Abstract
Identification of rational therapeutic targets is an important strategy to improve the cure rate of diffuse large B-cell lymphoma (DLBCL). We previously showed that inhibition of the phosphodiesterase 4B (PDE4B) unleashes cyclic-AMP (cAMP) inhibitory effects toward the PI3K/AKT pathway and induces apoptosis. These data raised important considerations as to which upstream regulators mediate cAMP inhibition of PI3K/AKT, and how identifying this signaling route could be translated into clinical initiatives. We found that in normal and malignant B cells, cAMP potently inhibit the phosphorylation and activity of the tyrosine kinase SYK. Using genetic models of gain- and loss-of-function, we demonstrated the essential role for PDE4B in controlling these effects in DLBCL. Furthermore, we used a constitutively active SYK mutant to confirm its central role in transducing cAMP effects to PI3K/AKT. Importantly, given SYK credentials as a therapeutic target in B-cell tumors, we explored the role of PDE4B in these responses. In multiple DLBCL models, we found that genetically, hence specifically, inhibiting PDE4B expression significantly improved the efficacy of SYK inhibitors. Our data defined a hitherto unknown role for cAMP in negatively regulating SYK and indicate that combined inhibition of PDE4B and SYK should be actively pursued.
Introduction
Recent genome-wide investigations provided important insight into the molecular basis for the clinical differences observed in diffuse large B-cell lymphomas (DLBCL).1-5 These studies led to recognition of logical targets for therapeutic interventions, spearheaded the implementation of clinical trials, and guided the identification of biomarkers that may define subpopulations of patients uniquely sensitive to these novel agents. In addition, full exploration of these data may uncover cross-talks between distinct signaling pathways that could be developed into rational combinatorial approaches for the treatment of DLBCL
We previously showed that the phosphodiesterase 4B (PDE4B) is expressed at significantly higher levels in fatal/refractory than in cured DLBCL.2 These findings were of interest because PDE4B, as a key phosphodiesterase in B lymphocytes,6,7 accounts for most of the hydrolysis and inactivation of cyclic-AMP (cAMP), a second-messenger with significant growth inhibitory activities in normal and malignant lymphocytes.8-10 In subsequent studies, we showed that cAMP, in a PDE4B-controlled manner, induced growth arrest and apoptosis in DLBCL via down-modulation of the PI3K/AKT pathway.11 Together, these data suggested that overexpression of PDE4B may contribute to the poor outcome of subsets of DLBCL by ablating the physiologic proapoptotic signals mediated by cAMP. The cross-talk between the cAMP/PDE4B and the PI3K/AKT pathway also raised important mechanistic considerations, in particular which upstream regulators form the basis for cAMP inhibition of the PI3K/AKT pathway and how clarifying this signaling route could be translated into clinically relevant initiatives. Addressing these questions is of special interest because PDE4 inhibitors are in advanced clinical testing for other nonmalignant conditions.
Herein, we show that in normal and malignant B cells, cAMP dramatically inhibits the phosphorylation and activity of the tyrosine kinase SYK, a known regulator of PI3K.12 Using genetic models of gain- and loss-of-function, we demonstrated a fundamental role of PDE4B in modulating cAMP-dependent SYK activity in DLBCL. Further, stable expression of a SYK constitutively active mutant defined that this tyrosine kinase is a key transducer of cAMP inhibitory signals toward PI3K/AKT pathway. More importantly, considering the strong credentials of SYK as a target for therapeutic inhibition in B-cell tumors,13 we also explored the role of PDE4B in these effects. We found that genetically, hence specifically, inhibiting PDE4B expression and activity significantly improved the efficacy of SYK inhibitors in DLBCL. Together, our data highlighted a hitherto unappreciated role of cAMP in negatively regulating SYK and indicate that combined pharmacologic inhibition of PDE4B and SYK may be beneficial in the treatment of DLBCL and related mature B-cell malignancies.
Methods
Cell lines and primary tumors
Human DLBCL cell lines (DHL6, DHL7, DHL10, WSU-NHL, OCI-Ly3, and OCI-Ly10) and the HEK-293 cell line were cultured at 37°C, 5% CO2 in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer.
Isolation of murine mature B cells
Spleens were harvested from wild-type C57BL/6 mice. Single mononuclear cell suspensions and red blood cell lysis were performed as we described.14 Subsequently, B cells were purified from the total mononuclear cell population using the Mouse B-Cell Enrichment kit in the RoboSep Automated Cell Separator (StemCell Technologies, Vancouver, BC). Purity and degree of enrichment was determined by fluorescence-activated cell sorting (FACS)–based measurement of CD19 positive cells, in pre- and postseparation aliquots. Typically, the B-cell fraction was enriched from 20% to more than 90%, and a single spleen yielded approximately 2 × 107 CD19+ cells. These primary cells were cultured in freshly prepared B-cell media (RPMI-1640 supplemented with 20% FBS, 100 μM β-mercaptoethanol, 10 mM HEPES, 2 μM l-glutamine, 0.1% penicillin/streptomycin, 20 μg/mL lipopolysaccharide [LPS], and 2.5 ng/mL murine interleukin-4 [IL-4]). The relevant experiments were normally performed within 24 hours of cell separation. Institutional Animal Care and Use Committee (IACUC) approval for the use of mice in the study was provided by the University of Texas Health Science Center at San Antonio.
