Abstract
The dock and lock (DNL) method is a new technology for generating multivalent antibodies. Here, we report in vitro and in vivo characterizations of 20-22 and 22-20, a pair of humanized hexavalent anti-CD20/22 bispecific antibodies (bsAbs) derived from veltuzumab (v-mab) and epratuzumab (e-mab). The 22-20 was made by site-specific conjugation of e-mab to 4 Fabs of v-mab; 20-22 is of the opposite configuration, composing v-mab and 4 Fabs of e-mab. Each bsAb translocates both CD22 and CD20 into lipid rafts, induces apoptosis and growth inhibition without second-antibody crosslinking, and is significantly more potent in killing lymphoma cells in vitro than their parental antibodies. Although both bsAbs triggered antibody-dependent cellular toxicity, neither displayed complement-dependent cytotoxicity. Intriguingly, 22-20 and 20-22 killed human lymphoma cells in preference to normal B cells ex vivo, whereas the parental v-mab depleted malignant and normal B cells equally. In vivo studies in Daudi tumors revealed 20-22, despite having a shorter serum half-life, had antitumor efficacy comparable with equimolar v-mab; 22-20 was less potent than 20-22 but more effective than e-mab and control bsAbs. These results indicate multiple advantages of hexavalent anti-CD20/22 bsAbs over the individual parental antibodies and suggest that these may represent a new class of cancer therapeutics.
Introduction
Specifically targeting cell-surface antigens with intact and fragmented monoclonal antibodies (mAbs) has become an effective approach for therapy of diverse human diseases,1 and the clinical success of rituximab has validated this modality for the treatment of B-cell non-Hodgkin lymphomas (NHLs) and autoimmune diseases.2,3 Because rituximab is a chimeric antibody that can show immunogenicity in some patient populations, such as in certain immune-disease patients,4 and has considerably long infusion times for the initial administration,2 efforts to introduce new anti-CD20 mAbs with improved characteristics are ongoing.5,6 Other endeavors include fusion of cytokines to anti-CD20 antibodies,7,8 the construction of multivalent antibodies having more than 2 binding arms to CD20,9-11 and bispecific antibodies (bsAbs) designed to link both CD20 and a different cell-surface antigen, such as CD2212 and CD3.13
The earlier methods used for the production of bsAbs involved either chemical cross-linking of IgG14 or Fab′ fragments,15 or quadromas made by the fusion of 2 hybridomas.16 Subsequent strategies focused on generating recombinant bsAbs composed of tandem single-chain Fv (scFvs) or diabodies.17 However, these Fc-lacking constructs in general suffered the limitations of low yields, heterogeneous compositions, elaborate purification strategies, insolubility, instability, aggregation, and poor pharmacokinetics. Because, for many applications, the presence of an Fc and its effector functions is beneficial, if not necessary, for improved in vivo properties, Fc-containing bsAbs as exemplified by a variety of novel designs also have been described.18-22
The dock and lock (DNL) platform technology23 has the potential for making a myriad of bioactive molecules with multivalency, multifunctionality, and defined composition. It uses the dimerization and docking domain (DDD) of cyclic adenosine monophosphate-dependent protein kinase24 and the anchoring domain (AD) of A-kinase anchoring proteins25 as linkers for specifically docking a DDD-containing module with an AD-containing module, with the resulting complex covalently locked with disulfide bonds.26
Because the combination of rituximab and epratuzumab (e-mab) showed improved antilymphoma efficacy without increased toxicity in patients27-29 whereas the combination of veltuzumab (v-mab) and e-mab also showed enhanced activity in a lymphoma xenograft model,30 we undertook to construct and evaluate bsAbs against both CD20 and CD22. We found previously that anti-CD20/CD22 bsAbs generated by fusing an anti-CD22 scFv to the carboxyl-terminus of the heavy chain of v-mab inhibited the in vitro growth of Daudi Burkitt lymphoma cells without the need for second-antibody crosslinking, and functioned synergistically with B-cell antigen receptor-mediated inhibition while also showing potent antilymphoma effects in vivo.12 In this study, we used DNL to generate a pair of hexavalent anti-CD20/22 bsAbs, designated 22-20 (formerly DNL1) and 20-22 (formerly DNL2), which consist of an IgG linked to 4 Fab fragments. Specifically, 22-20 comprises e-mab and 4 additional Fabs derived from v-mab, and is thus designed to bind CD22 bivalently and CD20 tetravalently. The 20-22 comprises v-mab and 4 additional Fabs derived from e-mab. We show that 22-20 and 20-22 have distinct properties compared with their parental counterparts, including enhanced antilymphoma activity in vitro and comparable efficacy in vivo despite showing shorter half-lives.
