Abstract
Ligation of inhibitory receptors renders natural killer (NK) cells inactive against autologous tumors. Recently, the proteasome inhibitor bortezomib was shown to sensitize tumors to autologous NK-cell cytotoxicity in vitro. Here, we show bortezomib augments the antitumor effects of syngeneic NK-cell infusions in tumor-bearing animals; this effect is further enhanced in regulatory T cell (Treg cell)–depleted hosts. In vitro, bortezomib-treated tumors had higher tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and perforin/granzyme-mediated caspase-8 activity, which enhanced their susceptibility to NK-cell lysis. Bioluminescence imaging of mice with established tumors showed treatment with bortezomib and syngeneic NK cells reduced tumor growth and prolonged survival compared with controls receiving bortezomib or NK cells alone. In contrast, tumor progression was not delayed when animals received bortezomib and perforin-deficient NK cells, showing drug-induced augmentation in NK-cell cytotoxicity was mediated through perforin/granzyme. Furthermore, tumor growth was slower in bortezomib-treated recipients when host Treg cells were eradicated with anti-CD25 antibody before infusing NK cells compared with mice without Treg-cell ablation (tumor doubling time, 16.7 vs 4.9 days, respectively; P = .02). These findings suggest that depletion of Treg cells followed by bortezomib-induced tumor sensitization to autologous NK cells could be used as a novel strategy to treat cancer.
Introduction
Recently, interest has grown in exploring the potential of natural killer (NK) cell–based immunotherapy regimens that use cellular effectors with antigen-independent tumor cytotoxicity.1 In vitro data,2-4 murine studies,3,5 retrospective data from allogeneic transplantation trials,6-8 and clinical trials evaluating adoptive allogeneic NK-cell infusions in patients with cancer9 have implicated a therapeutic role of NK cells in the treatment of malignant diseases. However, a number factors limit the ability of NK cells to kill malignant cells. Several studies have shown that Treg cells exert contact and TGF-β–dependent suppressive effects on endogenous NK-cell function. Athymic mice that lack Treg cells have long been known to have augmented NK-cell function.10 Similarly, severe combined immunodeficiency (SCID) mice have heightened NK cell–mediated rejection of allogeneic bone marrow cells that can be suppressed by infusing Treg cells.11,12 NK cell–inhibitory killer immunoglobulin-like receptors (KIRs) are also thought to be an important mechanism limiting the antitumor effects of autologous NK cells. Although allogeneic KIR ligand–incompatible NK cells may have greater tumor cytotoxicity than autologous NK cells, intense host immunosuppression must first be given before any measurable NK-cell engraftment can be achieved.9 Tumors can also use several KIR-independent mechanisms to evade NK-cell recognition and killing, including loss or down-regulation of ICAM-1 and NKG2D ligands, increased expression of granzyme B inhibitors, and HLA-E–dependent NK-cell inactivation.13-19
One strategy that has been recently explored to enhance the cytotoxic effects of adoptively infused NK cells is rendering tumor cells more susceptible to immune attack. Recently, we have shown that a variety of human tumors can be sensitized to lysis by autologous NK cells by pretreating malignant cells with the proteasome inhibitor bortezomib.20 In vitro studies showed that bortezomib up-regulated surface expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptors on tumor cells, enhancing their susceptibility to lysis by NK-cell TRAIL. Importantly, most tumors sensitized to NK cells had high major histocompatibility complex (MHC) class I surface expression, suggesting that bortezomib treatment could offset KIR ligand–mediated inhibition of autologous NK cells. Bortezomib has also been shown to reduce expression of cellular FLICE inhibitory protein (cFLIP) in several different human cancers, which also enhances TRAIL-mediated apoptosis.21-23 In murine studies, bortezomib augments in vitro NK-cell purging of leukemia cells by syngeneic NK cells.24 However, no data exists as to whether bortezomib can sensitize established tumors to the cytotoxic effects of adoptively infused NK cells in vivo. Likewise, no study has evaluated the effects of Treg cell depletion in a model of adoptive infusion of NK cells in recipients with established tumors.
In the present study, we show that treatment of murine tumors with bortezomib enhances NK-cell tumor cytotoxicity both in vitro and in vivo. In vitro, bortezomib-treated tumors had augmented susceptibility to NK-cell perforin/granzyme and recombinant TRAIL-mediated apoptosis resulting in enhanced caspase-8 activity. In tumor-bearing animals, treatment with bortezomib followed by adoptive infusions of syngeneic NK cells delayed tumor progression and prolonged survival compared with controls receiving bortezomib or NK cells alone. This potentiating effect was further augmented by first depleting host Treg cells and by exogenous IL-2 administration. These results suggest eradication of Treg cells, followed by bortezomib administration, could be used as a strategy to sensitize patients' tumors to the cytotoxic effects of adoptively infused autologous NK cells.
Methods
Preparation of NK cells
Murine NK cells were isolated from splenocytes by magnetic bead negative depletion (Miltenyi Biotec, Auburn, CA) and cultured in Dulbecco modified Eagle medium (DMEM) media supplemented with 10% fetal calf serum (FCS; HyClone, Logan, UT) and 500 U/mL recombinant human IL-2 (Roche, Nutley, NJ) for 4 to 7 days. Human NK cells were isolated by negative depletion of peripheral blood mononuclear cells (PBMCs) obtained from healthy volunteers using the CliniMacs NK-cell isolation kit (Miltenyi Biotec) and expanded with irradiated (100 Gy) Epstein-Barr virus–transformed B cells as feeder cells and 500 U/mL IL-2 as previously described.20 The purity of murine and human NK cells was typically greater than 85% as determined by staining for CD3 and DX5 or CD56 (BD Pharmingen, San Diego, CA), respectively, with 1% or less CD3+ T-cell contamination. All flow cytometric assays were acquired on a FACSCalibur (BD Pharmingen) and analyzed by FCS Express software (De Novo, Thornhill, ON). Written informed consent was obtained from healthy volunteers in accordance with the Declaration of Helsinki for the use of cells from leukapheresis products for research according to the requirements of the Institutional Review Board of the National Heart, Lung, and Blood Institute.