Reagents and antibodies
Forskolin and 8-bromo-cAMP were purchased from Sigma-Aldrich (St Louis, MO), and Piceatannol was purchased from Calbiochem (San Diego, CA). Affinity-purified goat anti–human and goat anti–mouse immunoglobulin G (IgG)/IgM were from Jackson ImmunoResearch Laboratories (West Grove, PA). Antibodies against total AKT, phospho-AKT (Ser473), phospho-SYK (Tyr525/526), phospho-SRC (Tyr416) were from Cell Signaling Technology (Danvers, MA), anti-total SYK from Santa Cruz Biotechnology (Santa Cruz, CA), and the anti–phospho-LYN (Tyr396) antibody was from Abcam (Cambridge, MA). Phycoerythrin (PE)-conjugated phospho-BLNK (Tyr84) and PE-conjugated p-SYK (Tyr348) were from BD Biosciences (San Jose, CA).
Genetic modulation of PDE4B expression in DLBCL cell lines
The generation of DHL6 cells expressing PDE4B2-WT, PDE4B2-phosphodiesterase inactive (PI) mutant (both FLAG-tagged), or empty vector (murine stem cell virus–enhanced green fluorescent protein [MSCV-eGFP]) was reported earlier.11 Here we used the same retrovirus-based system to generate stable DHL10-PDE4B2-WT and DHL10-PDE4B2-PI populations. To knockdown PDE4B expression, we used an oligonucleotide-based small interfering RNAs (siRNAs) strategy. Five PDE4B unique sequences were screened in Hela and HEK-293 cells (data not shown), and the 2 most efficient were selected for assays in DLBCL cell lines, named PDE4B siRNA no. 2, 5′- GCCUAAACAAUACAAGCAU-3′, and PDE4B siRNA no. 5, 5′-GCAUCUCACGCUUUGGAGU-3′. In all experiments, an irrelevant sequence (targeting the GFP gene, siRNA-ctrl 5′-AACGGCCACAAGUUCAGCGUG-3′) was used as a negative control. All oligonucleotides were purchased from Dharmacon (Lafayette, CO) and transiently transfected in the DLBCL cell line OCI-Ly3 by electroporation. Standardization of the siRNA transfection conditions in DLBCL cell lines was performed with a Cy3-labeled oligonucleotide (Ambion, Austin, TX) and included suspension of cells in reduced-serum Opti-MEM media (Invitrogen, Carlsbad, CA) at 5 × 106/mL and electroporation of 200 nM of the oligonucleotide in the Gene Pulser II System at 250 V, 950 μF (Bio-Rad Laboratories, Hercules, CA). Confirmation of the PDE4B knockdown was performed with quantitative real-time reverse transcription polymerase chain reaction (RT-PCR), as we described.11 In addition, these PDE4B-specific siRNA sequences were cloned into the pSilencer 4.1-CMV plasmid vector (Ambion), the constructs transfected by electroporation in the OCI-Ly3 cell line, and populations stably expressing the PDE4B-short hairpin RNAs (shRNAs) obtained with puromycin selection.
Genetic model of SYK gain-of-function in DLBCL cell lines
A gain-of-function mutant of SYK (Y629-31F; a gift from Hamid Band, Brigham and Women's Hospital, Boston, MA) was subcloned into the MSCV-eGFP retrovirus and transduced in the DHL6 cell line, as we described.11 Stably expressing highly purified cell populations (> 95% GFP+) were obtained by cell sorting. These 3 C-terminal tyrosil residues (amino acids 629-631) are responsible for the phosphorylation-dependent inhibitory conformational changes of SYK, and their mutation has been shown to constitutively activate the kinase function of this enzyme.15,16
Immunoblotting
Relevant cell lysates were isolated and subjected to electrophoresis in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as we described. For detection of phospho-SYK, phospho-LYN, and phospho-SRC, DLBCL cell lines were cultured overnight with medium supplemented with 2% FBS, pretreated with dimethyl sulfoxide (DMSO), 40 μM Forskolin, or 8-Br cAMP for 2 hours, followed by B-cell receptor (BCR) engagement with 10 μg/mL goat anti–human IgG/IgM for 10 minutes. Detection of pAKT(S473) was performed without BCR activation.
Intracellular measurements of p-BLNK (Tyr84) and p-SYK (pY348)—PhosphoFACS
Parental or genetically modified DLBCL cell lines were exposed to Forskolin, 8-Br-cAMP, or vehicle (DMSO) for various times with or without subsequent stimulation with goat anti–human IgG/IgM at 37°C for 10 minutes. Thereafter, the cells were fixed, permeabilized, and stained with relevant antibodies and controls. FACS analysis was performed using a BD FACSCalibur Flow Cytometer (BD Biosciences).
Intracellular cAMP quantification
Intracellular cAMP levels were measured using an enzyme-linked immunosorbent assay (ELISA)–based assay according to the manufacturer's instructions (Parameter cAMP assay; R&D Systems, Minneapolis, MN) at baseline or after exposure of the cells to the adenylyl cyclase activator Forskolin (40 μM, for 1-2 hours) or synthetic cAMP (0.5 and 1 mM), as previously described.11
Analysis of cell proliferation and apoptosis
The effects of genetic modulation of PDE4B expression on the growth inhibitory properties of the SYK inhibitor Piceatannol was determined with the CellTiter 96 AQueous Non-Radioactive Cell Proliferation assay (MTS; Promega, Madison, WI). Apoptosis was assessed with propidium iodide staining, as we previously described. In vitro culturing of DLBCL cell lines is accompanied by low/undetectable basal level of intracellular cAMP. Thus, to reliably measure the impact of PDE4B activity in these experiments, a fixed dose of 10 μM Forskolin was combined to vehicle or to increasing concentrations of Piceatannol. Subsequently, all growth inhibition data were normalized to the values obtained with Forskolin alone, thus effectively isolating the differential effects of Piceatannol in cells expressing distinct levels of PDE4B. Dose-response curves were fitted, and cellular 50% inhibitory concentration (IC50) was calculated by nonlinear regression analysis using Origin6 Software (Northampton, MA).