Methods
Antibodies, reagents, and culture media
Humanized antibodies provided by Immunomedics (Morris Plains, NJ) included v-mab (anti-CD20 IgG1), e-mab (anti-CD22 IgG1), labetuzumab (hMN-14, anti-CEACAM5 IgG1), and h734 (anti-indium-diethylenetriaminepentaacetic acid IgG1). Also provided by Immunomedics were rat anti–idiotype mAbs, WR1 and WR2 (both against v-mab), and WN (against e-mab). Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA), unless stated otherwise. Mouse anti–human IgM was purchased from Southern Biotech (Birmingham, AL). Heat-inactivated fetal bovine serum (FBS) was from HyClone Laboratories (Logan, UT). All other cell culture media and supplements were from Invitrogen (Carlsbad, CA). Restriction endonucleases and other enzymes were purchased from New England Biolabs (Ipswich, MA). Oligonucleotides were synthesized by Sigma-Genosys (The Woodlands, TX). PCR reactions were performed using Amplitaq polymerase (Applied Biosystems, Foster City, CA) and a PerkinElmer GeneAmp PCR system 9600 (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Cell lines
Sp/ESF cells, an enhanced variant of Sp2/0-Ag14 (ATCC, Manassas, VA), were maintained in hybridoma serum-free media supplemented with 2 mM l-glutamine and 100 units/mL penicillin-streptomycin. Daudi, Ramos, and Raji Burkitt lymphoma cells (ATCC) were maintained in RPMI 1640 media containing 10% FBS and supplemented with 1 mM sodium pyruvate, 10 mM l-glutamine, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 100 units/mL penicillin-streptomycin.
Hexavalent bsAbs made by DNL
Schematic representations of the expression cassettes and protein structures of the CH1-Fab-DDD2 and CH3-AD2-IgG modules, as well as the structure of a hexavalent bsAb, are shown in Figure 1. The specific modules used to generate each of the hexavalent mAbs are summarized in Table 1. A detailed description of the expression vectors, stable transgenic production cell lines, and purification of CH1-Fab-DDD2 and CH3-AD2-IgG modules, as well as the production of 22-20 and 20-22, is provided as supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Biochemical analyses
Size-exclusion high performance liquid chromatography (SE-HPLC) was performed on a Beckman System Gold Model 116 with a Bio-Sil SEC 250 column (Bio-Rad, Hercules, CA) and 0.04 M phosphate-buffered saline (PBS), pH 6.8, 1 mM ethylenediaminetetraacetic acid as the mobile phase. Reducing and nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses were performed using 4% to 20% gradient Tris-glycine gels (Lonza Walkersville, Walkersville, MD). Matrix-assisted laser desorption ionization time-of-flight mass spectrometry was performed in a sinapinic acid matrix by the Scripps Center for Mass Spectrometry (La Jolla, CA).
Competition ELISA
Microtiter plates were coated overnight with v-mab or e-mab at 5 μg/mL and blocked with 2% bovine serum albumin/PBS for 1 hour. The 22-20, 20-22, 20-20 (formerly Hex-hA20), 22-22 (formerly Hex-hLL2), e-mab, and v-mab, serially diluted in triplicate, were each mixed with WR2 or WN at 1 nM and added to the coated wells. The bound WR2 or WN was quantified with peroxidase-conjugated goat anti–rat IgG and o-phenylenediamine dihydrochloride.
Flow cytometry
Apoptosis, viable cell counting, cell binding, and off-rate measurements were performed by flow cytometry on a Guava PCA (Guava Technologies, Hayward, CA) using the manufacturer's reagents, protocols, and software. Additional information is provided in the figure legends.
Cell binding, off-rates, and internalization
The 22-20, 20-22, 20-20, v-mab, and e-mab were labeled with phycoerythrin (PE) using a Zenon R-Phycoerythrin human IgG labeling kit (Invitrogen, Z-25455), following the manufacturer's protocol. Measurement of off-rates from Raji cells was performed as described previously.9
To measure internalization, Raji cells (5 × 105) in 0.5 mL complete medium (phenol red-free RPMI 1640 media supplemented with 10% FBS) were incubated with 5 μg of PE-labeled mAb at 37°C for 60 minutes, pelleted at 400g, and washed 4 times with PBS. Labeled cells were incubated for 60 minutes in either PBS or 6.25 μg/mL trypsin, 10 mM reduced glutathione, PBS. Cells were washed twice with complete medium before analysis by flow cytometry using Guava Express software. Cell surface but not internalized PE-mAb was digested with trypsin, resulting in a loss of fluorescence. To quantify the degree of internalization, the mean fluorescence intensity (MFI) of the trypsin-treated cells was divided by the MFI obtained for the nontrypsinized cells for each PE-mAb–bound cell set.