Cells, reagents, and antibodies
The human tumor cell lines JOHW (National Heart, Lung, and Blood Institute [NHLBI]) and SK23 (National Cancer Institute [NCI]) and the murine tumor cell lines RENCA, CMS4 (NCI), T27A, BC3A, A20, WEHI-3, B16-F10, Lewis lung carcinoma (LLC1), 4T1, and CT26 (ATCC, Manassas, VA) were maintained in RPMI1640 (Cellgro, Herndon, VA) supplemented with 10% FCS. Bortezomib (Millennium Pharmaceuticals, Cambridge, MA), the caspase inhibitors Z-IETD-fmk (caspase-8) and Z-LEHD-fmk (caspase-9; R&D Systems, Minneapolis, MN), anti–mouse Fas-ligand (MFL3; Biolegend, San Diego, CA), anti–mouse TRAIL (N2B2; Biolegend), anti–human TRAIL (RIK-2; Biolegend), concanamycin A (Sigma-Aldrich, St Louis, MO), recombinant human TRAIL (PeproTech, Rocky Hill, NJ), recombinant mouse TRAIL (Biomol, Plymouth Meeting, PA), anti–mouse Fas (Jo2; BD Pharmingen), anti-NKG2D (R&D Systems) and anti–TGF-β (1D11; R&D Systems) were used at the indicated concentrations. Recombinant mouse TRAIL was used together with anti-polyhistidine antibody cross-link (2 μg antibody/μg recombinant TRAIL). Anti–mouse Fas (BD Pharmingen) was used at 10 μg/mL cross-linked with 10 μg/mL protein G (Sigma-Aldrich). For cell-surface staining, fluorochrome-conjugated anti–mouse DX5, H-2Dd, Fas, CD3, ICAM-1 (BD Pharmingen), anti–mouse TRAIL-R2, and secondary anti–goat PE-conjugated, anti–mouse Rae-1, anti–mouse Mult-1, anti–mouse H60, and secondary anti–rat APC (R&D Systems) were used at the recommended concentrations with appropriate isotype controls. A caspase-8 detection kit (EMD Bioscience, San Diego, CA) was used to measure caspase-8 activity.
Western blot
Cells were resuspended in lysis buffer (150 mM NaCl, 20 nM tris-HCl [pH 7.5], 1% NP-40, and protease inhibitor) and incubated for 30 minutes on ice and thereafter diluted in sample loading buffer (Quality Biological, Gaithersburg, MD) and boiled for 5 minutes. Protein (40 μg/well) was resolved on a 14% tris-glycine gel and transferred onto PVDF membranes and incubated with primary anti-Bid antibody (1:1000; BD Pharmingen). Membranes were washed and incubated with secondary β-actin (1:10 000; Cell Signaling, Danvers, MA) and thereafter developed using the enhanced chemiluminescence (ECL) system (Pierce, Rockford, IL).
Apoptosis, cytotoxicity, and proliferation/suppression assays
Apoptosis was measured by flow cytometry staining for annexin V (BD Pharmingen) and 7-AAD or propidium iodide (Beckman Coulter, Fullerton, CA). Chromium-51 (PerkinElmer Life and Analytical Sciences, Waltham, MA) release assays were performed plating 10 000 51Cr-labeled target cells/well (96-well plate) with NK cells. After a 4- to 18-hour incubation, the supernatants were harvested onto Luma plates (PerkinElmer) and analyzed using a MicroBeta scintillation counter (PerkinElmer). The effects of bortezomib on cell proliferation were analyzed by 3H-thymidine incorporation assay. Cells were cultured 2 to 5 days, and 3H-thymidine (0.037 MBq/well [1 μCi/well]) was added during the last 16 hours of incubation. Cells were thereafter lysed and harvested onto solid filters (MicroBeta filtermate 96 harvester; PerkinElmer) and analyzed by scintillation counting (MicroBeta; PerkinElmer). In Treg cell suppression assays, activated Treg cells were cocultured with syngeneic BALB/c NK cells for 3 days or were added directly to cytotoxicity assays at 1:1 to 1:9 Treg cell–NK cell ratios. Treg cells and conventional T cells (Tconvs) were isolated from BALB/c splenocytes by immunomagnetic bead selection of CD4/CD25+/+ cells and CD4+/CD25− cells, respectively (Miltenyi Biotec), and were then activated with human recombinant IL-2 (100 U/mL) and 5 μg/mL anti-CD3 (R&D Systems) for 3 to 4 days in RPMI with 10% FCS. Purity was assessed by antibody staining for CD4, CD25 (BD Pharmingen), and FoxP3 (eBioscience, San Diego, CA). Purified Treg cells were typically more than 90% CD4+/CD25+/FoxP3+ (data not shown).
In vivo tumor models
All mice (8-15 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in a pathogen-free facility in microisolator cages. All experiments were approved by the NHLBI animal care and use committee (protocol no. H-0112). BALB/c mice were injected with RENCA cells (105) intravenously (tail vein) or subcutaneously in the right flank. Bortezomib (5 μg) was injected 4 to 5 days after tumor injection, followed 24 hours later by an injection of syngeneic NK cells (1-10 × 106 cells). The bortezomib/NK cell treatment cycle was repeated twice with 1-week intervals between each injection. C57BL/6 mice were injected with 5 × 105 LLC1 tumor cells subcutaneously in the right flank. A single intraperitoneal injection of bortezomib (15 μg) was injected when tumor cells were palpable (1 mm3; approximately day 14), followed 24 hours later by a single injection of syngeneic NK cells (1-10 × 106 cells). Mice were monitored for growth of subcutaneous tumors and evaluated for tumor infiltration by manual counting the number of tumor nodules in resected lungs. For bioluminescence in vivo tumor imaging, RENCA tumor cells were stably transduced with a lentivirus containing the luciferase transgene (Lentigen, Gaithersburg, MD) at a multiplicity of infection of 10 in 8 μg/mL polybrene (Sigma-Aldrich) and cloned by limiting dilution to select for 100% transduced cells. During bioluminescent imaging, mice were anesthetized with isoflurane then injected intraperitoneally with 2 mg D-luciferin salt; mice were imaged using the IVIS Xenogen system (Caliper Life Sciences, Hopkinton, MA) for 1 minute every 3 to 7 days starting 1 to 2 weeks after tumor injection. An exponential fit was performed on luminescent data to derive the tumor doubling time. For in vivo depletion of Treg cells, mice were treated with a single 0.5 mg intraperitoneal injection of purified anti-CD25 antibody or rat IgG isotype control (Jackson ImmuoResearch Laboratories, West Grove, PA) 3 days after tumor injection and 1 day before treatment with bortezomib. The anti-CD25 (PC61) antibody was purified and tested for endotoxins by the Research Technologies Branch, Flow Cytometry Section (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). After PC61 injection, CD4+/CD25+ Treg cells remained undetectable in the blood of all treated mice for 2 to 3 weeks. Where indicated, IL-2 (105 U/mouse, intraperitoneally) was injected once daily for 4 days starting 1 day before NK cells were infused. The Student t test, Fisher exact test, 2-tailed log-rank test, or 1-way analysis of variance (ANOVA) with the Tukey multiple comparison post test was used to assess differences between groups. A value of P less than .05 was considered to be of significant difference between treatment groups.