Results
cAMP inhibits SYK activity in DLBCL
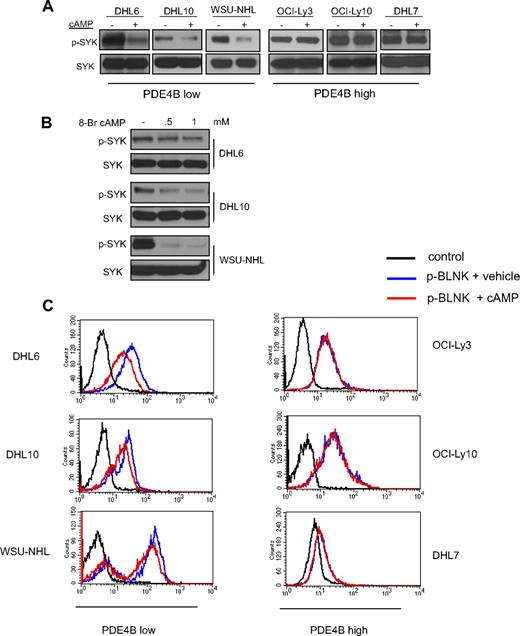
We previously showed that cAMP inhibits the PI3K/AKT pathway in DLBCL.11 As the tyrosine kinase SYK regulates PI3K activity in B cells, we investigated whether the cAMP inhibitory effects toward PI3K/AKT were transduced via SYK. We used a collection of well-characterized DLBCL cell lines with low and high PDE4B expression to test this hypothesis. Increasing cAMP intracellular levels, via pharmacologic activation of adenylyl cyclases with Forskolin, markedly decreased SYK phosphorylation (Tyr525/526) in DLBCL cell lines expressing low levels of PDE4B but had no effect in PDE4B-high cells (Figure 1A, and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). To confirm that these results were directly related to cAMP activities, we used a cell-permeable synthetic isoform of this second messenger, 8-bromo-cAMP, in the PDE4B-low cell lines and obtained identical results (Figure 1B). Tyrosines 525/526 are located in the catalytic site of SYK, and their phosphorylation level is a robust surrogate for this enzyme's activity.13,16 However, to further confirm that cAMP inhibited SYK activity, we measured the intracellular phosphorylation levels of one of its direct substrates, BLNK (Tyr84).17 Using phospho-FACS analyses, we showed an absolute correlation in the cAMP mediated decrease of SYK and BLNK phosphorylations (Figures 1C, S2), confirming that cAMP inhibits SYK activity. Finally, as we have shown before,11 cells lacking PDE4B were more sensitive to the growth inhibitory effects of cAMP than those expressing high levels of this enzyme (Figure S3).
PDE4B-dependent cAMP inhibition of SYK in DLBCL. (A) Western blot analyses show a marked inhibition of phospho-SYK (Tyr525/526) levels after elevation of intracellular levels of cAMP with the adenylyl cyclase activator Forskolin. These effects are present in PDE4B-low but not in PDE4B-high DLBCL cell lines. Total SYK expression confirms equal loading. (B) Exposure of DLBCL cell lines to the cell-permeable synthetic 8-Br-cAMP also significantly decreased SYK phosphorylation confirming the specificity of Forskolin effects. (C) FACS analysis shows that cAMP inhibits intracellular expression of phospho-BLNK (Tyr84), a direct SYK target, in a PDE4B-dependent manner. A 43%, 34%, and 37% decrease in the mean phospho-BLNK expression was detected in DHL6, DHL10, and WSU-NHL, respectively.
PDE4B-dependent cAMP inhibition of SYK in DLBCL. (A) Western blot analyses show a marked inhibition of phospho-SYK (Tyr525/526) levels after elevation of intracellular levels of cAMP with the adenylyl cyclase activator Forskolin. These effects are present in PDE4B-low but not in PDE4B-high DLBCL cell lines. Total SYK expression confirms equal loading. (B) Exposure of DLBCL cell lines to the cell-permeable synthetic 8-Br-cAMP also significantly decreased SYK phosphorylation confirming the specificity of Forskolin effects. (C) FACS analysis shows that cAMP inhibits intracellular expression of phospho-BLNK (Tyr84), a direct SYK target, in a PDE4B-dependent manner. A 43%, 34%, and 37% decrease in the mean phospho-BLNK expression was detected in DHL6, DHL10, and WSU-NHL, respectively.
In agreement with previous reports in DLBCL cell lines, the detection of SYK Tyr525/526 phosphorylation at baseline was below the Western blot analysis sensitivity.13 For these reasons, to reliably define the effects of cAMP on SYK phosphorylation, the experiments described above were performed in the presence of BCR engagement (10 μg/mL goat anti–human IgG/IgM for 10 minutes). Importantly, however, we showed that cAMP activities are likely to be directed at SYK because the phosphorylation of LYN and SRC, other B-cell relevant kinases activated by BCR engagement, were unaffected by cAMP (Figure S4). Furthermore, capitalizing on the higher sensitivity of the phospho-FACS assays, we showed that the cAMP inhibitory effects on SYK activity (defined by phospho-BLNK levels) were also evident in absence of in vitro BCR activation (Figure S5). Finally, we determined an additional layer of specificity as cAMP, although markedly modifying SYK's Tyr525/526 had no impact on the phosphorylation levels of Tyr348 (Figure S6).
