Abstract
We characterized the localization, phenotype, and some functions of plasmacytoid dendritic cells (pDCs) in the human spleen. pDCs were localized in the marginal zone and the periarteriolar region. Some were also found in the red pulp. pDCs were immature by phenotypic labeling, consistently with their capacity to internalize Dextran in a functional assay. In spleens from HIV-infected patients with thrombocytopenic purpura, these characteristics were unaffected. However, an accumulation of pDCs, but not myeloid dendritic cells (mDCs), was observed in some HIV+ patients, correlating with high proviral loads. Moreover, although undetectable in most HIV− patients, interferon-α (IFN-α) production was evidenced in situ and by flow cytometry in most HIV+ patients. IFN-α was located in the marginal zone. Surprisingly, IFN-α colocalized only with few pDCs, but rather with other cells, including T and B lymphocytes, mDCs, and macrophages. Therefore, pDCs accumulated in spleens from HIV+ patients with high proviral loads, but they did not seem to be the main IFN-α producers.
Introduction
Plasmacytoid dendritic cells (pDCs) are the major interferon-α (IFN-α) and IFN-β-producing cells in response to microbial stimuli. They were originally found in inflamed or infected lymph nodes, tonsils, and in circulating blood.1,2 Their localization, phenotype, and functions were described in mouse spleen, where they coexist with myeloid dendritic cells (mDCs), the other major DC subset.3,4
The spleen is divided into the red pulp, which ensures blood filtration, and the white pulp, which functions as a secondary lymphoid organ.5,6 The white pulp, organized around arterioles, is constituted of B- and T-cell areas. It is separated from the red pulp by the marginal zone, which contains B cells, macrophages,7 and fibroblasts that were suggested to guide lymphocyte immigration into the white pulp.6 The marginal zone is an important transit area for cells and antigens that leave the bloodstream and enter the white pulp6,7 where innate and adaptive immune responses are initiated.8
In mouse spleens, mDCs are located in the T-cell area surrounding the arterioles and in the marginal zone with few in the red pulp, whereas pDCs are in the T-cell area and in the red pulp with few in the marginal zone.4,9 Injection of murine cytomegalovirus (MCMV) or Toll-like receptor (TLR) ligands induces maturation and redistribution of mDC into the T-cell area,4,9,10 whereas pDCs either accumulate in the T-cell area or form clusters in the marginal zone depending on the stimulus.4 In human spleens, only mDCs were thoroughly characterized.11,12 They are mostly immature and distributed in the peri-arteriolar T-cell area and the marginal zone. In some cases of bacterial infection or multiple trauma, however, most mDCs are mature and accumulate in the T-cell area of the spleen,11,12 with a similar distribution to murine mDCs after TLR stimulation.4,9,10
During HIV or SIV infection, the spleen, as all secondary lymphoid organs, is a major site of viral replication and CD8+ T-cell accumulation, leading to architectural disruption at the AIDS stage.13,14 Circulating mDCs and pDCs are depleted,15-18 and type I IFN production in vitro is impaired in correlation with immune deficiency.19-22 An accumulation of DC-SIGN+CD40+ DCs is found in lymph nodes from acutely infected patients,23 of activated, CD83+ DCs in lymph nodes from macaques acutely infected with SIV24 and of CD123+ pDCs in lymph nodes from chronically infected patients under highly active antiretroviral therapy (HAART).25 This contrasts with a loss of mDCs and pDCs in lymph nodes and spleens from macaques with AIDS.24,26 In vitro, HIV stimulates IFN-α production by pDCs from healthy donors.27-29
pDCs have never been thoroughly studied in human spleen, and the effect of HIV infection on spleen DCs is unknown. Here we characterized pDCs in human spleen, established their localization, explored their functions, and analyzed the changes induced by HIV infection.
Methods
Patients and human tissues, sample preparation
Spleen samples from patients requiring therapeutic splenectomy were collected following national ethical guidelines regulating the use of human tissues, with informed consent obtained in accordance with the Declaration of Helsinki (clinical information is in Table 1), and prepared as described.11 Blocks of spleen were snap-frozen or cut into small pieces, forced through a sterile sieve mesh, and cells dissociated with type VII collagenase, DNase I (20 U/mL; Sigma-Aldrich, Saint-Quentin, Fallavier, France) and 10 mM ethylenediaminetetraacetic acid. Surface molecule expression was not affected by this enzymatic dissociation.30 Mononuclear cells were isolated from splenocyte suspensions or from blood by centrifugation over Diatrizoate-Ficoll (Eurobio, Les Ulis, France). Approval was obtained from the Comité de Protection des Personnes Ile de France III Institutional Review Board for these studies.
Flow cytometry
Cells were incubated with antibodies (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), washed in phosphate-buffered saline (PBS), fixed, and analyzed using a FACSCanto (BD Biosciences, Le Pont-de-Claix, France) and FlowJo (TreeStar, Ashland, OR). DR+ lineage− (CD3, 20, 19, 56, 14, 16) mononuclear cells were gated and mDCs and pDCs distinguished by CD11c or CD123 expression. For intracellular IFN-α–labeling, cells were saturated in PBS 5% AB human serum (HS) and incubated with surface antibodies (Table S1), washed in PBS, and fixed. Cells were then permeabilized with PBS containing 2% fetal calf serum (FCS), 5 mM ethylenediaminetetraacetic acid, 0.25% saponin, and 5% HS, incubated with IFN-α–specific or control antibody, washed, fixed, and analyzed using a LSRII flow cytometer (BD Biosciences) and FlowJo.