Results
Bortezomib sensitizes murine tumor cells to perforin/granzyme– and TRAIL-mediated apoptosis
Treatment of murine RENCA and LLC1 tumor cells with subapoptotic concentrations (5 to 20 nM) of bortezomib significantly decreased tumor proliferation and sensitized tumor cells to syngeneic NK-cell cytotoxicity. Similar results were observed in other murine cell lines, including 4T1 (breast cancer), B16 (melanoma), CMS4 (sarcoma), and the leukemia cell lines T27A and BC3A (data not shown). In contrast, no significant increase in susceptibility to NK-cell lysis was observed in the CT26 colon cancer cell line or the lymphoma and leukemia cell lines A20 or WEHI-3 after bortezomib treatment (data not shown). This potentiating effect was not related to changes in tumor surface expression of MHC class I, the death receptors DR5 and Fas, the adhesion molecule ICAM-1, or the NKG2D ligands rae-1 and Mult-1. However, there was an increase in the expression of the NKG2D ligand H60 in RENCA cells exposed to bortezomib (Figure 1). Blockade of NKG2D on NK cells resulted in loss of cytotoxicity against RENCA cells, although the relative reduction in lysis associated with NKG2D blocking was higher with untreated compared with bortezomib-treated RENCA cells (68% vs 38% reduction in lysis, respectively). These findings confirm observations by Abdool et al29 that interactions between NKG2D and its ligands represent a fundamental pathway that is important to NK-cell lysis of RENCA tumor cells. However, since the relative decrease in tumor lysis with NKG2D blocking was lower with bortezomib-treated RENCA cells compared with untreated RENCA cells, it is likely bortezomib sensitization to NK-cell lysis occurs through mechanisms that are independent of NKG2D and its ligands (Figure 2).
Bortezomib decreases proliferation and sensitizes RENCA tumor cells to syngeneic NK-cell lysis, but does not induce tumor apoptosis or alter cell-surface expression of MHC class I, DR5, Fas, Rae-1, or ICAM-1. RENCA or LLC1 tumor cells were treated with 20 nM bortezomib for 18 hours and were analyzed for (A) syngeneic BALB/c or C57BL/6 NK cell lysis in a 4-hour 51Cr release assay; (B) surface expression of MHC class I, Fas, DR5, Rae-1, Mult-1, H60, and ICAM-1 (x-axis shows log fluorescence); (C) proliferation (after 72 hours of bortezomib treatment [0.5-20 nM]);and (D) induction of apoptosis (assessed by staining for annexin V and propidium iodide). y- and x-axes show log fluorescence. Error bars in panels A and C depict SD.
Bortezomib decreases proliferation and sensitizes RENCA tumor cells to syngeneic NK-cell lysis, but does not induce tumor apoptosis or alter cell-surface expression of MHC class I, DR5, Fas, Rae-1, or ICAM-1. RENCA or LLC1 tumor cells were treated with 20 nM bortezomib for 18 hours and were analyzed for (A) syngeneic BALB/c or C57BL/6 NK cell lysis in a 4-hour 51Cr release assay; (B) surface expression of MHC class I, Fas, DR5, Rae-1, Mult-1, H60, and ICAM-1 (x-axis shows log fluorescence); (C) proliferation (after 72 hours of bortezomib treatment [0.5-20 nM]);and (D) induction of apoptosis (assessed by staining for annexin V and propidium iodide). y- and x-axes show log fluorescence. Error bars in panels A and C depict SD.
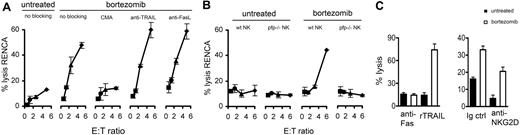
Bortezomib sensitizes RENCA tumor cells to NK-cell perforin granzyme-mediated apoptosis and to recombinant TRAIL (rTRAIL), but not FasL-mediated apoptosis. RENCA tumor cells were treated with 20 nM bortezomib for 18 hours and analyzed for susceptibility to (A) wild-type BALB/c NK-cell lysis of RENCA tumors in the presence or absence of CMA (20 nM), neutralizing antibodies to TRAIL (N2B2; 10 μg/mL), or FasL (MFL3; 10 μg/mL). (B) Wild-type (wt) or perforin deficient (pfp−/−) BALB/c NK-cell lysis of RENCA tumors in a 4-hour 51Cr release assay. (C) RENCA tumor cells were treated with 20 nM bortezomib for 18 hours and analyzed for susceptibility to lysis by (left panel) anti-Fas antibody (Jo2; 10 μg/mL) or rTRAIL (1 μg/mL) in an 18-hour 51Cr release assay, or to (right panel) syngeneic NK cells in the presence of neutralizing antibodies to NKG2D (2 μg/mL). Error bars depict SD.
Bortezomib sensitizes RENCA tumor cells to NK-cell perforin granzyme-mediated apoptosis and to recombinant TRAIL (rTRAIL), but not FasL-mediated apoptosis. RENCA tumor cells were treated with 20 nM bortezomib for 18 hours and analyzed for susceptibility to (A) wild-type BALB/c NK-cell lysis of RENCA tumors in the presence or absence of CMA (20 nM), neutralizing antibodies to TRAIL (N2B2; 10 μg/mL), or FasL (MFL3; 10 μg/mL). (B) Wild-type (wt) or perforin deficient (pfp−/−) BALB/c NK-cell lysis of RENCA tumors in a 4-hour 51Cr release assay. (C) RENCA tumor cells were treated with 20 nM bortezomib for 18 hours and analyzed for susceptibility to lysis by (left panel) anti-Fas antibody (Jo2; 10 μg/mL) or rTRAIL (1 μg/mL) in an 18-hour 51Cr release assay, or to (right panel) syngeneic NK cells in the presence of neutralizing antibodies to NKG2D (2 μg/mL). Error bars depict SD.
Bortezomib sensitization of murine tumors to NK-cell apoptosis was predominantly perforin/granzyme dependent; blocking assays showed bortezomib-induced augmentation of NK-cell cytotoxicity was lost when concanamycin A (CMA) but not neutralizing anti-TRAIL or anti-Fas ligand (FasL) antibodies were added to cultures (Figure 2). Moreover, bortezomib did not augment in vitro tumor killing by NK cells isolated from perforin-deficient BALB/c mice. Similar to human tumors, bortezomib also sensitized murine tumors to TRAIL-mediated apoptosis; apoptosis increased in bortezomib-treated RENCA cells compared with untreated cells upon addition of recombinant TRAIL but not agonistic anti-Fas antibodies. In summary, these data show bortezomib sensitized RENCA tumor cells to both perforin/granzyme- and TRAIL-mediated apoptosis, although sensitization to NK-cell cytotoxicity was predominantly perforin/granzyme dependent.