cAMP inhibits SYK activity in normal B cells
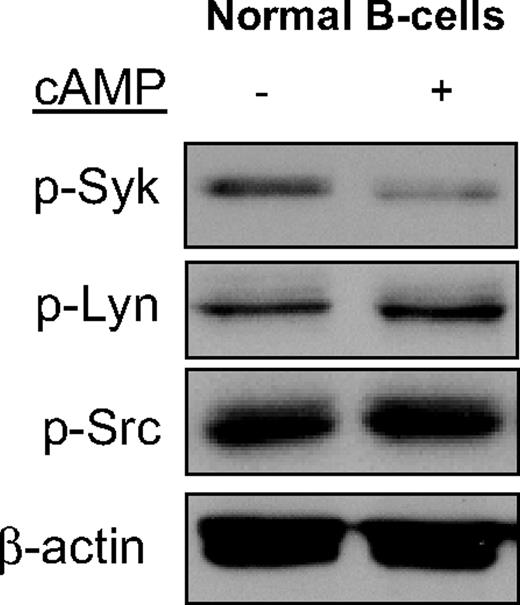
To confirm that the cAMP-mediated SYK inhibition encountered in DLBCL is physiologically relevant, we evaluated normal mature B cells. As shown in Figure 2, elevation of cAMP levels in freshly isolated and highly purified normal B cells resulted in a marked down-regulation of Syk's Tyr525/526 phosphorylation. Importantly, there were no changes in the phosphorylation levels of Lyn and Src, again highlighting the specificity of cAMP activities.
cAMP specifically inhibits SYK phosphorylation in normal mature Bcells. Phosphorylation of SYK, but not of the LYN and SRC kinases, was significantly inhibited by cAMP in highly purified murine normal mature B cells. β-Actin immunoblotting confirms equal loading.
cAMP specifically inhibits SYK phosphorylation in normal mature Bcells. Phosphorylation of SYK, but not of the LYN and SRC kinases, was significantly inhibited by cAMP in highly purified murine normal mature B cells. β-Actin immunoblotting confirms equal loading.
PDE4B controls the cAMP-mediated SYK inhibition in DLBCL
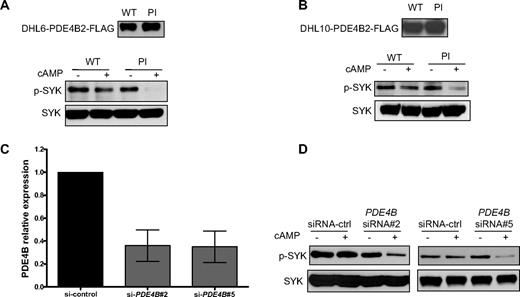
PDE4B is a key phosphodiesterase in normal and malignant B cells.2,6,7,11 In support to this well-established concept, we found that in DLBCL cell lines, the expression of other cAMP-specific phosphodiesterases, such as PDE4D and PDE7A, did not correlate with the cAMP levels and the observed downstream effects toward SYK/BLNK (Figure S7). These data indicate that these PDEs do not contribute significant cAMP hydrolysis in DLBCL. In addition, in agreement with earlier work,18,19 but contrary to a recent report in chronic lymphoid leukemia (CLL),20 we found that PDE7B is not expressed in lymphoid cells (DLBCL) and that the PDE7-specific inhibitor BRL-50481 is largely ineffective in DLBCL cell lines (data not shown). These data suggested that the observed cAMP-mediated inhibition of SYK is directly controlled by PDE4B expression/activity. To objectively test this hypothesis, we generated gain- and loss-of-function models of PDE4B in DLBCL cell lines. The DHL6 and DHL10 cell lines, which have null/low PDE4B expression (Figure S1), were genetically modified to stably express a PDE4B-WT or a PI mutant11 ; measurements of intracellular cAMP levels functionally validate these constructs (Figure S8). Subsequently, Western blot analysis was used to determine the phospho-SYK (Tyr525/526) levels after cAMP induction. Expression of PDE4B-WT in DHL6 and DHL10 DLBCL cell lines rendered SYK resistant to the inhibitory effects of cAMP, whereas in cells expressing a PDE4B-PI mutant gene, cAMP markedly inhibit phospho-SYK levels (Figure 3A,B). Again, we validated these findings by measuring phospho-BLNK levels (Figure S9). In complimentary experiments, we used 2 independent siRNA sequences to knockdown PDE4B expression in the DLBCL cell line OCI-Ly3 (Figure 3C); we confirmed the functional relevance of inhibiting PDE4B expression by demonstrating a marked elevation in intracellular cAMP levels in these cells (Figure S10). Finally, we showed that inhibiting PDE4B expression and activity rendered the aggressive ABC-type cell line OCI-Ly3 sensitive to the cAMP-mediated inhibition of the oncogenic SYK tyrosine kinase (Figure 3D). Thus, combining genetic models of gain- and loss-of-function, we showed that PDE4B plays a central role in controlling SYK activity in DLBCL.
PDE4B controls the cAMP-mediated SYK inhibition in DLBCL. (A,B) Stable expression of PDE4B-WT but not a PI mutant blocked cAMP-mediated inhibition of SYK phosphorylation in the DLBCL cell lines DHL6 and DHL10. Immunoblotting with total anti-SYK antibody confirmed equal loading, and anti-FLAG demonstrate the ectopic expression of PDE4B in these cell lines. (C) Quantitative real-time RT-PCR shows an approximate 65% decrease in PDE4B expression in the OCI-Ly3 cell line, 72 hours after 200 nM siRNA oligonucleotide transfection. Results are displayed as expression relative to siRNA-control transfected cells, determined using the ΔΔcycle threshold (CT) method and reported as 2−ΔΔCT. (D) siRNA-based knockdown of PDE4B expression with 2 independent sequences in the DLCBL cell line OCI-Ly3 led to a marked cAMP-mediated decrease in SYK Tyr525/526 phosphorylation. Immunoblotting with total SYK confirms equal loading. In these experiments, induction of cAMP was performed by incubating the cell lines with 40 μM Forskolin for 1 hour.