In situ immunofluorescence
Blocks of spleen (5 × 5 × 5 mm) were snap-frozen in OCT in liquid nitrogen then stored at −80°C; 10-μm sections were prepared using an AS 620 SME cryotome (Thermo Shandon, Cergy-Pontoise, France) and fixed in acetone. Nonspecific sites and endogenous biotins were blocked with PBS/0.3% bovine serum albumin/0.5% HS for 30 minutes and with avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Sections were incubated with primary antibodies (1 hour 30 minutes; Table S2), washed twice in PBS/0.5% Tween 20 (Sigma-Aldrich), and incubated with secondary antibodies (1 hour, biotinylated goat anti–mouse IgG1 at 0.5 μg/mL, Caltag/Invitrogen Carlsbad, CA; and either Alexa 546–conjugated GAMIgG2a or Alexa 546–conjugated GAMIgG2b at 5 μg/mL, Invitrogen; or tetramethyl rhodamine isothiocyanate–conjugated GAMIgG at 10 μg/mL, Invitrogen). Biotinylated antibodies were revealed with streptavidin–Alexa Fluor 488 (1 μg/mL, 15 minutes; Invitrogen). Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (Sigma-Aldrich) and incubated in PBS-glycine (0.5 M, 30 minutes). Slides were mounted with the Fluoromount Kit (Dako North America, Trappes, France). Sections were analyzed with a Leica microscope equipped with a DC 300F camera (digital module R, IM 1000; Leica, Rueil-Malmaison, France) or an Axiovert 100M Zeiss microscope (Carl Zeiss, Le Pecq, France) equipped with an Orca ER camera (−20°C, pixels 1344/1024; Hamamatsu, Bridgewater, NJ). Photographs were colorized with Adobe Photoshop (Microsoft) and ImageJ 1.38. Quantification of pDCs was performed using the ImageJ “analyze particle” function on the BDCA2-labeled areas. Nonspecific labeling of the elastic intima of the arterioles was not taken into account. Statistical analysis was performed using GraphPad Prism Software (La Jolla, CA; Mann-Whitney, Spearman tests).
Dextran endocytosis
A total of 106 peripheral blood mononuclear cells were incubated with dextran-fluorescein isothiocyanate in RPMI/10% fetal calf serum (specific uptake: 1 mg/mL, 1 hour, 37°C, nonspecific binding: 4°C, negative control: no dextran at 37°C). Cells were washed, resuspended in RPMI/10% human AB serum, labeled on ice for HLA-DR, lineage, CD11c and CD123, and analyzed by FACSCanto. All experiments were done on frozen cells, as endocytosis by fresh or frozen DCs did not differ.
Cell-associated HIV-1 DNA quantification, cell quantification and statistics
HIV DNA was measured by nested real-time quantitative polymerase chain reaction (PCR; LightCycler; Roche Diagnostics, Meylan, France).31 Given the low infection levels in some patients, PCR quantification was performed 3 to 10 times per patient.