Caspase-8 activity is enhanced in bortezomib-treated murine and human tumors sensitized to NK-cell apoptosis
We investigated the underlying apoptotic signaling pathways that resulted in bortezomib-treated tumors having increased susceptibility to both perforin/granzyme- and TRAIL-mediated apoptosis. There was no evidence that bortezomib altered the mitochondrial apoptotic pathway, as cytochrome c levels did not change in bortezomib-treated tumors (data not shown). Furthermore, there were no changes in levels of the proapoptotic molecule Bid or its truncated p15 fragment when bortezomib-treated RENCA tumor cells were cocultured with NK cells (Figure 3). Instead, caspase-8 activity was augmented in tumors treated with bortezomib; after coculture with syngeneic BALB/c NK cells, caspase-8 levels were significantly higher in bortezomib-treated RENCA tumors compared with nontreated tumors. Augmentation of caspase-8 activity was dependent on perforin/granzyme, as no activation of caspase-8 was observed in bortezomib-treated tumors cocultured with either perforin-deficient NK cells or NK cells in the presence of CMA (Figure 3). Moreover, inhibition of caspase-8 with Z-IETD-fmk inhibited bortezomib-induced tumor sensitization to NK-cell cytotoxicity. In contrast, when blocking caspase-9 with Z-LEHD-fmk, no reduction in cytotoxicity of bortezomib-treated tumors was observed. Similar results were observed when experiments were performed using the murine leukemia cell line T27A (data not shown).
Bortezomib sensitizes murine and human RCC cells to NK-cell lysis through activation of caspase-8. RENCA tumors and human RCC cells (JOHW tumor cells) were treated with 20 nM and 10 nM bortezomib, respectively, for 18 hours, and then washed and cocultured with syngeneic murine and allogeneic human NK cells, respectively (3:1 effector-target [E/T] ratio) for an additional 5 hours and analyzed for (A,B) caspase-8 activity or (C) susceptibility to NK-cell lysis (E/T ratio = 1:1) in the presence of caspase-8 (Z-IETD)– or caspase-9 (Z-LEHD)–blocking reagents (20 μM). (D) Untreated or bortezomib-treated (20 nM bortezomib for 18 hours) RENCA cells were cocultured with syngeneic NK cells (E/T ratio = 3:1) for 5 hours and analyzed for expression of Bid. (E) Cytotoxicity of freshly isolated and expanded human NK cells against human RCC cells at E/T ratios of 15:1 and 3:1, respectively. CMA indicates treatment with CMA (20 nM); α-TRAIL, anti–mouse TRAIL antibody (N2B2; 10 μg/mL) or anti–human TRAIL (RIK-2) antibody (10 μg/mL); wt, wild-type murine NK cells; and pfp−/−, perforin-deficient murine NK cells. x-axis in panel A shows log fluorescence of caspase-8. Each data point represents a separate experiment in panel B, (horizontal bars show mean) and P values were calculated by one-way ANOVA with the Tukey multiple comparison post test.
Bortezomib sensitizes murine and human RCC cells to NK-cell lysis through activation of caspase-8. RENCA tumors and human RCC cells (JOHW tumor cells) were treated with 20 nM and 10 nM bortezomib, respectively, for 18 hours, and then washed and cocultured with syngeneic murine and allogeneic human NK cells, respectively (3:1 effector-target [E/T] ratio) for an additional 5 hours and analyzed for (A,B) caspase-8 activity or (C) susceptibility to NK-cell lysis (E/T ratio = 1:1) in the presence of caspase-8 (Z-IETD)– or caspase-9 (Z-LEHD)–blocking reagents (20 μM). (D) Untreated or bortezomib-treated (20 nM bortezomib for 18 hours) RENCA cells were cocultured with syngeneic NK cells (E/T ratio = 3:1) for 5 hours and analyzed for expression of Bid. (E) Cytotoxicity of freshly isolated and expanded human NK cells against human RCC cells at E/T ratios of 15:1 and 3:1, respectively. CMA indicates treatment with CMA (20 nM); α-TRAIL, anti–mouse TRAIL antibody (N2B2; 10 μg/mL) or anti–human TRAIL (RIK-2) antibody (10 μg/mL); wt, wild-type murine NK cells; and pfp−/−, perforin-deficient murine NK cells. x-axis in panel A shows log fluorescence of caspase-8. Each data point represents a separate experiment in panel B, (horizontal bars show mean) and P values were calculated by one-way ANOVA with the Tukey multiple comparison post test.
Similar to murine tumors, caspase-8 activity was significantly up-regulated in bortezomib-treated renal cell carcinoma tumors of human origin after coculture with IL-2–activated human NK cells. Neutralization of TRAIL but not perforin inhibited NK cell–mediated caspase-8 activation in bortezomib-treated tumors (Figure 3). Furthermore, the caspase-8 blocking reagent Z-IETD-fmk prevented bortezomib from augmenting NK-cell tumor cytotoxicity. This reduction in caspase-8 activity and tumor cytotoxicity was only observed with IL-2–expanded NK cells and did not occur in cultures containing freshly isolated NK cells. In contrast to expanded IL-2 activated NK cells, which killed tumor targets predominantly through TRAIL, freshly isolated NK cells killed bortezomib-treated human tumor cells predominantly through perforin/granzyme-mediated apoptosis. The results of these experiments were reproduced when using the human melanoma cell line SK23 (data not shown).
Bortezomib enhances NK cell–mediated antitumor effects in vivo
We next evaluated whether bortezomib would enhance the antitumor effects of adoptively infused NK cells in tumor-bearing mice. Tumor growth was delayed in BALB/c animals with subcutaneous RENCA tumors treated with the combination of bortezomib and syngeneic NK cells compared with mice receiving NK cells or bortezomib alone (Figure 4). There was a similar delay in the growth of subcutaneous LLC1 tumors in C57BL/6 mice treated with bortezomib and syngeneic NK cells compared with mice receiving NK cells or bortezomib alone. In animals injected intravenously with RENCA tumor cells, pulmonary tumor burden (P < .01) was significantly lower after treatment with 3 cycles of bortezomib and adoptive syngeneic NK-cell infusions compared with mice receiving either NK cells alone, bortezomib alone, or no treatment (Figure 4). These results demonstrate that bortezomib-induced tumor sensitization to NK-cell cytolytic function observed in vitro also occurs in vivo. The delay in tumor growth associated with combining bortezomib and NK cells was abolished when mice were treated with bortezomib followed by infusion of perforin-deficient NK cells (Figure 4). These in vivo observations are consistent with the in vitro finding that enhanced NK-cell cytolytic activity against bortezomib-treated murine tumors is mainly mediated through perforin/granzyme.