PDE4B controls the cAMP-mediated SYK inhibition in DLBCL. (A,B) Stable expression of PDE4B-WT but not a PI mutant blocked cAMP-mediated inhibition of SYK phosphorylation in the DLBCL cell lines DHL6 and DHL10. Immunoblotting with total anti-SYK antibody confirmed equal loading, and anti-FLAG demonstrate the ectopic expression of PDE4B in these cell lines. (C) Quantitative real-time RT-PCR shows an approximate 65% decrease in PDE4B expression in the OCI-Ly3 cell line, 72 hours after 200 nM siRNA oligonucleotide transfection. Results are displayed as expression relative to siRNA-control transfected cells, determined using the ΔΔcycle threshold (CT) method and reported as 2−ΔΔCT. (D) siRNA-based knockdown of PDE4B expression with 2 independent sequences in the DLCBL cell line OCI-Ly3 led to a marked cAMP-mediated decrease in SYK Tyr525/526 phosphorylation. Immunoblotting with total SYK confirms equal loading. In these experiments, induction of cAMP was performed by incubating the cell lines with 40 μM Forskolin for 1 hour.
SYK controls the effects of cAMP/PDE4B on AKT
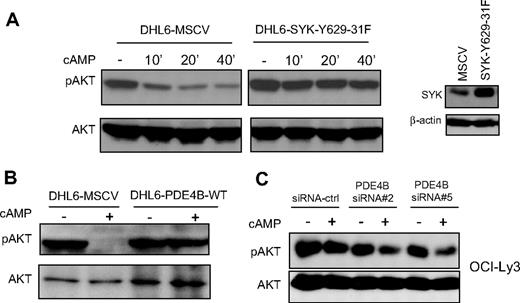
In earlier studies, we showed that cAMP, in a PDE4B-dependent manner, inhibits the PI3K/AKT pathway.11 SYK is a known regulator of PI3K activity in B lymphocytes.12 Therefore, our findings that cAMP/PDE4B also impinge on SYK activity suggest that this tyrosine kinase may control cAMP-mediated inhibition of PI3K/AKT in DLBCL. To test this hypothesis, we constitutively expressed a well-characterized SYK gain-of-function mutant (SYK-Y629-31F)15,16 in the PDE4B-null DHL6 cell line. We reasoned that if the cAMP-mediated inhibition of AKT was in fact relayed by SYK, expression of this mutant would limit or abrogate these effects. Indeed, we found that elevating cAMP levels resulted in marked decrease in phosphorylation of AKT (S473) in the DHL6 cells expressing an empty vector (DHL6-MSCV), whereas these inhibitory events were largely absent in DHL6 cells expressing SYK-Y629-31F (Figure 4A). Importantly, as both cell populations lack PDE4B expression, these data suggest that constitutive activation of SYK may mimic PDE4B effects on PI3K/AKT pathway. In support of this idea, we used our gain- or loss-of-function models and confirmed that similarly to the SYK Y629-31 mutant, high PDE4B expression limited cAMP-mediated AKT down-modulation (Figure 4B,C). Together, these data indicate that cAMP, SYK, and AKT signals may be transduced in a linear fashion in DLBCL. Consequently, one of the manners by which abnormally elevated SYK activity may contribute to lymphomagenesis is by limiting the physiologic cAMP-mediated inhibition of AKT.
SYK and PDE4B control the effects of cAMP on AKT phosphorylation. (A) In the PDE4B-null DHL6 cell line, elevation of cAMP levels inhibit AKT phosphorylation (Ser473; left panel). Stable expression of a SYK gain-of-function mutant blocked cAMP-mediated inhibition of phospho-AKT. Anti-SYK immunoblot demonstrate the ectopic expression of this protein in the DHL6 cell line. These data suggest that constitutive activation of SYK may mimic the anti-inhibitory effects of PDE4B in DLBCL. (B,C) Western blot analyses of PDE4B overexpression in the DHL6 and knockdown in the OCI-Ly3 cell line, confirmed the central role of this phosphodiesterase in regulating cAMP-mediated AKT inhibition.
SYK and PDE4B control the effects of cAMP on AKT phosphorylation. (A) In the PDE4B-null DHL6 cell line, elevation of cAMP levels inhibit AKT phosphorylation (Ser473; left panel). Stable expression of a SYK gain-of-function mutant blocked cAMP-mediated inhibition of phospho-AKT. Anti-SYK immunoblot demonstrate the ectopic expression of this protein in the DHL6 cell line. These data suggest that constitutive activation of SYK may mimic the anti-inhibitory effects of PDE4B in DLBCL. (B,C) Western blot analyses of PDE4B overexpression in the DHL6 and knockdown in the OCI-Ly3 cell line, confirmed the central role of this phosphodiesterase in regulating cAMP-mediated AKT inhibition.