Outer primer pairs: HIVgag-out5′: CCCTTCAGACAGGATCAGAA; HIVgag-out3′: ATTCTGCAGCTTCCTCATTGAT. Inner primers: HIVgag-in5′: TAGAGGTAAAAGACACCAAGGA; HIVgag-in3′: ATGTCCCCCCACTGTGTTTA. Fluorescence resonance energy transfer (FRET) probes: P1HIVgag AGAAGAGAAGGCTTTCAGCCCAGAAGTAATACCCf; P2HIVgag (red640) TGTTTTCAGCATTATCAGAAGGAGCCACCCCACAp). Results were standardized to one CD3 copy.
Results
In situ analysis of pDC distribution in human spleen
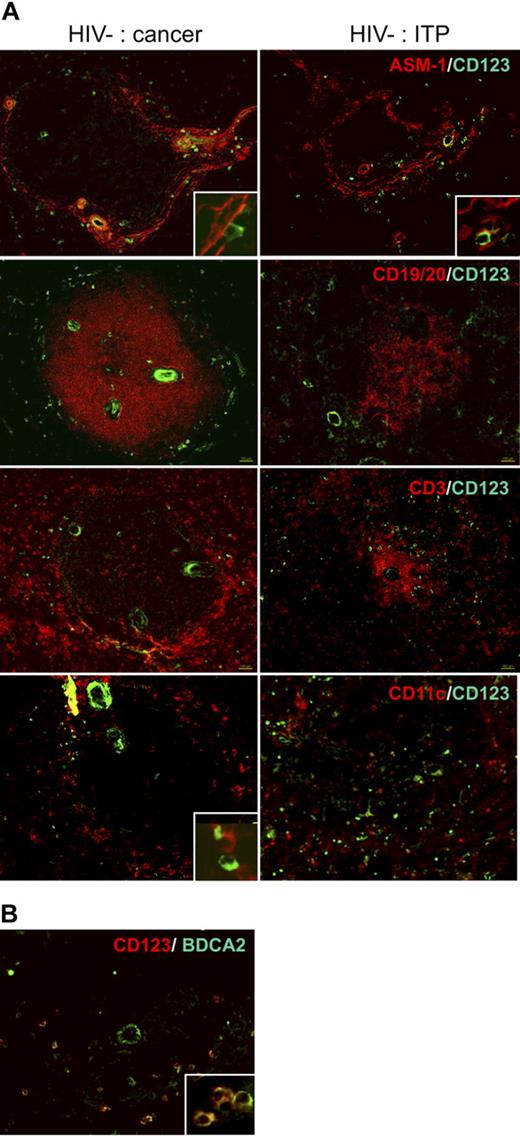
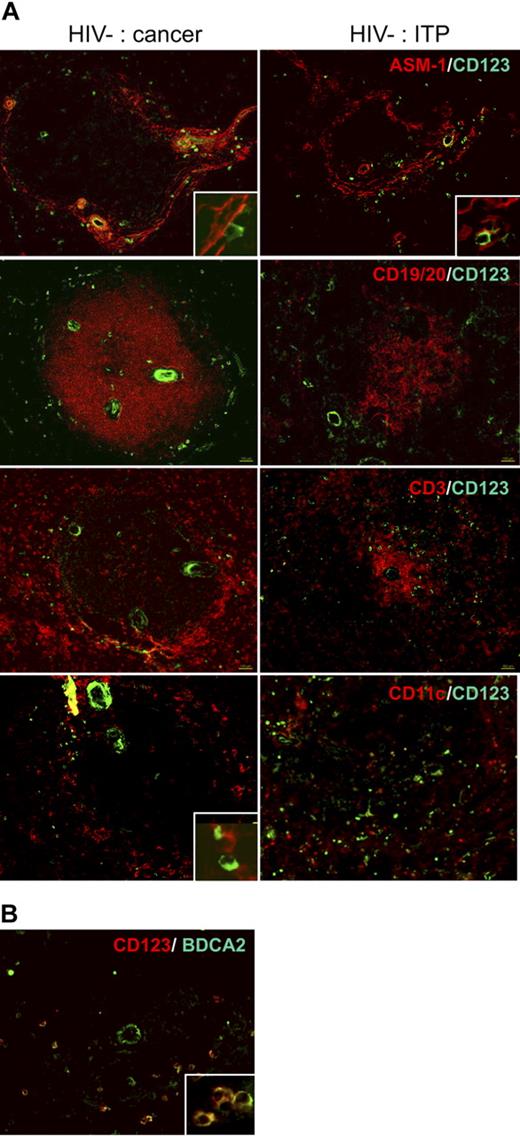
We analyzed pDC distribution in situ in normal spleens from cancer patients, who underwent splenectomy because of tumor adhesion to the spleen (patients C, F, H, I, and J; Table 1). ASM1 labeling of myofibroblasts (Figure 1 top left panels; controls in Figure S1) allows localization of the marginal and periarteriolar zones.6 Most CD123+ cells were located around the arterioles and within the network of AMS1+ cells, whereas few were in the red pulp. Interestingly, they were very close to ASM1+ cells (Figure 1A inset in top panels). In accordance with their localization in the marginal zone, CD123+ cells formed a ring around the B-cell zone (CD19, 20+), whereas very few were found inside (Figure 1A second panels). Most of the CD123+ cells of the white pulp were located within the CD3+ T-cell area, around the arteriole (Figure 1A third panels). They frequently made contact with mDCs (Figure 1A inset in bottom panels). Therefore, CD123+ cells were localized in the T-cell area and in the marginal zone, and very few in the B-cell area. This localization was similar in spleens from patients with idiopathic thrombopenic purpura (ITP) or hemolytic anemia (patients A, D, E, G, and K; Table 1, Figure 1A right panels), and from an atypical patient (patient B) with splenomegaly and angioma, as well as from organ donors (not shown). This localization was similar to that of CD11chi mDCs previously characterized in spleens from organ donors.11 In blood, CD123+ cells comprise pDCs and basophils. Double labeling showed that CD123+ cells also expressed BDCA2, a specific pDC surface lectin32 (Figure 1B), confirming that they were indeed pDCs. Thus, for the rest of our study, we used BDCA2 to label pDCs.
Distribution of pDCs in human spleen. (A) Labeling of serial spleen sections from a patient (patient C) with pancreatic cancer (left) and a patient (patient A) with ITP (right). Original magnification ×100; inset, ×400. (B) Colabeling of pDCs with CD123 and BDCA2. Original magnification ×200; inset, ×400.
Distribution of pDCs in human spleen. (A) Labeling of serial spleen sections from a patient (patient C) with pancreatic cancer (left) and a patient (patient A) with ITP (right). Original magnification ×100; inset, ×400. (B) Colabeling of pDCs with CD123 and BDCA2. Original magnification ×200; inset, ×400.