Bortezomib sensitizes RENCA and LLC1 to syngeneic NK-cell lysis in vivo. (A left panel) Tumor size by palpation in BALB/c mice injected with RENCA tumor cells (100 000 cells subcutaneously) and treated with bortezomib (5 μg/mouse intravenously) on days 3, 10, and 17, followed by injection of IL-2–activated syngeneic NK cells (106 cells intravenously) on days 4, 11, and 18. Each symbol represents an individual mouse on day 29 after tumor injection. (Right panel) Tumor size by palpation in C57BL/6 mice injected with LLC1 tumor cells (500 000 cells subcutaneously) that were treated with bortezomib (15 μg/mouse intraperitoneally) on day 14, followed by injection of IL-2–activated syngeneic NK cells (1 × 106 cells intravenously) on day 15. Each symbol represents an individual mouse on day 28 after tumor injection (horizontal bars show mean). (B) Quantification of pulmonary tumor nodules in RENCA tumor–bearing BALB/c mice after treatment with bortezomib (days 5, 12, and 19) and wild-type (wt) or perforin-deficient (pfp−/−) NK cells (2 × 106 cells; days 6, 13, and 20). All animals received IL-2. Animals were killed on day 28 (left panel) and on day 30 (right panel) and evaluated for number of tumor nodules in the lung by manual counting (horizontal bars show mean). (C) In vivo bioluminescence assay and survival of animals treated with bortezomib and/or NK-cell infusions. Luciferase-transduced RENCA cells were injected, and mice were treated with 9 weekly injections of bortezomib and 2 to 5 × 106 syngeneic NK cells starting 4 days after tumor injection. Values in bioluminescence graph (left panel) represent tumor doubling time. Values in survival graph (right panel) represent median survival days. Images show animals on day 39 after tumor injection. Values below each image represent the average tumor flux (photons/second [p/s]; × 103) ± SD. All experiments were performed with 5 or more mice per group and repeated at least once. P values were calculated by 2-tailed unpaired t test or 2-tailed log-rank test.
Bortezomib sensitizes RENCA and LLC1 to syngeneic NK-cell lysis in vivo. (A left panel) Tumor size by palpation in BALB/c mice injected with RENCA tumor cells (100 000 cells subcutaneously) and treated with bortezomib (5 μg/mouse intravenously) on days 3, 10, and 17, followed by injection of IL-2–activated syngeneic NK cells (106 cells intravenously) on days 4, 11, and 18. Each symbol represents an individual mouse on day 29 after tumor injection. (Right panel) Tumor size by palpation in C57BL/6 mice injected with LLC1 tumor cells (500 000 cells subcutaneously) that were treated with bortezomib (15 μg/mouse intraperitoneally) on day 14, followed by injection of IL-2–activated syngeneic NK cells (1 × 106 cells intravenously) on day 15. Each symbol represents an individual mouse on day 28 after tumor injection (horizontal bars show mean). (B) Quantification of pulmonary tumor nodules in RENCA tumor–bearing BALB/c mice after treatment with bortezomib (days 5, 12, and 19) and wild-type (wt) or perforin-deficient (pfp−/−) NK cells (2 × 106 cells; days 6, 13, and 20). All animals received IL-2. Animals were killed on day 28 (left panel) and on day 30 (right panel) and evaluated for number of tumor nodules in the lung by manual counting (horizontal bars show mean). (C) In vivo bioluminescence assay and survival of animals treated with bortezomib and/or NK-cell infusions. Luciferase-transduced RENCA cells were injected, and mice were treated with 9 weekly injections of bortezomib and 2 to 5 × 106 syngeneic NK cells starting 4 days after tumor injection. Values in bioluminescence graph (left panel) represent tumor doubling time. Values in survival graph (right panel) represent median survival days. Images show animals on day 39 after tumor injection. Values below each image represent the average tumor flux (photons/second [p/s]; × 103) ± SD. All experiments were performed with 5 or more mice per group and repeated at least once. P values were calculated by 2-tailed unpaired t test or 2-tailed log-rank test.
We next evaluated the growth kinetics of pulmonary tumor metastases by bioluminescence assays. Tumor doubling time assessed 19 to 34 days after tumor injection was reduced in recipients treated with bortezomib or NK cells alone (2.7 and 2.2 days, respectively) compared with untreated mice (1.3 days), but survival did not differ among cohorts. Mice treated with the combination of bortezomib and NK cells had nearly a 50% reduction of tumor growth (tumor doubling = 5.1 days) compared with mice treated with bortezomib or NK cells alone. Importantly, animals treated with combined bortezomib/NK infusions had a significantly longer survival (median, 60 days; P = .01) than untreated mice or mice treated with bortezomib alone or NK cells alone (median survival, 41 days in all 3 cohorts),
There was no clinical evidence of toxicity nor significant differences in total body weights between BALB/c mice treated with 3 consecutive weekly intravenous injections of bortezomib followed by 107 IL-2–activated NK cells (approximately 4 × 108 NK cells/kg body weight) compared with untreated controls (no bortezomib and no NK cells); treated animals gained an average of 0.26 g/day compared with untreated controls, who gained an average of 0.22 g/day. Autopsies showed no evidence for organ damage associated with this therapy, specifically tissues from the liver, kidney, spleen, lymph nodes (mesenteric and inguinal), heart, gut (small and large intestine), stomach, pancreas, skin, adrenal gland, thyroid, spinal cord, gonads, brain, bone marrow, retina, and peripheral nerves (sciatic).
In vivo depletion of Treg cells enhances the antitumor effects of adoptively infused NK cells
Treg cells have been reported to inhibit endogenous NK-cell cytotoxicity against tumor targets in mice. Therefore, we investigated if CD4+/CD25+/FoxP3+ Treg cells would likewise suppress the antitumor effects of ex vivo–expanded adoptively infused syngeneic NK cells in tumor-bearing mice after treatment with bortezomib. In vitro experiments showed NK-cell numbers decreased significantly when NK cells were cocultured with activated BALB/c Treg cells compared with cocultures with activated BALB/c CD4+CD25− Tconv cells (data not shown). Moreover, NK cells cocultured with Treg cells had significantly lower in vitro cytolytic activity against RENCA tumors compared with NK cells cultured with Tconv cells (Figure 5). Treg cell–mediated inhibition of NK-cell cytotoxicity was dependent on TGF-β, as cytotoxicity was restored when neutralizing antibodies to TGF-β were added to NK cell–Treg cell cocultures. Incubating NK cells with supernatants collected from activated Treg cell cultures did not reduce NK- cell cytotoxicity or proliferation, suggesting that this inhibition was cell-contact dependent (data not shown).