PDE4B expression modulates the efficacy of a SYK inhibitor in DLBCL
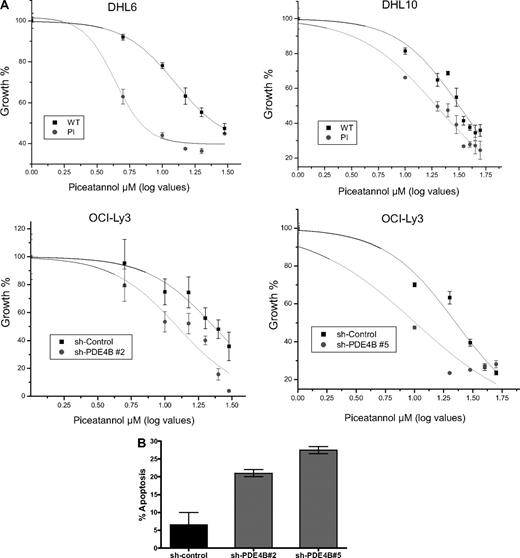
Therapeutic inhibition of the tyrosine kinase SYK is one of the most promising novel approaches for the treatment of mature B-cell malignancies.13,21,22 The uncovering of a prominent role for cAMP in modulating SYK activity suggested that PDE4B inhibition could improve the efficacy of SYK inhibitors in DLBCL. To test this hypothesis in a manner that specifically assesses the role of PDE4B, we used isogenic DLBCL cell lines with overexpression or down-regulation of this enzyme. Thus, we generated dose-response curves and measured the cellular IC50 for the SYK inhibitor Piceatannol in 4 independent DLBCL models of PDE4B gain- or loss-of-function. In agreement with our hypothesis, the concentration of Piceatannol required to inhibit the proliferation of DHL6-PDE4B-WT cells by 50% (IC50) was approximately 3-fold higher than that needed to inhibit their isogenic counterparts expressing a PDE4B-inactive mutant (12.5 ± 0.7 μM vs 4.32 ± 0.7 μM, P < .01). Likewise, DHL10 cells expressing a PDE4B-WT gene were significantly more resistant to Piceatannol than the PDE4B-PI-expressing cells (IC50s, 31.7 ± 1.5 μM vs 19.3 ± 1.0 μM, P < .01; Figure 5A top panel). In confirmatory experiments, stable expression of 2 distinct PDE4B shRNA constructs in the DLBCL cell line OCI-Ly3 (Figure S11), significantly improved the efficacy of Piceatannol in this aggressive DLBCL cell line: sh-PDE4B no. 2 (IC50, 12.3 ± 1.5 μM vs 23.1 ± 1.2 μM, sh-control cells) and sh-PDE4B no. 5 (IC50, 10.1 ± 2.7 μM vs 22.6 ± 1.9 μM, sh-control cells; P < .01; Figure 5A bottom panel). Finally, we defined the impact of the genetic inhibition of PDE4B on the rate of apoptosis induced by the SYK inhibitor Piceatannol; the apoptotic fraction was significantly larger in OCI-Ly3 cells expressing the PDE4B shRNAs than in shRNA-control cells (P < .05, 1-way analysis of variance [ANOVA]; Figure 5B). Together, these data provide a strong, mechanistically based, rationale to combine PDE4 and SYK inhibitors in the treatment of DLBCL and related mature B-cell malignancies.
PDE4B expression modulates the activity of the SYK inhibitor Piceatannol. (A) Dose-response curves and cellular IC50 of Piceatannol for DLBCL cell lines genetically modified to express high or low levels of PDE4B. (Top panel) Constitutive expression of PDE4B-WT in DHL6 and DHL10 significantly impaired the growth inhibitory efficacy of the SYK inhibitor Piceatannol. IC50s of 12.5 ± 0.7 μM versus 4.32 ± 0.7 μM and 31.7 ± 1.5 μM versus 19.3 ± 1.0 μM were found for DHL6 and DHL10, expressing PDE4B-WT and PI mutant, respectively (P < .01, log IC50 comparisons). (Bottom panel) Likewise, stable expression of 2 distinct PDE4B shRNA constructs in the DLBCL cell line OCI-Ly3, significantly improved Piceatannol efficacy; sh-PDE4B no. 2 (IC50, 12.3 ± 1.5 μM vs 23.1 ± 1.2 μM, sh-control cells) and sh-PDE4B no. 5 (IC50, 10.1 ± 2.7 μM vs 22.6 ± 1.9 μM, sh-control cells; P < .01, log IC50 comparisons). In the x axis, the log values of the Piceatannol concentration are shown in a linear scale. All growth inhibition curves were generated in triplicate. (B) Apoptosis rate in OCI-Ly3 cells stably expressing a shRNA-control or sh-RNA directed to PDE4B. Knockdown of PDE4B expression significantly enhances the 10 μM Piceatannol-induced apoptosis in DLBCL (P < .05, one-way ANOVA). Data shown is the mean + SE of 2 independent experiments for each unique PDE4B shRNA sequence.
PDE4B expression modulates the activity of the SYK inhibitor Piceatannol. (A) Dose-response curves and cellular IC50 of Piceatannol for DLBCL cell lines genetically modified to express high or low levels of PDE4B. (Top panel) Constitutive expression of PDE4B-WT in DHL6 and DHL10 significantly impaired the growth inhibitory efficacy of the SYK inhibitor Piceatannol. IC50s of 12.5 ± 0.7 μM versus 4.32 ± 0.7 μM and 31.7 ± 1.5 μM versus 19.3 ± 1.0 μM were found for DHL6 and DHL10, expressing PDE4B-WT and PI mutant, respectively (P < .01, log IC50 comparisons). (Bottom panel) Likewise, stable expression of 2 distinct PDE4B shRNA constructs in the DLBCL cell line OCI-Ly3, significantly improved Piceatannol efficacy; sh-PDE4B no. 2 (IC50, 12.3 ± 1.5 μM vs 23.1 ± 1.2 μM, sh-control cells) and sh-PDE4B no. 5 (IC50, 10.1 ± 2.7 μM vs 22.6 ± 1.9 μM, sh-control cells; P < .01, log IC50 comparisons). In the x axis, the log values of the Piceatannol concentration are shown in a linear scale. All growth inhibition curves were generated in triplicate. (B) Apoptosis rate in OCI-Ly3 cells stably expressing a shRNA-control or sh-RNA directed to PDE4B. Knockdown of PDE4B expression significantly enhances the 10 μM Piceatannol-induced apoptosis in DLBCL (P < .05, one-way ANOVA). Data shown is the mean + SE of 2 independent experiments for each unique PDE4B shRNA sequence.