Numeration and maturation status of human spleen pDCs
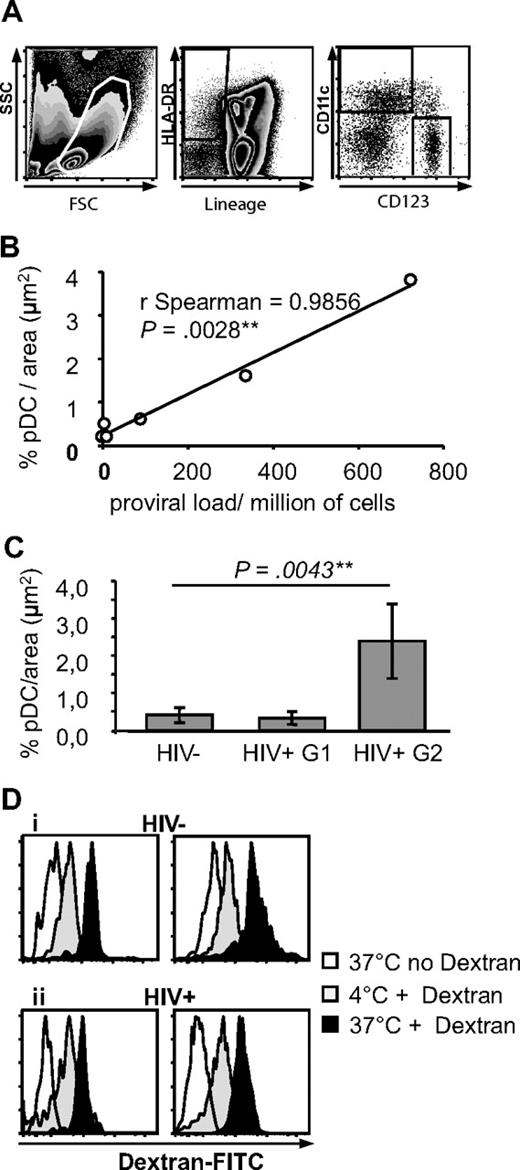
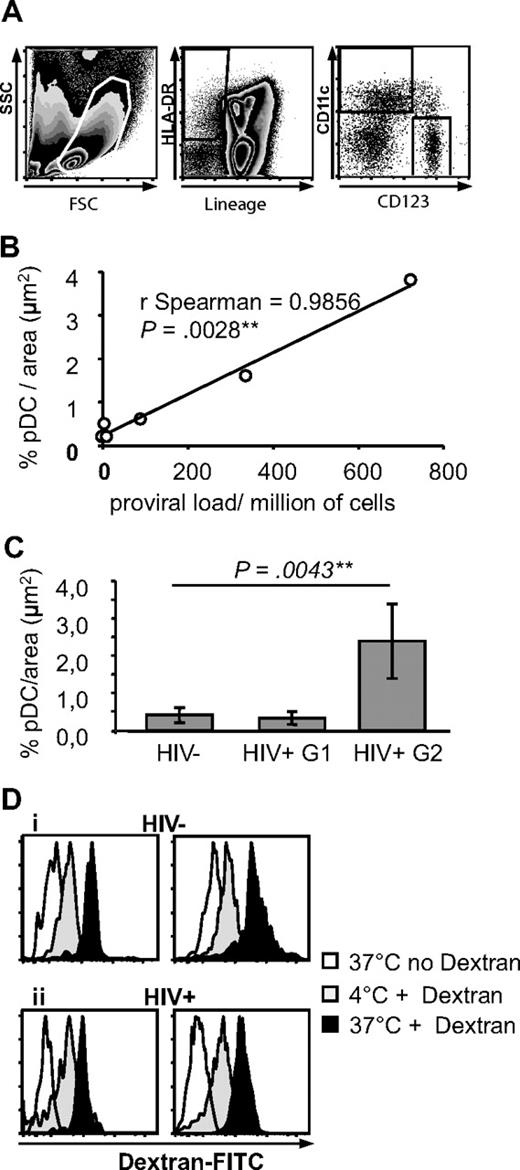
By rare event analysis of flow cytometry data (Figure 2A), pDCs represented 0.23% plus or minus 0.19% of spleen mononuclear cells (n = 10). As we previously observed for mDCs in human spleen,33 pDCs from organ donor, cancer, or ITP patients did not express CD83 or CD86 and therefore were immature (Figure 3B).
Characterization of pDCs and mDCs in spleens from HIV− and HIV+ patients. (A) Definition of pDCs and mDCs by flow cytometry. Spleen mononuclear cells were gated by size and granularity (left panel). Lineage− HLA-DR+ cells were gated (middle panel) and CD11c and CD123 expression analyzed on this population (right panel). (B) Quantitative analysis of pDCs in HIV+ patients. Correlation between pDC density and proviral load in 6 HIV+ patients (proviral load was not available for patient R). (C) Quantitative analysis of BDCA2 specific labeling on spleen sections. Results obtained from 5 HIV− patients (total sections analyzed: 17, total area analyzed: 55 574 729 μm2), and 6 HIV+ patients (for groups 1 and 2, respectively 3 patients each, total sections analyzed: 9 and 11, total area analyzed: 29 421 386 and 35 959 471 μm2). (D) Endocytosis capacity of spleen pDCs and mDCs. Endocytosis of dextran-fluorescein isothiocyanate by spleen pDCs (left) and mDCs (right) in an HIV− (i) and HIV+ (ii) patient. pDCs and mDCs were defined as in panel A. Graphs are representative of n = 6 HIV− and n = 3 HIV+ patients.
Characterization of pDCs and mDCs in spleens from HIV− and HIV+ patients. (A) Definition of pDCs and mDCs by flow cytometry. Spleen mononuclear cells were gated by size and granularity (left panel). Lineage− HLA-DR+ cells were gated (middle panel) and CD11c and CD123 expression analyzed on this population (right panel). (B) Quantitative analysis of pDCs in HIV+ patients. Correlation between pDC density and proviral load in 6 HIV+ patients (proviral load was not available for patient R). (C) Quantitative analysis of BDCA2 specific labeling on spleen sections. Results obtained from 5 HIV− patients (total sections analyzed: 17, total area analyzed: 55 574 729 μm2), and 6 HIV+ patients (for groups 1 and 2, respectively 3 patients each, total sections analyzed: 9 and 11, total area analyzed: 29 421 386 and 35 959 471 μm2). (D) Endocytosis capacity of spleen pDCs and mDCs. Endocytosis of dextran-fluorescein isothiocyanate by spleen pDCs (left) and mDCs (right) in an HIV− (i) and HIV+ (ii) patient. pDCs and mDCs were defined as in panel A. Graphs are representative of n = 6 HIV− and n = 3 HIV+ patients.