Depletion of Treg cells enhances the antitumor effects of adoptively infused syngeneic NK cells in tumor-bearing mice. (A) Treg cells and Tconv cells were isolated from BALB/c spleens using immunomagnetic beads selecting for CD4+/CD25+ Treg cells and CD4+/CD25− Tconv cells. T cells were then activated in vitro with anti-CD3 and IL-2 for 4 days. There was a depletion of Treg cells, but not DX5+/CD3− NK cells, in mice receiving anti-CD25 antibody or rat IgG isotype antibody. Peripheral blood was harvested 4 days after antibody treatment and was stained for CD25+ and CD4+ (Treg cells, gated on CD3+) or CD3− and DX5+ (NK cells). y- and x-axes show log fluorescence (horizontal bars show mean). (B) NK-cell cytotoxicity of RENCA tumor cells in presence of Tconv cells or Treg cells at various T cell–NK cell ratios (1:1, 1:3, and 1:9). A total of 30 μg/mL neutralizing anti–TGF-β antibody was added to 1:1 cocultures of NK cells and Treg cells. BALB/c mice were injected with RENCA (day 0) and treated with anti-CD25 antibody (day 3) and bortezomib (day 4) and syngeneic NK cells (day 5). Tumor progression (C) and survival (D) in mice receiving 3 weekly injections of bortezomib and NK cells; all mice received IL-2. All experiments were performed with 5 or more mice per group and repeated at least once. Results in panel D are pooled from 2 experiments with a total of 8 to 13 animals per group. Values in panels C and D represent tumor-doubling time and median survival days in mice receiving bortezomib and NK cells with anti-CD25 treatment or without anti-CD25 treatment, respectively. P values were calculated by unpaired t test or 2-tailed log-rank test.
Depletion of Treg cells enhances the antitumor effects of adoptively infused syngeneic NK cells in tumor-bearing mice. (A) Treg cells and Tconv cells were isolated from BALB/c spleens using immunomagnetic beads selecting for CD4+/CD25+ Treg cells and CD4+/CD25− Tconv cells. T cells were then activated in vitro with anti-CD3 and IL-2 for 4 days. There was a depletion of Treg cells, but not DX5+/CD3− NK cells, in mice receiving anti-CD25 antibody or rat IgG isotype antibody. Peripheral blood was harvested 4 days after antibody treatment and was stained for CD25+ and CD4+ (Treg cells, gated on CD3+) or CD3− and DX5+ (NK cells). y- and x-axes show log fluorescence (horizontal bars show mean). (B) NK-cell cytotoxicity of RENCA tumor cells in presence of Tconv cells or Treg cells at various T cell–NK cell ratios (1:1, 1:3, and 1:9). A total of 30 μg/mL neutralizing anti–TGF-β antibody was added to 1:1 cocultures of NK cells and Treg cells. BALB/c mice were injected with RENCA (day 0) and treated with anti-CD25 antibody (day 3) and bortezomib (day 4) and syngeneic NK cells (day 5). Tumor progression (C) and survival (D) in mice receiving 3 weekly injections of bortezomib and NK cells; all mice received IL-2. All experiments were performed with 5 or more mice per group and repeated at least once. Results in panel D are pooled from 2 experiments with a total of 8 to 13 animals per group. Values in panels C and D represent tumor-doubling time and median survival days in mice receiving bortezomib and NK cells with anti-CD25 treatment or without anti-CD25 treatment, respectively. P values were calculated by unpaired t test or 2-tailed log-rank test.
We next evaluated whether depletion of Treg cells would further enhance the antitumor effects of adoptively infused NK cells after bortezomib treatment. Bioluminescence imaging of BALB/c mice bearing luciferase-transduced RENCA tumors showed no difference in tumor growth (assessed by calculating tumor-doubling times between days 19 and 34) or survival in mice treated with a Treg cell–depleting anti-CD25 antibody given alone or in combination with either NK cells or bortezomib compared with untreated mice (data not shown). However, ablation of host Treg cells before treatment with bortezomib and adoptive NK-cell infusions significantly reduced the growth of RENCA tumors and prolonged survival compared with mice receiving bortezomib and NK cells without Treg cell ablation (tumor-doubling time, 16.7 vs 4.9 days, respectively; P = .02; median survival, 69 vs 59 days, respectively; P = .028; Figure 5). Flow cytometry performed on blood samples collected from mice after anti-CD25 treatment showed near complete eradication of circulating Treg cells with similar NK-cell numbers compared with controls not receiving treatment with anti-CD25 antibodies.
Discussion
Inactivation of NK cells via their inhibitory KIRs and low-level surface expression of apoptosis-inducing death receptors on tumor cells limit autologous NK-cell immunity against cancer. These obstacles may account for the poor response of patients with cancer to adoptive autologous NK-cell infusions. In vitro, bortezomib has been shown to sensitize tumors to TRAIL- and FasL-dependent lysis by autologous NK cells.20,24 This study demonstrates that bortezomib enhances the antitumor effects of syngeneic NK-cell infusions in animals with established tumors, resulting in delayed tumor progression and prolonged survival. Furthermore, a pronounced augmentation in NK-cell killing was observed in bortezomib-treated recipients when host Treg cells were eradicated before infusing NK cells. Notably, this treatment strategy appeared to be nontoxic and safe, with no clinical or histologic evidence of normal tissues becoming sensitized to NK-cell cytotoxicity.
Several experiments performed in this study provided insight into the mechanisms by which bortezomib sensitized tumors to autologous killing. Unlike human tumors,20 expression of the death receptor DR5 and Fas did not change in any of the murine tumors (RENCA, LLC1, 4T1, CMS4, CT26, and B16) exposed to bortezomib. Furthermore, blocking experiments showed antagonistic antibodies TRAIL and FasL did not reduce the augmented NK- cell killing of bortezomib-treated tumors. In contrast, NK-cell cytotoxicity was reduced to similar levels observed against untreated tumors when the perforin inhibitor CMA was added to bortezomib-treated tumors (RENCA, T27A, and B16), or when perforin-deficient NK cells were used in cytotoxicity assays. Furthermore, in contrast to syngeneic wild-type NK cells, infusions of syngeneic perforin-deficient NK cells did not confer tumor protection in bortezomib-treated mice, confirming that bortezomib sensitized tumors to perforin-mediated NK-cell killing in vivo. Still, tumors treated with bortezomib in vitro were found to have increased sensitivity to killing by recombinant TRAIL. Investigators have previously shown that murine NK cells require NKG2D to recognize RENCA cells, ICAM-1 to mediate perforin/granzyme-dependent apoptosis, and are inhibited by MHC class I expressed on tumor cells.25 In our analysis, no changes in expression of MHC class I or ICAM-1 occurred in bortezomib-treated tumors. As observed by others, the blockade of NKG2D resulted in global reduction of NK cell–mediated cytotoxicity, confirming the importance of NKG2D signaling for RENCA lysis. Although we did observe an up-regulation in the surface expression of the NKG2D ligand H60 when RENCA cells were exposed to bortezomib, blocking NKG2D reduced NK-cell cytotoxicity of bortezomib-treated RENCA cells to a smaller degree than untreated RENCA cells, suggesting that sensitization to NK cell–mediated lysis did not occur as a consequence of up-regulation of NKG2D ligands at a surface level. Whether bortezomib increases susceptibility to NK cell–mediated lysis via Syk-PTK–independent or –dependent pathways was not studied in this analysis.
