression coefficient, 0.02; P = .05) and presence of high-hyperdiploidy (P = .07) were related to an increased risk of an SMN (overall P value of the Cox model, .02). Finally, inclusion of the VHR patients, most of whom only received LSA2L2 maintenance therapy, yielded the same coefficients for the duration of MTX/6MP maintenance therapy (regression coefficient, 0.02; P = .02) and presence of high-hyperdiploidy (P = .07; overall P value of the Cox model, .003). Within the individual SR-, IR-, and HR-ALL risk groups, the patients who did or did not develop SMN did not differ significantly in their actual duration of MTX/6MP maintenance therapy (P > .2 in all comparisons).
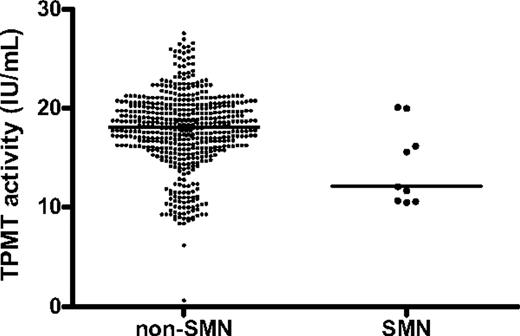
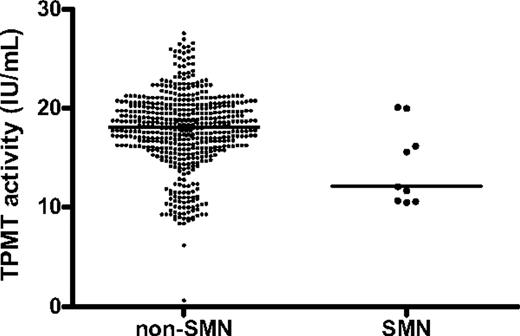
With the limitation that data on MTX and 6MP doses, erythrocyte metabolite levels, or blood counts were only available for the 545 patients who participated in the randomized NOPHO ALL-92 maintenance therapy study or developed an SMN (Figure 1), the patients who developed an SMN did not differ significantly from the remaining population with respect to their doses of 6MP (median, 62.4; n = 16 vs 59.4 mg/m2 l n = 526; P = .18) or MTX (median, 16.6; n = 16 vs 15.3 mg/m2; n = 526; P = .21), their erythrocyte MTX levels (median, 4.9; n = 8 vs 5.6 nmol/mmol hemoglobin [Hb]; n = 514; P = .17), their erythrocyte 6TGN levels (median, 297 vs 170 nmol/mmol Hb; P = .11), or their average leukocyte (median: 3.4; n = 17 vs 3.3 × 109/L; n = 527; P = .35) or absolute neutrophil counts (median, 2.1; n = 17 vs 2.0 × 109/L; n = 529; P = .91). In contrast, the patients who developed an SMN had significantly lower TPMT activity than did the remaining patients (median, 12.1 vs 18.1 IU/mL; P = .02; Figure 3). The patients who developed an SMN received 10% to 15% higher 6MP doses than those that did not develop an SMN, and this was the case both among patients classified as TPMT low activity (median, 55.5; n = 3 vs 50.3 mg/m2; n = 57; P = .31) and those classified as TPMT wild-type (median, 69.7; n = 9 vs 60.4 mg/m2; n = 418; P = .03). The patients with lower TPMT activity tended to be diagnosed with t-AML/MDS earlier than patients with higher TPMT activity, but this tendency did not reach statistical significance in Spearman correlation analysis (rS = 0.67, P = .07). Four of the 76 patients classified as TPMT low activity developed an SMN (pSMN12y = 6% ± 3%), compared with 9 of 533 classified as TPMT wild-type patients (pSMN12y = 2% ± 1%; P = .06).
Red blood cell thiopurine methyltransferase activity (TPMT) for patients who developed a second malignant neoplasm compared with patients who did not. Nine patients developed a SMN (right) compared with 515 patients who did not (left). Each dot represents 1 patient (median, 12.1 vs 18.1 IU/mL; P = .02).
Red blood cell thiopurine methyltransferase activity (TPMT) for patients who developed a second malignant neoplasm compared with patients who did not. Nine patients developed a SMN (right) compared with 515 patients who did not (left). Each dot represents 1 patient (median, 12.1 vs 18.1 IU/mL; P = .02).
The overall risk of SMN in the randomized NOPHO ALL-92 maintenance therapy study did not differ between the 2 treatment arms (4 cases among 269 patients in each arm) and did not differ from that of 1024 patients who did not receive a transplant who did not participate in the study (P = .9).