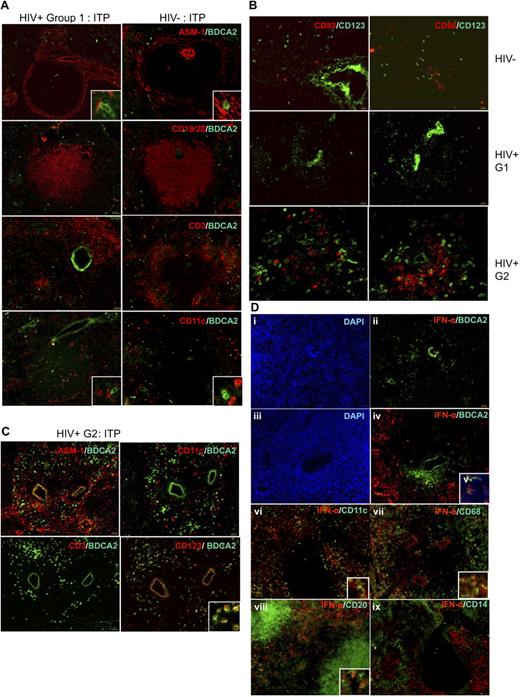
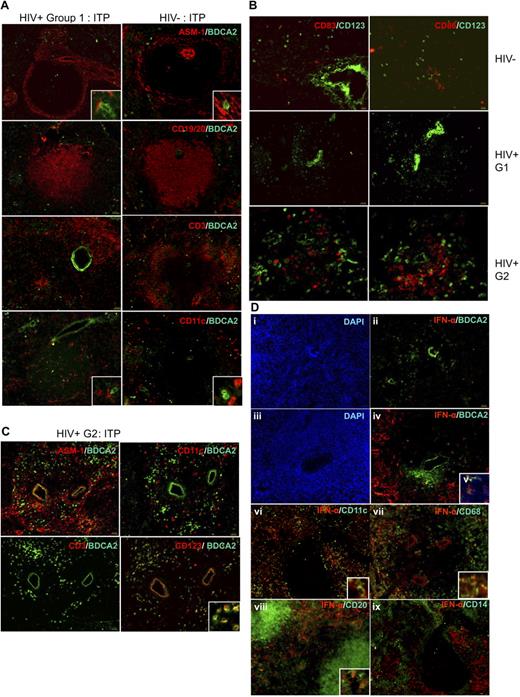
Localization and phenotype of pDC in HIV+ human spleens. (A) Comparative distribution of pDCs on serial sections from HIV− patient A (right panels) and HIV+ patient M (except for CD3/BDCA2 labeling done in patient N, left panel), both with ITP. Original magnification, ×100; inset, ×400. Results are representative of those found in 6 HIV− and 7 HIV+ patients. (B) pDCs have a nonactivated phenotype in HIV− and HIV+ patients. Serial spleen sections were labeled with CD83 and CD123 (left panels), CD86 and CD123 (right panels) in HIV− patient C (top panels), HIV+ group 1 patient O (middle panels), and HIV+ group 2 patient S (bottom panels). Original magnification ×100; bottom panels, ×200. (C) pDC accumulation in HIV+ patient S from group 2. Original magnification ×100; inset, ×400. (D) Most HIV+ patients, not HIV− patients, have IFN-α–producing cells in the marginal zone. Serial spleen sections were labeled for IFN-α and BDCA2, CD11c, CD68, CD20, or CD14. Sections from HIV− patient A (i,ii) and from HIV+ patients R (iii-v), M (vi,ix), and O (vii,viii). Original magnification ×100; insets, ×400; v, ×400. Pictures are representative of 4 HIV− and 6 HIV+ patients.
Localization and phenotype of pDC in HIV+ human spleens. (A) Comparative distribution of pDCs on serial sections from HIV− patient A (right panels) and HIV+ patient M (except for CD3/BDCA2 labeling done in patient N, left panel), both with ITP. Original magnification, ×100; inset, ×400. Results are representative of those found in 6 HIV− and 7 HIV+ patients. (B) pDCs have a nonactivated phenotype in HIV− and HIV+ patients. Serial spleen sections were labeled with CD83 and CD123 (left panels), CD86 and CD123 (right panels) in HIV− patient C (top panels), HIV+ group 1 patient O (middle panels), and HIV+ group 2 patient S (bottom panels). Original magnification ×100; bottom panels, ×200. (C) pDC accumulation in HIV+ patient S from group 2. Original magnification ×100; inset, ×400. (D) Most HIV+ patients, not HIV− patients, have IFN-α–producing cells in the marginal zone. Serial spleen sections were labeled for IFN-α and BDCA2, CD11c, CD68, CD20, or CD14. Sections from HIV− patient A (i,ii) and from HIV+ patients R (iii-v), M (vi,ix), and O (vii,viii). Original magnification ×100; insets, ×400; v, ×400. Pictures are representative of 4 HIV− and 6 HIV+ patients.
Accumulation of pDCs, but not mDCs, in spleens from HIV+ patients with a high proviral load
Because the spleen is an important site of HIV replication and immune response,34 we compared the distribution of pDCs in the spleens from ITP patients infected or not with HIV. In HIV+ patients (Figure 3A), pDCs were localized as in HIV− patients, in the marginal zone, in the periarteriolar T-cell area, and in the red pulp. They were also close to ASM1+ cells and CD11chi mDC. Strikingly however, in 3 of 7 HIV-infected patients, the density of pDCs, but not mDCs, seemed much higher, without changes in the distribution (Figure 3C).