Since both TRAIL and granzyme B cleave Bid as well as caspase-8,26-29 we investigated whether these 2 key molecules were involved in bortezomib-induced sensitization. Experiments revealed caspase-8 activity increased substantially in both murine and human bortezomib-treated tumors upon coculture with NK cells in the absence of any changes in mitochondrial apoptotic pathways. Along these lines, bortezomib has previously been shown to reduce expression of cellular FLICE inhibitory protein (cFLIP) in several different cancers, rendering tumor cells susceptible to TRAIL-mediated apoptosis.21-23 However, Liu et al recently challenged these findings, reporting cFLIP levels actually increased in lung cancer cell lines treated with bortezomib, with enhanced TRAIL-mediated apoptosis occurring rather as the consequence of up-regulation of TRAIL-R2 expression.30 Previously, we showed that increased surface expression of TRAIL-R2 occurred in a variety of human tumors exposed to bortezomib and correlated with sensitization to NK-cell lysis; tumors that failed to up regulate TRAIL-R2 after bortezomib treatment did not become sensitized to NK cells.20 Taken altogether, these findings show the heightened NK-cell antitumor responses against bortezomib-treated murine tumors were predominantly mediated through perforin/granzyme and were dependent on caspase-8.
Several studies have shown Treg cells mediate contact and TGF-β–dependent suppressive effects on endogenous NK-cell cytotoxicity.31,32 In this study, we also found that Treg cells suppressed the tumor cytotoxicity of IL-2–activated NK cells in vitro. Furthermore, we extend this finding, showing that the antitumor effect of adoptively infused IL-2–activated syngeneic NK cells after bortezomib treatment can be potentiated in tumor-bearing animals by depleting Treg cells, resulting in a substantial prolongation in survival.
Pilot trials in humans have shown that adoptively infused autologous NK cells can mediate antitumor effects in a subset of patients with cancer. However, dominant NK cell–inhibitory receptors such as NKG2A and KIR likely hinder the antitumor effects of NK cells in vivo. Our study is the first to show that the activity of syngeneic NK cells against cancer can be substantially bolstered by combining adoptive NK-cell infusions with bortezomib treatment. Recently, in vivo data showing that bortezomib can enhance TRAIL-mediated antitumor immunity has also been reported. Sayers et al reported in vitro treatment with bortezomib enhanced recombinant TRAIL-mediated leukemia purging, resulting in improved tumor-free survival in mice that received transplants of tumor-purged bone marrow.23 Similarly, treatment with bortezomib and agonistic antibodies to DR5 was shown to reduce the number of pulmonary tumor metastases in tumor-bearing animals compared with controls receiving either bortezomib or DR5 agonistic antibodies alone.33 In the study by Hallet et al, bortezomib was shown to augment in vitro NK-cell purging of leukemia cells.24 However, in contrast to our study, the effect of combining bortezomib with adoptive NK-cell infusions was not evaluated in either of these studies.
In vitro experiments conducted in the current study highlight differences in effector function between murine and human NK cells. In contrast to mice, human tumors were sensitized to TRAIL-mediated killing by IL-2–activated NK cells after bortezomib treatment. This differed from fresh nonactivated human NK cells, where lysis of bortezomib-treated tumors was mediated predominantly by perforin/granzyme. Furthermore, fresh NK cells had significantly lower tumor cytotoxicity compared with IL-2–activated/expanded NK cells. The surface expression of FasL and TRAIL is low on freshly isolated human NK cells and is substantially up-regulated upon in vitro expansion and/or activation of NK cells with IL-2.34 This likely accounted for our finding that tumor sensitization to cytotoxicity by human NK cells after bortezomib treatment was mediated predominantly by perforin/granzyme in fresh human NK cells and by TRAIL in IL-2–activated/expanded human NK cells. Further, these results suggest that IL-2–activated/expanded NK cells may have greater clinical efficacy against bortezomib-treated tumors compared with fresh NK cells.
In summary, our study shows that bortezomib enhances the antitumor effects of adoptively infused syngeneic NK cells, delaying tumor growth and substantially prolonging survival in mice with established tumors. This antitumor effect was further potentiated by eradicating Treg cells and by IL-2 administration. This novel treatment strategy could be used as an approach to potentiate the antitumor effects of adoptively infused autologous NK cells in patients with cancer.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article iw hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John Andersson (National Institute of Allergy and Infectious Diseases [NIAID], NIH) for providing the PC61 hybridoma; Larry Lantz (Research and Technologies Branch, NIAID, NIH) for purification and endotoxin testing of the anti-CD25 antibody; Dr Timothy Back (NCI, NIH) for providing the perforin-deficient spleens from BALB/c mice; Dr Michael Eckhaus (Veterinary Resources Program, National Center for Research Resources, NIH) for evaluating histopathology in bortezomib- and NK cell–treated mice; and the NHLBI animal facility staff technicians in Buildings 50 and 10/8C and the mouse imaging facility.
This research was supported by the intramural research program of NIH, NHLBI, Hematology Branch. We also wish to acknowledge Action to Cure Kidney Cancer (ACKC) and The Dean R. O'Neill Memorial Fellowship for generous contributions supporting this research.
National Institutes of Health
Authorship
Contribution: A.L. designed, performed, and analyzed the research and wrote the paper; H.Y., A.S., and M.B. performed the research; and R.C. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Childs, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Rm 3-5140, Bldg 10-CRC, 10 Center Dr, MSC 1202, Bethesda, MD 20892-1202; e-mail: childsr@nih.gov.