CDC and ADCC
Complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) assays were performed using Daudi cells as described previously.9 Blood specimens were collected under a protocol approved by the New England Institutional Review Board (Wellesley, MA), and informed consent was obtained in accordance with the Declaration of Helsinki.
Ex vivo depletion of B and T cells compared also with Daudi and Raji lymphoma cells
The effects of 22-20 and 20-22 on peripheral blood lymphocytes from healthy volunteers were evaluated ex vivo using flow cytometry and compared with those of v-mab, rituximab, and 20-20. Labetuzumab was used as a negative control mAb. Raji or Daudi (5 × 104 cells) were mixed with heparinized whole blood (150 μL) and incubated with test mAbs for 2 days. Cells were incubated for 30 minutes with fluorescein isothiocyanate–labeled anti-CD3, anti-CD19, anti-κ light chain, or mouse IgG1 (BD Biosciences, San Jose, CA). After lysis of erythrocytes, cells were analyzed using a FACSCalibur (BD Biosciences) with CellQuest software. Both Daudi and Raji cells separate from lymphocytes on forward-scatter versus side-scatter flow cytometry dot plots and are gated with the monocyte population. Daudi are identified as κ light chain–positive cells in the monocyte gate. Raji are identified as CD19+ cells in the monocyte gate. The normal B cells and T cells are identified as CD19+ and CD3+ cells, respectively, in the lymphocyte gate. In these experiments, Student t test was used to evaluate statistical significance (P < .05).
In vivo analyses
All animal studies were approved by the Center for Molecular Medicine and Immunology's Institutional Animal Care and Use Committee and performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care, US Department of Agriculture, and US Department of Health and Human Services regulations. A detailed description of the protocols used for all of the in vivo analyses is provided as supplemental methods.
Results
Hexavalent bsAbs made by DNL
Six different hexavalent antibodies of the same architecture were produced using the DNL method. Specific combinations of CH3-AD2-IgG and CH1-DDD2-Fab modules were mixed under mild redox conditions and then purified with protein A affinity chromatography. A standardized nomenclature is used to identify each construct, wherein the antigen code for the CH3-AD2-IgG module, which provides 2 specific binding Fabs, is indicated first, followed by a dash and then the antigen code for the CH1-DDD2-Fab module, which contributes 4 specific Fabs. All of the hexavalent DNL structures used in this study are summarized in Table 1. The 2 anti-CD20/CD22 bsAbs, 22-20 and 20-22, were generated by mixing CH1-DDD2-Fab-v-mab and CH3-AD2-IgG-e-mab or CH1-DDD2-Fab-e-mab and CH3-AD2-IgG-v-mab, respectively (Figure 1). The 20-20 and 22-22 are monospecific hexavalent structures for CD20 and CD22, respectively. CH3-AD2-IgG-h734 and CH1-DDD2-Fab-hMN-14 modules, which comprise Fabs derived from mAbs that do not bind B-lymphoma cells, were used to make 734-20 and 20-14, which are CD20-binding structural controls for 22-20 and 20-22, respectively.
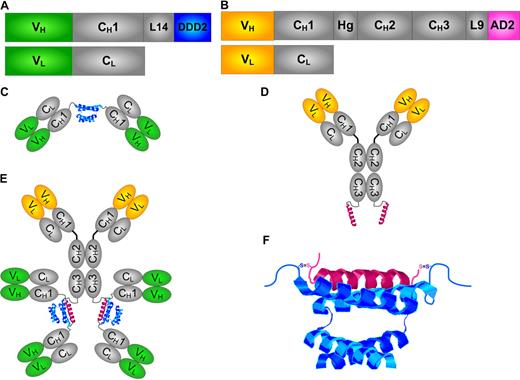
Schematic diagrams of DNL modules and structures. (A) The design for CH1-DDD2-Fab module. (B) The design for CH3-AD2-IgG modules. (C) The structure of CH1-DDD2-Fab as a dimer. (D) The structure of CH3-AD2-IgG as a monomer. (E) The structure of a bispecific hexavalent construct generated by DNL from reacting CH1-DDD2-Fab with CH3-AD2-IgG. (F) The interaction of the DDD2 and AD2 peptides. The variable domains for the heavy and light chains (VH + VL) are shown in green or orange. The constant domains of the heavy and light chains (CH + CL), the hinge (Hg), the 14-amino-acid residue (L14), and 9-amino-acid residue peptide linkers (L9) are shown in gray. The DDD2 and AD2 peptides are shown in blue and pink, respectively.