Discussion
Today, the most effective treatment protocols for children with ALL result in cure rates of 80% to 85%.30,31 In this perspective the life-threatening complications of antileukemic therapy such as therapy-related SMN increase their relative effect on the overall survival. Although the risk of SMN within the first 15 years from diagnosis is only a few percent in most protocols,1,3-5,13,32,33 subsets of patients with significantly higher risk have been identified. These subsets include patients exposed to topoisomerase II inhibitors or alkylating agents, in which the genotoxicity and relation to malignant transformation is well established.5,34 Furthermore, after CNS irradiation the cumulative risk of head and neck secondary neoplasms and CNS tumors, including meningiomas, may be well above 10% at 25 to 30 years from diagnosis of ALL.1,35 Finally, several drugs have been suggested to modify the risk of SMNs by interference with DNA repair or by promoting the growth of a malignant clone. Such agents include l-asparaginase,36 granulocyte colony-stimulating factor,37 and thiopurines.7,8,38
The present population-based, retrospective study indicates that maintenance therapy, and especially 6MP pharmacotherapy, influenced the development of SMNs. This conclusion is based on several lines of evidence. First, the strongest indication that 6MP influenced the risk of the SMN is the known DNA-damaging effect of 6MP39-41 and the correlation of the risk of t-AML/MDS to (1) longer duration of 6MP/MTX maintenance therapy, (2) low TPMT activity, (3) higher doses of 6MP for TPMT wild-type patients, and (4) higher 6TGN levels (although nonsignificant). During oral 6MP therapy 1:103 to 1:104 DNA nucleotides in leukocytes have been shown to be substituted with 6TGN (DNA-6TGN),42,43 but at present it is unknown whether the risk of t-AML/MDS is related to the individual leukocyte DNA-6TGN levels. Second, none of the patients on NOPHO ALL-92 received epipodophyllotoxins, which are known to be leukemogenic, at least in part because of inhibition of topoisomerase II.34,44 Third, although all patients received anthracyclines during induction therapy and delayed intensification, the total dose for SR-ALL patients was only 120 mg/m2. However, malignant clones may arise a few months after anthracyclines have been administered, and the subsequent therapy may then have modified the risk of whether it develops into overt t-AML/MDS,45 and anthracycline therapy during induction could thus have been a contributing factor for the development of t-AML/MDS in the present cohort. Fourth, only the patients with IR-ALL, HR-ALL, and VHR-ALL received an alkylating agent (3.0-6.6 g/m2 of cyclophosphamide), and none of the SMNs occurred among the CNS-irradiated patients who did not receive a transplant. Finally, oral low-dose MTX has rarely been reported to induce secondary MDS/AML.46 However, HD-MTX could have been a contributing factor through its inhibition of purine de novo synthesis and enhancement of DNA-6TGN incorporation during the concurrently administered 6MP.47,48 Accordingly, patients with low TPMT activity are more prone to develop severe myelotoxicity after HD-MTX with concurrently administered 6MP.49
As a result of a common genetic polymorphism, approximately 90% of whites are homozygous for high TPMT activity (TPMT wild-type), 5% to 10% are heterozygous and express intermediate TPMT activity, and 1:300 are TPMT deficient.11 Levels of TPMT activity in red blood cells, lymphocytes, and leukemic blasts are generally closely correlated, although TPMT gene copy number changes may modify the TPMT phenotype of the leukemic cells.50
TPMT methylates 6MP and other 6MP metabolites such as 6-thioinosine 5′-monophosphate, producing 6-methyl-mercaptopurine and 6-methyl-thioinosine 5′-monophosphate (MeTIMP), respectively.11 MeTIMP is a strong inhibitor of purine de novo synthesis,11 and MeTIMP levels increase with higher doses of 6MP,12 which may explain the positive correlation between the dose of 6MP and the risk of SMN among the TPMT wild-type patients in the present study, because inhibition of purine de novo synthesis could have enhanced DNA-6TGN incorporation.