Discussion
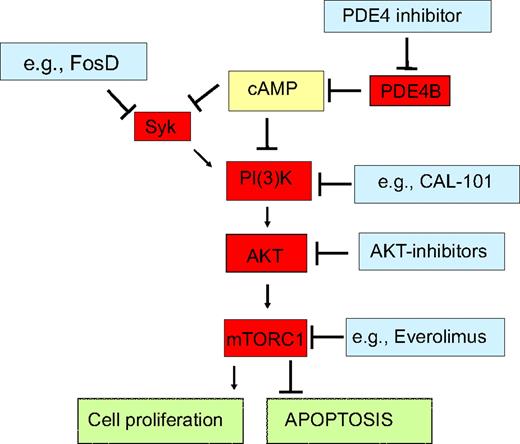
In this report, we describe a hitherto unknown regulatory axis present in normal and malignant B lymphocytes that links the second messenger cAMP to the lymphomagenic tyrosine kinase SYK. These findings refine our understanding of the physiologic control of B-lymphocytes activation and open extraordinary opportunities for the development of rational combinatorial therapeutic modalities in DLBCL (Figure 6).
PDE4B signaling interactions highlight novel opportunities for combined therapeutic approaches in DLBCL. We showed that in normal and malignant B-lymphocytes cAMP, at least in part via down-modulation of the tyrosine kinase SYK, inhibits the PI3K/AKT/mTOR pathway, thus curtailing cell proliferation and inducing apoptosis. As PDE4B inactivates cAMP, it abrogates these growth inhibitory effects, and in subsets of DLBCL, it may contribute to the abnormally elevated activity of SYK and PI3K/AKT/mTOR. Pharmacologic inhibition of PDE4B should restore the intracellular levels of cAMP, reestablish growth suppression and apoptosis, and potentially improve the efficacy of various agents that impinge on this pathway. Indeed, in proof-of-principle experiments, we showed that genetic modulation of PDE4B increase the effectiveness of a SYK inhibitor. This concept is timely and its clinical implementation a realistic goal as exemplified by availability of series of relevant compounds already in clinical trials for lymphomas (http://clinicaltrials.gov/).
PDE4B signaling interactions highlight novel opportunities for combined therapeutic approaches in DLBCL. We showed that in normal and malignant B-lymphocytes cAMP, at least in part via down-modulation of the tyrosine kinase SYK, inhibits the PI3K/AKT/mTOR pathway, thus curtailing cell proliferation and inducing apoptosis. As PDE4B inactivates cAMP, it abrogates these growth inhibitory effects, and in subsets of DLBCL, it may contribute to the abnormally elevated activity of SYK and PI3K/AKT/mTOR. Pharmacologic inhibition of PDE4B should restore the intracellular levels of cAMP, reestablish growth suppression and apoptosis, and potentially improve the efficacy of various agents that impinge on this pathway. Indeed, in proof-of-principle experiments, we showed that genetic modulation of PDE4B increase the effectiveness of a SYK inhibitor. This concept is timely and its clinical implementation a realistic goal as exemplified by availability of series of relevant compounds already in clinical trials for lymphomas (http://clinicaltrials.gov/).
The relevance of the cAMP signaling network in B-cell lymphomas was first appreciated when we found that PDE4B, the main phosphodiesterase in B lymphocytes, was significantly overexpressed in fatal DLBCL.2,11 As PDE4B abrogates cAMP's growth inhibitory and proapoptotic effects, we reasoned that elevated expression of this phosphodiesterase contributed to the poor outcome of subsets of DLBCL. In subsequent studies, we preclinically validated PDE4B as a bona fide target for therapeutic intervention in these tumors and identified an important interplay between cAMP/PDE4B and the PI3K/AKT pathway.11 Those findings raised important considerations, including which upstream regulators mediated cAMP inhibition of PI3K/AKT and how identifying this signaling route could be translated into clinically relevant initiatives.
The tyrosine kinase SYK, which transduces and amplifies signals derived from the BCR,13,16,21 has been reported to activate PI3K.12 Furthermore, the activity of SYK's T-cell cognate kinase, ZAP-70, was previously reported to be inhibited by cAMP.23 These seemingly unrelated data led us to hypothesize that the cAMP effects toward PI3K/AKT could be mediated by SYK. Our data conclusively showed that cAMP inhibits SYK activity in DLBCL and highlighted the central role of PDE4B in controlling these events. Furthermore, by ectopically expressing a constitutively active SYK mutant, we confirmed that this kinase is upstream of PI3K/AKT and transduces most of the cAMP inhibitory effects toward AKT. Together, these data also suggest that abnormally high PDE4B expression contributes to lymphomagenesis by blocking the physiologic down-modulation of SYK/PI3K/AKT by cAMP (Figure 6).