Schematic diagrams of DNL modules and structures. (A) The design for CH1-DDD2-Fab module. (B) The design for CH3-AD2-IgG modules. (C) The structure of CH1-DDD2-Fab as a dimer. (D) The structure of CH3-AD2-IgG as a monomer. (E) The structure of a bispecific hexavalent construct generated by DNL from reacting CH1-DDD2-Fab with CH3-AD2-IgG. (F) The interaction of the DDD2 and AD2 peptides. The variable domains for the heavy and light chains (VH + VL) are shown in green or orange. The constant domains of the heavy and light chains (CH + CL), the hinge (Hg), the 14-amino-acid residue (L14), and 9-amino-acid residue peptide linkers (L9) are shown in gray. The DDD2 and AD2 peptides are shown in blue and pink, respectively.
Each of the CH3-AD2-IgG and CH1-DDD2-Fab modules was produced by myeloma cells in batch roller bottle cultures. DNL conjugation of 22-20, 20-22, and each of the structural control constructs (20-20, 22-22, 20-14, and 734-20) resulted in highly purified covalent structures of the expected size and composition. A detailed description of the biochemical characterization of 22-20, 20-22, and the 4 DNL modules used for their synthesis is provided in supplemental results and Figure S1.
Binding avidity
The 22-20 and 20-22 retain the binding properties of their parental Fab/IgGs with all 6 Fabs apparently capable of binding simultaneously. Competitive enzyme-linked immunosorbent assays (ELISAs) compared the binding avidities of 22-20 and 20-22 for CD20 with those of v-mab and 20-20 (Figure 2A), or for CD22 with e-mab and 22-22 (Figure 2B). Rat anti-idiotype mAbs to v-mab (WR2) and e-mab (WN) were used to evaluate CD20 and CD22 binding, respectively. To assess binding, ELISA plates were coated with v-mab or e-mab and the test mAbs were allowed to compete with the immobilized IgG for WR2 or WN binding, respectively.
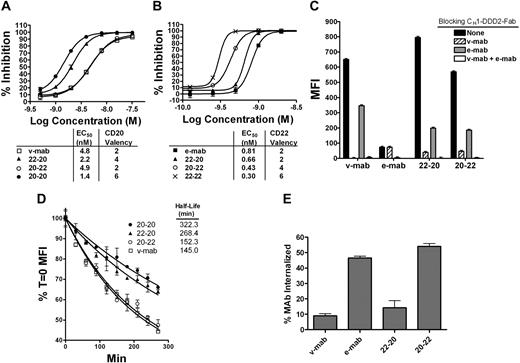
Binding properties of 22-20 and 20-22. (A) Competition ELISA showing relative binding avidity of v-mab, 22-20, 20-22, and 20-20 for binding to WR2, the anti-Id antibody to v-mab. (B) Competition ELISA showing relative binding avidity of e-mab, 22-20, 20-22, and 22-22 for binding to WN, the anti-Id antibody to e-mab. (C) Cell-binding analysis by flow cytometry. Raji cells were preincubated with CH1-DDD2-Fab-v-mab, CH1-DDD2-Fab-e-mab, or both at 1 mg/mL before staining with PE-22-20, PE-20-22, PE-v-mab, or PE-e-mab. (D) Analysis of dissociation rates from live Raji. Cells were saturated with PE-mAbs, and the fluorescence intensity was measured over time by flow cytometry. Percentage maximal binding (MFI T = 0) was calculated by dividing MFI T = x into MFI T = 0 and plotted versus time. (E) Internalization of PE mAbs was measured using flow cytometry by comparison of the MFI of trypsinized cells versus control cells after a 1-hour incubation at 37°C.
Binding properties of 22-20 and 20-22. (A) Competition ELISA showing relative binding avidity of v-mab, 22-20, 20-22, and 20-20 for binding to WR2, the anti-Id antibody to v-mab. (B) Competition ELISA showing relative binding avidity of e-mab, 22-20, 20-22, and 22-22 for binding to WN, the anti-Id antibody to e-mab. (C) Cell-binding analysis by flow cytometry. Raji cells were preincubated with CH1-DDD2-Fab-v-mab, CH1-DDD2-Fab-e-mab, or both at 1 mg/mL before staining with PE-22-20, PE-20-22, PE-v-mab, or PE-e-mab. (D) Analysis of dissociation rates from live Raji. Cells were saturated with PE-mAbs, and the fluorescence intensity was measured over time by flow cytometry. Percentage maximal binding (MFI T = 0) was calculated by dividing MFI T = x into MFI T = 0 and plotted versus time. (E) Internalization of PE mAbs was measured using flow cytometry by comparison of the MFI of trypsinized cells versus control cells after a 1-hour incubation at 37°C.