We performed a quantitative analysis of pDC in HIV+ spleen sections. pDC density varied from 0.15% to 3.8% per μm2. The proviral loads from these 6 different patients varied from 0.96 to 723 HIV-1 DNA copies/million of spleen mononuclear cells, and they strongly correlated with pDC density (Figure 2B, ρ = 0.99, P = .003). The patients with high proviral load had not been treated by HAART. Therefore, HIV+ patients were divided into 2 groups: patients treated by HAART, with proviral loads lower than 200 copies/million cells (group 1; patients M, N, O, and P), and patients untreated by HAART with proviral loads higher than 200 copies/million cells (group 2; patients Q, R; cells from donor S were not available). Patients from group 2 had a significantly higher density of pDCs than patients from group 1 and than HIV− patients (2.4 pDC/μm2 ± 1, 0.3 ± 0.17 and 0.4 ± 0.2, P = .004, Figure 2C). By flow cytometry, pDCs from HIV+ group 2 patients represented 0.99% of mononuclear cells (n = 2), a percentage higher than that from HIV− patients (0.23% ± 0.19%, n = 10) and from HIV+ group 1 patients (0.19% ± 0.15%, n = 4), although not significantly because few samples were available. There was a strong correlation between in situ and flow cytometric quantification (ρ = 0.86, P = .004). By contrast, the percentages of mDCs among mononuclear cells in the 3 groups were undistinguishable (not shown). pDCs from both groups of HIV+ patients were immature, that is, CD83− and CD86− (Figure 3B), like most mDCs (data not shown). Therefore, in some HIV+ patients, pDCs, but not mDCs, accumulate in the spleen, in correlation with local proviral load.
Human spleen pDCs and mDCs perform dextran endocytosis
Human circulating mDCs endocytose dextran,35 but this function was never shown in pDCs or human spleen DCs. Spleen pDCs and mDCs were able to internalize dextran at 37°C, with higher internalization in mDCs than in pDCs (difference between intensity at 4°C and 37°C). This is consistent with their immature phenotype. Interestingly, the capacity of spleen mDCs and pDCs to endocytose dextran was not affected by HIV infection, in accordance with their immaturity (Figure 2D).
IFN-α is found in spleen marginal zones from most HIV+ patients and not HIV− patients, mostly in BDCA2− and CD123− cells
IFN type I production is the major function of pDCs. Although pDCs were fairly detectable (Figure 3Dii), we did not detect any IFN-α in spleens of 3 of 4 HIV− patients. The fourth patient (J), who displayed IFN-α splenic production, had a pancreatic tumor with Wirsung duct obstruction and pancreatitis. In contrast with most HIV− patients, many cells were positive for IFN-α in the spleens of 5 of 7 HIV+ patients (Figure 3Diii-ix). IFN-α production was not related with proviral loads. The 2 HIV+ patients (N and S) who had undetectable IFN-α production had hemophagocytosis or a pre-Castleman syndrome, but patient Q who had IFN-α production also had hemophagocytosis. IFN-α was located mostly around the white pulp, in the marginal zone; some positive cells were within the white pulp. Surprisingly, only few IFN-α+ cells expressed BDCA2 (ie, pDCs, Figure 3Div,v). Some were CD11c+ mDCs (Figure 3Dvi), CD68+ marginal zone macrophages (Figure 3Dvii), or CD20+ B lymphocytes (Figure 3Dviii) and even CD3+ T lymphocytes (not shown).
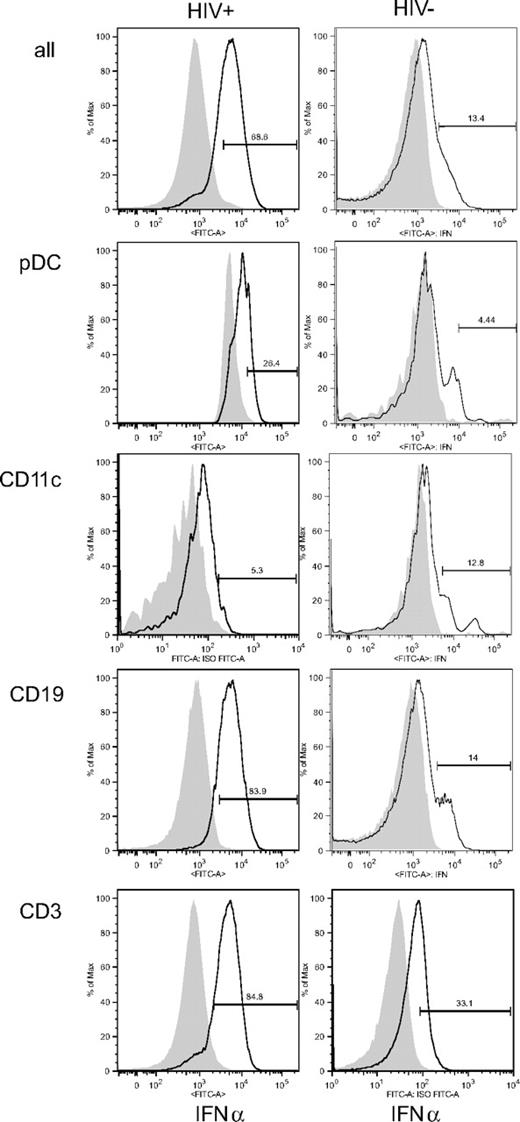
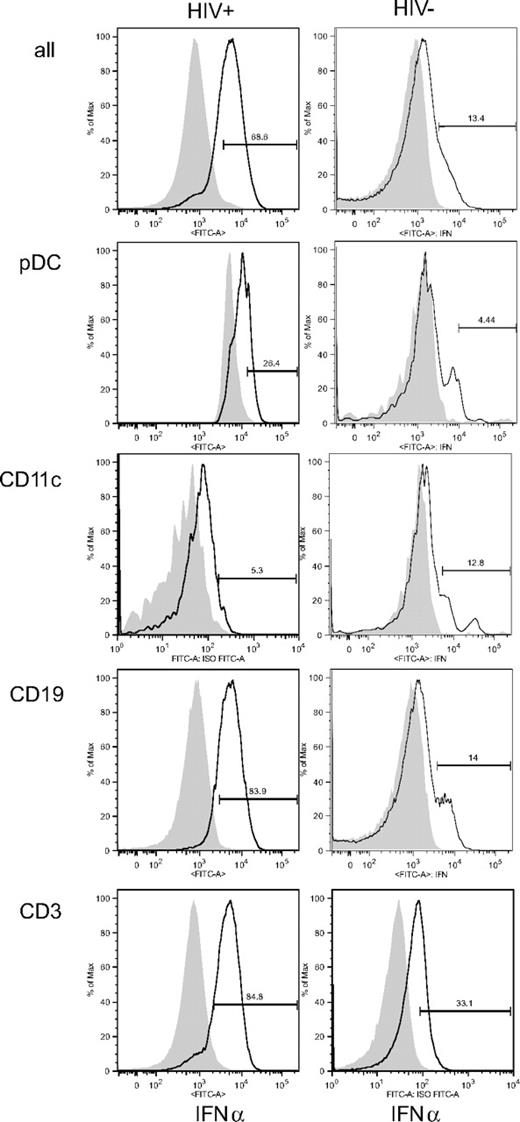
To assess the intrinsic presence of functional protein in each cell type, we analyzed IFN-α intracellularly by flow cytometry (Figure 4; Table 2). HIV+ patients who had detectable IFN-α in situ (HIV+ IFN-α+) had more IFN-α+ splenocytes by flow cytometry than HIV− patients. Labeling was mostly found in CD19+ B and CD3+ T lymphocytes. pDCs, however, defined by the expression of CD123 and BDCA4 (Figures 4, S2), were only weakly labeled for IFN-α, as well as CD11c+ mDCs (Figure 4; Table 2), CD14+ monocytes/macrophages, or CD56+CD16+CD3− NK cells (Table 2). These populations represented 39.63% to 83.52% of IFN-α–expressing splenocytes (Table 2). Therefore, intriguingly, the cells most strongly labeled for IFN-α were not pDCs but rather B or T lymphocytes.