Reduced TPMT activity increases the availability of 6MP for 6TGN formation, resulting in higher E-6TGN, increased myelotoxicity, and a higher cure rate.16,21,51 Because (1) only the 2 most common low-activity polymorphism were analyzed in the present cohort, (2) the concordance between TPMT genotype and phenotype is incomplete, and (3) erythrocyte TPMT activity may fluctuate during therapy because of variation in the life span of red blood cell,52 it remains to be explored whether TPMT genotyping or phenotyping is superior in prediction of the risk of SMNs. Since 2001, determination of TPMT genotype/phenotype has been mandatory in NOPHO protocols, and during the initial maintenance therapy 6MP dose is reduced to 50 mg/m2 for TPMT heterozygous patients. With longer follow-up it will be explored whether this approach has reduced the risk of SMNs for such patients without increasing their low relapse rate.21
In support of the present findings, St Jude Children's Research Hospital has previously reported that the interval between the diagnosis of ALL and of t-AML/MDS was correlated with TPMT activity.38 In contrast, the German Berlin-Frankfurt-Münster (BFM) study group failed to show a significant relation between low-activity TPMT genotypes and the risk of SMNs.53 However, (1) only genotyping and not phenotyping was used in the BFM study; (2) the backbone maintenance therapy dose of 6MP was 75 mg/m2 in the NOPHO protocol compared with only 50 mg/m2 in the BFM protocol; (3) the total duration of therapy for the SR-ALL patients was 27 months in NOPHO ALL-92, whereas maintenance therapy lasts only approximately 18 months for SR-patients in BFM protocols54 ; and (4) the BFM protocol only give 6MP 25 mg/m2 concurrent with HD-MTX (so-called protocol M), whereas the NOPHO ALL-92 protocol prescribed HD-MTX (5 g/m2) 5 times together with concurrent 6MP at 75 mg/m2 during maintenance therapy, which could have increased 6MP-induced DNA damage.49,55
Although the pattern of second cancers may change with longer follow-up, the high relative frequency of t-AML/MDS and their genetic aberrations in the present study is different from many previous reports, in which solid tumors were relatively more common and t-AML/MDS frequently harbored 11q23-aberrations because of more extensive exposure to topoisomerase II inhibitors such as epipodophyllotoxins and anthracyclines.34,44,54 Furthermore, t-AML/MDS with deletions of all or part of chromosomes 5 and 7 have previously been linked to exposures to alkylating agents or radiation therapy.5 Note that only one 11q23-rearranged case was found in the present cohort [a del(11q23)], whereas 14 cases had chromosome 5 or 7 aberrations. A linkage between high-hyperdiploid ALL and SMN, including t-AML/MDS with −7/7q-, has not previously been reported, but thiopurine therapy has been linked to t-AML/MDS with monosomy 7 in patients with benign disorders.41,56 It is uncertain to what extent the ALL cases with missing or normal karyotypes in reality had a high-hyperdiploid clone, but it is well known that conventional chromosome analysis may fail to identify high-hyperdiploidy because of poor in vitro growth of leukemic cells in this ALL subset.23 Because both high-hyperdiploidy and monosomy 7 represent mitotic nondisjunction, the linkage could represent an individual propensity for such mitotic errors similar to that identified in mothers of children with Down syndrome.57
Partly explained by pharmacogenetic polymorphisms, children with ALL vary significantly in their drug disposition and treatment response,58 and some, although not all, studies have linked excessive risks of the development of SMN to specific predisposing genetic polymorphisms such as low-activity glutathione S-transferase, methylenetetrahydrofolate reductase, NAD(P)H:quinone oxidoreductase 1, or TPMT polymorphisms.7,8,38,59-64 However, little is known about how to modify therapy to counteract such genetic predisposition. If the linkage of t-AML/MDS with high-hyperdiploidy, TPMT low activity, and 6MP treatment intensity is confirmed by others, studies are needed to explore whether individualized 6MP dose adjustments can reduce their risk of this sequelae to antileukemic therapy without increasing their rate of relapse.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the Nordic pediatric oncology centers that have supported this study with registration of patient data.
This was supported by The Danish Childhood Cancer Foundation; The University Hospital Rigshospitalet; The Children's Cancer Foundation of Sweden (grants 53/91, 62/94, 72/96, 98/59, 04/002); The Danish Cancer Society (grants 91-048, 92-017, 93-017, 95-100-28); The Lundbeck Foundation (grant 38/99); NovoNordisk Foundation; Home Secretary Research Grant for Individualised Therapy; Danish Research Council for Health and Disease; The Nordic Cancer Union (grants 56-9257, 56-100-03-9102); The Otto Christensen Foundation; and the United States National Institutes of Health (NIH; grants R01-GM28157, U01 GM61388).
K.S. holds the Danish Childhood Cancer Foundation Research Professorship in Pediatric Oncology.
National Institutes of Health
Authorship
Contribution: K.S. designed the study and wrote the paper; R.W. performed most of the TPMT analyses; K.S. and J.N. performed the rest of the pharmacologic analyses; M.K.A. performed karyotyping; E.F. (together with the Nordic Society for Paediatric Haematology and Oncology cytogenetic group) scrutinized the karyotypes of all patients; H.H. was responsible for verifying t-AML/MDS cases; H.H., I.A-M., M.B., J.K., M.H., K.V., and R.N. were responsible for providing clinical data for the patients; K.S. and A.L.S. performed the statistical analyses; and all authors commented and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Nordic Society for Paediatric Haematology and Oncology study group appears in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Kjeld Schmiegelow, The Pediatric Clinics, Juliane Marie Center 5704, University Hospital Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; e-mail: kschmiegelow@rh.dk.