Comparison of EC50 values of nonlinear regression curves demonstrated that each construct possesses the functional valency as designed and that each Fab binds with a similar affinity to those of the parental mAb. For analysis of CD20 binding avidity (Figure 2A), the EC50 for 20-22 (4.9 nM), which has 2 CD20-binding Fabs, is consistent with that of v-mab (4.8 nM). The EC50 values for 22-20 (2.2 nM) and 20-20 (1.4 nM) are approximately 2- and 3-fold lower, consistent with mAbs having 4 and 6 CD20-binding Fabs, respectively. Analysis of CD22 binding avidity (Figure 2B) resulted in EC50 values for 22-20 (0.66 nM), 20-22 (0.43 nM), and 22-22 (0.30 nM) that are consistent with mAbs possessing 2, 4, and 6 CD22 Fabs, respectively, compared with e-mab (0.81 nM).
Cell binding and internalization
Flow cytometry was used to investigate bispecific cell binding (Figure 2C). Raji cells were preincubated with excess CH1-DDD2-Fab-v-mab, CH1-DDD2-Fab-e-mab, or both DDD2 modules to block CD20, CD22, and CD20/CD22 binding, respectively. Cells were then stained with a saturating amount of PE-conjugated 22-20, 20-22, v-mab, or e-mab, and the fluorescence intensity for the CD20/CD22-blocked cells was compared with that of unblocked cells. Without blocking, v-mab gave an approximately 10-fold higher MFI compared with e-mab, indicating that CD20 is expressed at a considerably higher level than CD22 on Raji cells. CH1-DDD2-Fab-v-mab and CH1-DDD2-Fab-e-mab completely inhibited CD20 binding of v-mAb and CD22 binding of e-mab, respectively. The combination of both DDD2 modules completely blocked binding of all PE-mAbs. Whereas CH1-DDD2-Fab-e-mab partially blocked (∼ 50%) CD20 binding to v-mab, CH1-DDD2-Fab-v-mab had no effect on CD22 binding to e-mab. That the individual DDD2 modules did not completely block binding of 22-20 and 20-22 demonstrates that the bsAbs bind to both CD20 and CD22 on the cells. Blocking CD20 reduces binding of 22-20 and 20-22 considerably more than blocking CD22, again indicating a substantially greater antigen density of CD20 in Raji.
We have shown previously that 20-20 dissociates markedly slower from live cells than v-mab.9 Similar off-rate experiments with Raji compared 22-20 and 20-22 with v-mab and 20-20 (Figure 2D). We found that the relative T1/2 correlated with the number of CD20-binding arms and that the number of CD22-binding arms had a negligible effect. This is probably because of the relatively greater CD20 antigen density compared with CD22 on these cells. The 20-22 (T1/2 = 152 minutes), which has 2 CD20 binding arms, had a statistically similar off-rate as v-mab (T1/2 = 145 minutes; P = .381). The 22-20 (T1/2 = 268 minutes), which has 4 CD20 binding arms, had a significantly slower off-rate than v-mab (P < .001) but was faster than 20-20 (T1/2 = 322 minutes; P = .004), which has 6 CD20 binding arms.
Ligation of e-mab to CD22, but not v-mab to CD20, results in the rapid internalization of the antibody-antigen complex.30 We investigated the extent of internalization of 22-20 and 20-22 by flow cytometry (Figure 2E). Live cells were incubated with PE-mAbs at 37°C for 1 hour before trypsin digestion to remove noninternalized mAbs. The MFI of cells stained with PE-v-mab and PE-22-20 was reduced by 90% and 85%, respectively, indicating that 22-20 behaves similarly to v-mab and has a slow internalization rate. Conversely, approximately 50% of the 20-22 internalized, similar to the results for e-mab. These data are consistent with observations by immunofluorescence microscopy (data not shown).