IFN-α intracellular labeling. Histograms represent IFN-α+ cells among total spleen mononuclear cells (all), CD123+BDCA4+ cells (pDCs), HLA-DR+CD19−CD11c+ cells (mDCs), CD19+ B cells, or CD3+ T cells. Data are from HIV+ patient M and from HIV− patient A, except for CD3 labeling, which is from patient L. This figure is representative of 4 HIV+ patients who were positive for IFN-α in situ and 3 HIV− patients who were negative for IFN-α in situ.
IFN-α intracellular labeling. Histograms represent IFN-α+ cells among total spleen mononuclear cells (all), CD123+BDCA4+ cells (pDCs), HLA-DR+CD19−CD11c+ cells (mDCs), CD19+ B cells, or CD3+ T cells. Data are from HIV+ patient M and from HIV− patient A, except for CD3 labeling, which is from patient L. This figure is representative of 4 HIV+ patients who were positive for IFN-α in situ and 3 HIV− patients who were negative for IFN-α in situ.
Discussion
At steady state, pDCs were located mainly in the marginal zone and the T-cell areas of the human spleen. This was found in cancer patients and in organ donors. This localization paralleled that of mDCs in human spleen at steady state.11 The marginal zone is the place where dendritic cells meet antigens arriving from blood.5,6 Close proximity of marginal zone pDCs with mDCs hints that interactions (mutual survival, maturation stimuli, antigen exchange for cross-presentation) may occur in vivo. Close proximity with myofibroblasts suggests that these cells may direct the migration of pDCs toward the marginal zone or the white pulp.5,6
The localization of human spleen pDCs in the marginal zone contrasts with that of murine pDCs, which are found in the marginal zone only after specific stimulation.4 In our patients, cancer or ITP may have stimulated pDC migration through stress signals.10 However, pDC localization was the same in organ donor spleens, where mDCs are most often immature,11 and costimulation molecules were not overexpressed. Therefore, this location is probably a human specificity, like the presence of a lining of T cells separating the inner and the outer parts of the marginal zone.5 A small proportion of human mDCs in organ donors are mature, especially in the periarteriolar T-cell zone.11 Here, pDCs did not show any sign of maturation, correlating with their good endocytic capacity, shown here for the first time in human spleen DCs.
The changes brought by HIV infection were mostly represented by IFN-α production in 5 of 7 HIV+ patients compared with 1 of 4 HIV− patients, whose pancreatitis may explain IFN-α production. Moreover, a specific accumulation of pDCs, and not mDCs, was evidenced in 3 HIV+ patients. This is in accordance with previous results showing an accumulation of pDCs in lymph nodes from patients with chronic HIV infection, either in density compared with other cell populations,25,36 or in absolute value, resulting from lymph node hyperplasia.37 In contrast, patients with more advanced disease38 or macaques with AIDS had lymph node DC depletion, perhaps because of destruction of the lymph node architecture or to increased cell death.24,26,37 In SIV-infected cynomolgus macaques, pDCs seem to home into the lymph nodes as their proportions and absolute counts change inversely in blood and lymph nodes.39 In SIVagm-infected African green monkeys, pDCs also seem to home transiently into the lymph nodes just before their numbers drop in the blood.40 Whether the accumulation of pDCs in the spleen is the cause or the consequence of high viral loads in HIV+ patients remains to be determined. However, the correlation with local proviral load, which somehow integrates the replication history of the patient and not only instant viral replication, such as RNA viral load,41 may indicate that replicating virus attracts pDCs into the spleen. Accumulation of pDCs in the spleen may be related to increased expression of chemokine receptors or integrins, such as CCR7 or CD54, enhanced survival, local differentiation from precursors, or less likely, pDC multiplication, or potentiation of HIV replication by pDCs.