![Figure 1. Bortezomib decreases proliferation and sensitizes RENCA tumor cells to syngeneic NK-cell lysis, but does not induce tumor apoptosis or alter cell-surface expression of MHC class I, DR5, Fas, Rae-1, or ICAM-1. RENCA or LLC1 tumor cells were treated with 20 nM bortezomib for 18 hours and were analyzed for (A) syngeneic BALB/c or C57BL/6 NK cell lysis in a 4-hour 51Cr release assay; (B) surface expression of MHC class I, Fas, DR5, Rae-1, Mult-1, H60, and ICAM-1 (x-axis shows log fluorescence); (C) proliferation (after 72 hours of bortezomib treatment [0.5-20 nM]);and (D) induction of apoptosis (assessed by staining for annexin V and propidium iodide). y- and x-axes show log fluorescence. Error bars in panels A and C depict SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/24/10.1182_blood-2008-11-190421/4/m_zh80180934310001.jpeg?Expires=1765945686&Signature=sXlPAQJv0oBbDOis10vYLcEzBtxwQJrnBBBL76DzKSF2o1KW~VBX54UiuwEQ7LNfLoSsHbMvYCc1PL8AeFN5WX6IwbIPNrL1btPLEE~I1OxRLzqJTcBvcATAwBVm4mMktpBq~251TY80at44TkSzM3HzCWyQOLo~k3C0J3GHAlQqLVJSs5cRdH7slHZ-0itGfZ-UZqu-IU9E9MLVem08-aBjeyH8-LdRh7XDi8TARZav5~SY-AKg7FHWy~vf4sH0shNESYg2DhrP9clASVV6SL5n-AOTz5tXFCb3JsU1smcXjEIBe74Q8jAzi6J6jU8hFBq1qUFGPpBV7EVb2m0jyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Bortezomib sensitizes murine and human RCC cells to NK-cell lysis through activation of caspase-8. RENCA tumors and human RCC cells (JOHW tumor cells) were treated with 20 nM and 10 nM bortezomib, respectively, for 18 hours, and then washed and cocultured with syngeneic murine and allogeneic human NK cells, respectively (3:1 effector-target [E/T] ratio) for an additional 5 hours and analyzed for (A,B) caspase-8 activity or (C) susceptibility to NK-cell lysis (E/T ratio = 1:1) in the presence of caspase-8 (Z-IETD)– or caspase-9 (Z-LEHD)–blocking reagents (20 μM). (D) Untreated or bortezomib-treated (20 nM bortezomib for 18 hours) RENCA cells were cocultured with syngeneic NK cells (E/T ratio = 3:1) for 5 hours and analyzed for expression of Bid. (E) Cytotoxicity of freshly isolated and expanded human NK cells against human RCC cells at E/T ratios of 15:1 and 3:1, respectively. CMA indicates treatment with CMA (20 nM); α-TRAIL, anti–mouse TRAIL antibody (N2B2; 10 μg/mL) or anti–human TRAIL (RIK-2) antibody (10 μg/mL); wt, wild-type murine NK cells; and pfp−/−, perforin-deficient murine NK cells. x-axis in panel A shows log fluorescence of caspase-8. Each data point represents a separate experiment in panel B, (horizontal bars show mean) and P values were calculated by one-way ANOVA with the Tukey multiple comparison post test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/24/10.1182_blood-2008-11-190421/4/m_zh80180934310003.jpeg?Expires=1765945686&Signature=lUN2IRcxI458Szo8m7BNrN8KXqs8H1zXjlzSJYeDFVFdWyH3fk5OnftQBi57GwOWLU84vG6-a4KFA2Thvf1obtFnqQu~kDQHMGGFX81tXxJTqne1Y1kYyikSrVDTgqbAXtzPBgKO-Gptofb0NawkQgIMyn4EW-3gsTIJU4X3s6H67tWNSc22n9dsvQCDhLgCJcXUqbNm6bbvUNumT3VaxvTxLM~TtfPP~x~vIeEBR-wthwrCMeokBOiaQsvTyMMmOxuG3OJHGocccCetqfY4GPPNPrlN5UKZT1pxvqJGp1pYtDnSoE3xc6fYDTM0PKrgSTe0dWdWUIXuw1j5cmOTnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Bortezomib sensitizes RENCA and LLC1 to syngeneic NK-cell lysis in vivo. (A left panel) Tumor size by palpation in BALB/c mice injected with RENCA tumor cells (100 000 cells subcutaneously) and treated with bortezomib (5 μg/mouse intravenously) on days 3, 10, and 17, followed by injection of IL-2–activated syngeneic NK cells (106 cells intravenously) on days 4, 11, and 18. Each symbol represents an individual mouse on day 29 after tumor injection. (Right panel) Tumor size by palpation in C57BL/6 mice injected with LLC1 tumor cells (500 000 cells subcutaneously) that were treated with bortezomib (15 μg/mouse intraperitoneally) on day 14, followed by injection of IL-2–activated syngeneic NK cells (1 × 106 cells intravenously) on day 15. Each symbol represents an individual mouse on day 28 after tumor injection (horizontal bars show mean). (B) Quantification of pulmonary tumor nodules in RENCA tumor–bearing BALB/c mice after treatment with bortezomib (days 5, 12, and 19) and wild-type (wt) or perforin-deficient (pfp−/−) NK cells (2 × 106 cells; days 6, 13, and 20). All animals received IL-2. Animals were killed on day 28 (left panel) and on day 30 (right panel) and evaluated for number of tumor nodules in the lung by manual counting (horizontal bars show mean). (C) In vivo bioluminescence assay and survival of animals treated with bortezomib and/or NK-cell infusions. Luciferase-transduced RENCA cells were injected, and mice were treated with 9 weekly injections of bortezomib and 2 to 5 × 106 syngeneic NK cells starting 4 days after tumor injection. Values in bioluminescence graph (left panel) represent tumor doubling time. Values in survival graph (right panel) represent median survival days. Images show animals on day 39 after tumor injection. Values below each image represent the average tumor flux (photons/second [p/s]; × 103) ± SD. All experiments were performed with 5 or more mice per group and repeated at least once. P values were calculated by 2-tailed unpaired t test or 2-tailed log-rank test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/24/10.1182_blood-2008-11-190421/4/m_zh80180934310004.jpeg?Expires=1765945686&Signature=uWmsnYawwgxjFiJd7vKwB0jiOXUBr0Rgg7GDb6W2eDXl1OvjZLeZhZgFJSbHkdPjkLU65ktNRfOOzWyQyTmTBBLt7fqpy86zWRSln1svBeoGbVcTwHbHO86BdLvR4Dh-9LQqrb7-KGvEvIX~-vm~YZvzTDIpdYrYl209Uwp9g5IZrQSxWFl2qREDQesnyY~2xHEhY36Z34MXmYSUX-X4kX2lI7-aITeW5yTBr0MOHOrxNc6lw4yvbiezaN8VI1oLiXkkGNBxTxThysQCUxlPghRosA8JkmUK9cuks60orjKC0bIGPMhOSYgNzIgpWj3xCTmKwvMp5zdgrC3qAJv6pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