Growth inhibition of lymphoma cells
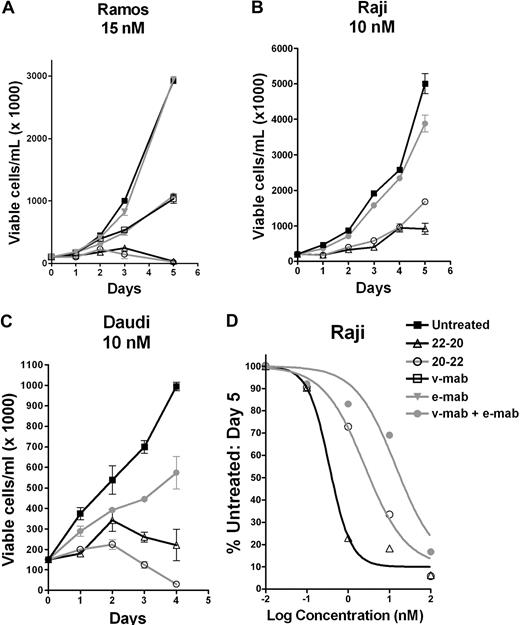
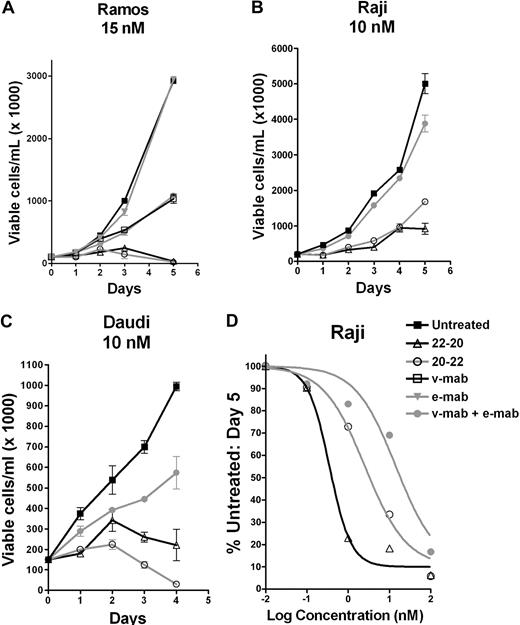
As shown by a cell counting assay, 22-20 and 20-22 effectively inhibited the growth of 3 Burkitt lymphoma cell lines, Ramos, Raji, and Daudi, at 15 nM or lower, whereas under the same conditions the individual parental antibodies were either ineffective (e-mab) or not as effective (v-mab alone or combined with e-mab). Dose-response curves obtained for Raji (Figure 3D), Ramos, and Daudi were used to calculate EC50 values (Table 2), which indicate that, in general, 22-20 was more potent than 20-22 in the 3 lymphoma cell lines examined. Whereas the addition of a crosslinking anti–human Fc antibody markedly increased the activity of v-mab, hypercrosslinking of 20-22 or 22-20 did not significantly enhance their antiproliferative activity (data not shown).
In vitro antiproliferation determined by the cell-counting assay. Cells were seeded in T-flasks at 100 000 cells/mL and treated with 22-20, 20-22, v-mab, e-mab, or a combination of v-mab and e-mab at the indicated concentrations. Viable cell densities (VCD) were determined daily by flow cytometry using Guava Viacount. On day 3, cultures were split 1:2 to maintain logarithmic growth over the course of the assay. The data are plotted as the VCD corresponding to the undiluted culture. (A) Ramos. (B) Raji. (C) Daudi. (D) Dose-response curves generated from cell counts at day 5. Raji cells were treated with various concentrations of 22-20, 20-22, or v-mab + e-mab, and the percentage of untreated cells was plotted versus mAb concentration.
In vitro antiproliferation determined by the cell-counting assay. Cells were seeded in T-flasks at 100 000 cells/mL and treated with 22-20, 20-22, v-mab, e-mab, or a combination of v-mab and e-mab at the indicated concentrations. Viable cell densities (VCD) were determined daily by flow cytometry using Guava Viacount. On day 3, cultures were split 1:2 to maintain logarithmic growth over the course of the assay. The data are plotted as the VCD corresponding to the undiluted culture. (A) Ramos. (B) Raji. (C) Daudi. (D) Dose-response curves generated from cell counts at day 5. Raji cells were treated with various concentrations of 22-20, 20-22, or v-mab + e-mab, and the percentage of untreated cells was plotted versus mAb concentration.
Control constructs, 20-14, 734-20, and 22-22, which have the same structural architecture as 20-22 and 22-20, were assayed for growth inhibition of Ramos with quantification by 3-(4,5-dimethylthiazole-2-yl-5-3-carboxymethoxyphenyl)-2-4-sulfophenyl)-2H-tetrazolium MTS (Figure S2). The effect of 20-14 (control structure for 20-22) was similar to that of v-mab, indicating that crosslinking of CD20 and CD22 at the cell surface is required for the enhanced cytotoxicity of 20-22. We have shown previously that mAbs capable of trivalent, tetravalent, or hexavalent CD20 binding have enhanced antiproliferative potency compared with bivalent (IgG) binding.9 This was confirmed with the tetravalent CD20-binding structure 734-20, which showed enhanced growth inhibition compared with v-mab. There was no statistical difference between dose-response curves for 734-20 and 22-20. Like e-mab, the hexavalent anti-CD22 construct, 22-22, did not inhibit growth of the lymphoma cell lines (data not shown).