The current model of SYK regulation involves the opposing activities of the BCR and protein tyrosine phosphatases (PTPs). Although the mechanism by which cAMP inhibits SYK is still unknown, our data can instruct on the most likely possibilities. We found that cAMP effects in normal and malignant B lymphocytes are remarkably specialized and consistently spared LYN and SRC, upstream tyrosine kinases that phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) of Ig-α and Ig-β.24 In addition, cAMP did not modify the phosphorylation of SYK Tyr348, a primary target of the SRC kinases,25 but consistently inhibited Tyr525/526, autophosphorylated residues that are at the core of SYK activity. Thus, it is improbable that the inhibitory activity of cAMP is directed to upstream kinases or the BCR complex. Instead, it is more likely that cAMP specifically activates a PTP that directly dephosphorylates Tyr525/526. However, we cannot currently exclude the possibility that this putative PTP activated by cAMP also dephosphorylates the tyrosine residues at the ITAM, which serve as docking/activation sites for SYK. The use of unbiased approaches, such as siRNA-based screens targeting proximal components of the BCR signaling pathway, should identify the molecule(s) that mediate cAMP effects toward SYK.
Pharmacologic inhibition of SYK is a promising therapeutic modality for the treatment of DLBCL and related mature B-cell malignancies.13,21,26 Therefore, improved understanding of this enzyme's regulation and activity may lead to optimal use of SYK inhibitors, including the identification of biomarkers for clinical response and the development of rational combined targeting strategies. The discovery that cAMP inhibits SYK suggested that inhibition of PDE4B may augment the efficacy of SYK inhibitors. In this study, using multiple DLBCL models of PDE4B gain- or loss-of-function, we showed that this phosphodiesterase impinges on the efficacy of the SYK inhibitor Piceatannol. In particular, when exposed to Piceatannol, DLBCL cell lines with reduced PDE4B expression displayed a significantly lower IC50 and higher apoptosis than their counterparts expressing high PDE4B levels. Importantly, with the use of isogenic DLBCL cell lines that differed exclusively in respect to PDE4B expression, we guaranteed that the known genetic heterogeneity of DLBCLs did not play a role in the observed results. Thus, we propose that implementation of clinical trials that combine PDE4 and SYK inhibitors is a rational therapeutic strategy for the treatment of mature B-cell tumors. Furthermore, our findings also suggest that PDE4B expression/activity may be a biomarker for response to SYK inhibitors in DLBCL. Importantly, the fact that the cAMP/PDE4B signals also modulate the PI3K/AKT pathway and its downstream targets (Figure 6) considerably expands the array of, mechanistically supported, combinatorial therapeutic approaches involving PDE4 inhibitors in DLBCL. Finally, current data suggest that tonic or ligand-induced BCR signaling effects renders DLBCLs sensitive to agents that target SYK.13 The 3 independent cell lines (DHL6, DHL10, and OCI-Ly3) that we used to demonstrate the benefits of the combinatorial PDE4 and SYK inhibition are all dependent on tonic BCR survival signals. Thus, as we developed this combinatorial targeting concept further, it will be of interest to determine whether PDE4B inhibition could also play a therapeutic role in patients resistant to SYK inhibition or in instances where SYK up-regulation is independent of BCR signals.
Phosphodiesterases of the PDE4 family, in particular PDE4B2, is highly expressed and accounts for most of the cAMP hydrolysis in immune cells.6,7,11 This finding underscores the relevance of PDE4B overexpression in fatal DLBCL and indicates that PDE4 inhibitors are likely to have a significant impact in reestablishing the cAMP inhibitory effects in normal and malignant lymphocytes. In agreement with this idea, efforts to develop PDE inhibitors for inflammatory and autoimmune conditions (asthma, chronic obstructive pulmonary disease [COPD], psoriasis, multiple sclerosis, etc) are centered in agents with specificity to the PDE4 family.27,28 It should be noted that the multiple phosphodiesterases expressed in mammalian tissues have rather unique architecture at their active site. Therefore, it is possible to develop very selective PDE inhibitors with minimal nonspecific side effects, a concept fully established by the successful clinical use of PDE5 inhibitors for erectile dysfunction.28 This notion is particularly important because the adverse effects noted in clinical trials of PDE4 inhibitors, may be related to PDE4D, not PDE4B, inhibition.29 Therefore, it will be important to eventually identify inhibitors with preferential activity toward PDE4B.
The observation that PDE inhibition could be beneficial in the treatment of lymphoid malignancies is a long standing concept. Indeed, in 1976, Hait and Weiss8 suggested that “it may be possible to inhibit specifically the predominant forms of this enzyme in leukemic lymphocytes, cause elevation of cAMP, and consequently a reduction in their growth rate.” The improved mechanistic understanding of this signaling axis that we provided here, and the preclinical validation of novel opportunities for rational therapeutic combinations in DLBCL, should rekindle interest in this field and, hopefully, redirect strategic plans to also consider the testing of clinical grade PDE4 inhibitors in oncology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by a grant from the National Cancer Institute (R21CA112043 to R.C.T.A.). R.C.T.A. is a Scholar of the American Society of Hematology.
National Institutes of Health
Authorship
Contribution: S.-W.K. performed research, designed assays, interpreted results, and cowrote the manuscript; D.R. and M.R.M. performed research; and R.C.T.A. designed the study, analyzed and interpreted the results, and wrote the manuscript. All authors read the manuscript and agreed with its contents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ricardo Aguiar, Division of Hematology and Medical Oncology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr, MC7880, San Antonio, TX 78229; e-mail: aguiarr@uthscsa.edu.