In any case, pDCs and mDCs from HIV+ patient spleens were not more mature than those from HIV− patients. This is paradoxical given that HIV stimulates TLR3 and TLR7 and induces IFN-α production, which triggers mDC maturation.27 However, in vitro HIV induces only semimaturation of mDCs,21 unless high amounts of purified virus are used,42 and inhibits TLR9-mediated stimulation.43 We found no activation of DC ex vivo in the spleens from HIV+ patients, but a defect in CD83 up-regulation on culture,33 consistent with observations in lymph node DCs from SIV-infected macaques24 and lymph node mDCs and pDCs from HIV-infected patients.37
pDCs were expected to be the main IFN-α producers in response to HIV infection.27-29 Surprisingly, in the HIV+ patients studied here, they did not seem to contribute much to this production. The cells labeled in situ for IFN-α were mostly B lymphocytes or CD68+ marginal zone macrophages. Protein labeling indicates function, but not necessarily production by a specific cell type, because IFN-α is secreted. Flow cytometry showed intrinsic protein labeling of the same cells and of CD3+ T lymphocytes, and only weak labeling of pDCs and mDCs. Was this loss of specific markers a result of activation in the lymphoid tissue, as suggested in front of similar data in lymph nodes from patients with acute HIV infection or with chronic progressive disease?44 It is doubtful that the markers disappeared even intracellularly. Moreover, different pDC-specific molecules were labeled: CD123, BDCA2, and BDCA4. Was this really a defect in IFN-α production by pDCs, and was this really production by other cells? Here in the spleen, IFN-α–labeled cells were in the marginal zone, as expected from mouse data after infection with influenza or MCMV.2,4,45,46 In HSV infection, at a time when pDCs were not yet identified, splenic marginal metallophilic macrophages and marginal zone macrophages were found to be the major IFN-α or -β producers.8 In influenza or MCMV infection in mice, pDCs are the main IFN-α producers,2,46 but in LCMV infection or in Newcastle disease virus pulmonary infection, this is not the case,45,47 and in the absence of the main IFN-α–producing cells, other cell types in turn take up this function.47 In reovirus infection, Peyer patch mDCs and pDCs produce IFN-α.48 In vitro, HIV-1 induces IFN-α secretion by peripheral blood pDCs, but it was shown that gp120 inhibits TLR9-mediated signaling,43 and accumulated data show a defect of IFN-α production in vitro by peripheral blood mononuclear cells from patients with chronic, late-stage infection.19-22 HIV-1 chronic infection may stimulate continuously pDCs for IFN-α production so that it is down-modulated as a homeostatic mechanism,36 and other cell types may relay this production. Alternatively, pDCs might still be the main producers of IFN-α during HIV-1 infection, but IFN-α would be rapidly dispersed and taken up by other cell types. Intracellular labeling argues for presence of the functional protein inside the cells. To distinguish between intrinsic production or uptake by IFN-α receptors, we attempted to perform purification of cell populations and quantitative reverse-transcribed PCR to assess for intrinsic IFN-α mRNA transcription, but it was technically unsatisfactory in our rare frozen samples.
In conclusion, human spleen pDCs at the steady state were found in the marginal zone and in the T-cell zone as immature cells that do not produce IFN-α. HIV infection induced IFN-α production and selective accumulation of pDCs, but not mDCs, correlating with proviral loads. Surprisingly, cells other than pDCs were mostly labeled with IFN-α. More attention should be devoted now to IFN-α production by types other than pDCs. Manipulating type I IFN responses locally in the lymphoid organs where viral replication occurs might help decrease viral production.49,50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Milon, Y. Richard, and all members of the Dendritic Cells work group from the Agence Nationale de Recherche sur le Sida (AC19/AC31) for helpful discussions; J. F. Fléjou, P. Dartigues, Ms Fauchon, Ms Balthazard, Ms Chenitz, P. Richard, and P. E. Boucher for spleen samples; E. Souil for teaching histology methods; M. A. Blaton for technical help; P. Bourdoncle for help with fluorescence microscopy and in situ quantification; and M. Andrieu, I. Kamga, G. Hoeffel, S. Kahi, L. Develioglu, L. Fery, N. Bonilla, and D. Matheoud for help in processing spleen samples.
This work was supported by Agence Nationale de Recherche sur le Sida, Sidaction, and CNRS.
Authorship
Contribution: M.N., L.P., and A.H. designed research and wrote the manuscript; M.N., L.P., A.H., L.C., S.D., L.K., R.C., S.L., J.P., and O.A.-A. performed research and analyzed data; J.S. and S.B. contributed reagents and analytic tools; and L.G., B.F., A.B., J.-P.M., T.J.M., J.-P.V., and E.O. provided samples and clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Hosmalin, Institut Cochin, Département d'Immunologie, Bâtiment Gustave Roussy, 27 Rue du Faubourg Saint Jacques, Paris, France; e-mail: anne.hosmalin@inserm.fr.
References
Author notes
*M.N. and L.P. contributed equally to this study.