Apoptosis and role of caspase and calcium
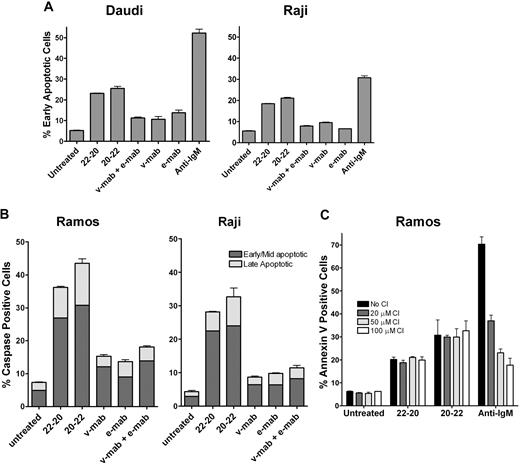
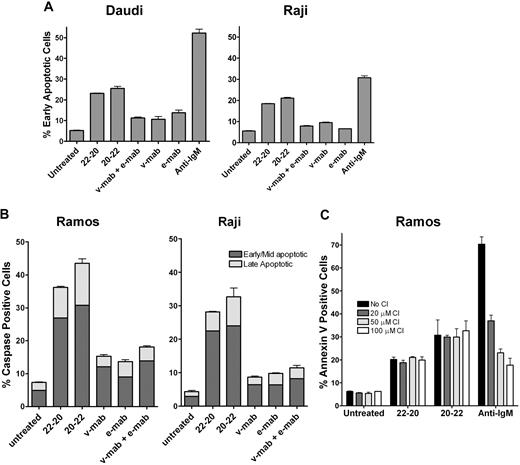
Phosphatydylserine translocation in Daudi and Raji cells was analyzed by the Guava Nexin assay after 24 hours of incubation (Figure 4). For both cell lines, treatment with 22-20 or 20-22 resulted in more cells in early apoptosis (18%-25%) compared with v-mab or e-mab, either alone or in combination (7%-10%), and the untreated control (5%). Caspase activation was investigated to corroborate the Guava Nexin results. The results of Guava MultiCaspase assays with Ramos and Raji cells (Figure 4B) were consistent with those determined with the Guava Nexin assay using similar treatment conditions. The effect of Z-VAD-FMK (a broad spectrum caspase inhibitor) on apoptosis induced by 22-20 and 20-22 was examined in Ramos. The results shown in Figure 4C indicate that Z-VAD-FMK inhibited apoptosis induced by antihuman IgM in a dose-dependent manner but had no effect on 22-20– or 20-22–induced apoptosis, suggesting the involvement of caspase-independent pathways.
Induction of apoptosis. Cells were cultured for 24 hours in the presence of 22-20, 20-22, v-mab, e-mab, or a combination of v-mab and e-mab at 50 nM. (A) Apoptosis was measured by Guava Nexin for Daudi and Raji. The percentage of early apoptotic cells (annexin V–PE+/7-AAD−) is shown. (B) Apoptosis was measured by Guava MultiCaspase for Ramos and Raji after staining with SR-VAD-FMK. (C) The effect of caspase inhibitor (CI) on apoptosis of Ramos induced by 22-20, 20-22, or anti-IgM (5 μg/mL, a positive control for caspase-dependent apoptosis). Cells were cultured for 24 hours in the presence the indicated reagent alone or in the presence of Z-VAD-FMK at 20, 50, or 100 μM, and then analyzed by Guava Nexin.
Induction of apoptosis. Cells were cultured for 24 hours in the presence of 22-20, 20-22, v-mab, e-mab, or a combination of v-mab and e-mab at 50 nM. (A) Apoptosis was measured by Guava Nexin for Daudi and Raji. The percentage of early apoptotic cells (annexin V–PE+/7-AAD−) is shown. (B) Apoptosis was measured by Guava MultiCaspase for Ramos and Raji after staining with SR-VAD-FMK. (C) The effect of caspase inhibitor (CI) on apoptosis of Ramos induced by 22-20, 20-22, or anti-IgM (5 μg/mL, a positive control for caspase-dependent apoptosis). Cells were cultured for 24 hours in the presence the indicated reagent alone or in the presence of Z-VAD-FMK at 20, 50, or 100 μM, and then analyzed by Guava Nexin.
Similar to v-mab and rituximab, 22-20 and 20-22 induced a detectable increase in intracellular calcium only on crosslinking with a second antibody. However, the Ca2+ flux observed with 22-20 and 20-22 was considerably less (25%-50%) than that of v-mab under the same conditions.
Homotypic adhesion
Both 22-20 and 20-22 induced strong homotypic adhesion of Daudi cells, resulting in incorporation of most cells into medium- to large-size aggregates, whereas under the same conditions, cells treated with either v-mab or rituximab did not form aggregates (Figure S3).
Serum stability
Both 22-20 and 20-22 are stable in human serum (Figure S3). As described in the supplemental methods, this assay specifically measures the concentration of intact bsAb because the anti-CD22-binding groups bind immobilized anti-idiotype to e-mab and the CD20-binding groups positioned at the opposite end of the bsAb are probed with an anti-idiotype antibody to v-mab. The data demonstrate that there is no significant loss of either mass or bispecific binding activity over at least 5 days in human sera (22-20, P = .528; 20-22, P = .195).
Effector functions
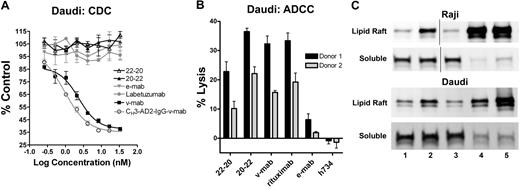
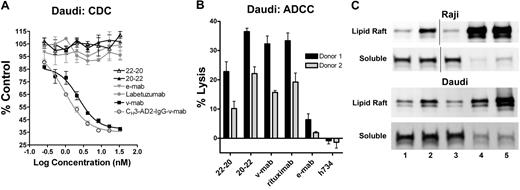
CDC was evaluated in vitro using human complement and Daudi cells (Figure 5A). V-mab, but not e-mab, exhibited potent CDC.30 Similarly, CH3-AD2-IgG-v-mab,9 but not CH3-AD2-IgG-e-mab (data not shown), induced CDC. Because the addition of 4 Fab fragments of v-mab to CH3-AD2-IgG-v-mab abolished CDC, as shown previously for 20-20,9 it is not surprising that neither 22-20 nor 20-22 induces CDC.
Effector functions of 22-20 and 20-22. (A) Complement-dependent cytotoxicity. Daudi cells were incubated with serial dilutions (3.33 × 10−8 to 2.6 × 10−10 M) of 22-20 (△), 20-22 (▲), e-mab (▼), v-mab (■), CH3-AD2-IgG-v-mab (○), or labetuzumab (●) in the presence of human complement. The percentage complement control (number of viable cells in the test sample compared with cells treated with complement only) was plotted versus the log of the nM concentration. (B) Antibody-dependent cellular cytotoxicity. Daudi cells (target) were incubated with 22-20, 20-22, v-mab, e-mab, or h734 at 5 μg/mL in the presence of freshly isolated peripheral blood mononuclear cells (effector). A 100% lysis reference was generated by the addition of detergent to wells only containing target cells. The percentage lysis obtained for each of 2 effector cell donors is represented in the bar graph. (C) Evaluation of membrane distribution of CD22 after treatment of Raji (top 2 panels) or Daudi (bottom 2 panels) with untreated (lane 1), e-mab (lane 2), v-mab (lane 3), 22-20 (lane 4), or 20-22 (lane 5). Cells were lysed in buffer containing 1% Triton X-100 and fractionated by sucrose density-gradient ultracentrifugation. The lipid rafts were collected from the interface of 5% and 30% sucrose, and the soluble fractions from the 40% sucrose at the bottom of the tubes. The lipid raft and soluble fractions were analyzed by anti-CD22 immunoblot. Vertical lines have been inserted to indicate a repositioned gel lane.
Effector functions of 22-20 and 20-22. (A) Complement-dependent cytotoxicity. Daudi cells were incubated with serial dilutions (3.33 × 10−8 to 2.6 × 10−10 M) of 22-20 (△), 20-22 (▲), e-mab (▼), v-mab (■), CH3-AD2-IgG-v-mab (○), or labetuzumab (●) in the presence of human complement. The percentage complement control (number of viable cells in the test sample compared with cells treated with complement only) was plotted versus the log of the nM concentration. (B) Antibody-dependent cellular cytotoxicity. Daudi cells (target) were incubated with 22-20, 20-22, v-mab, e-mab, or h734 at 5 μg/mL in the presence of freshly isolated peripheral blood mononuclear cells (effector). A 100% lysis reference was generated by the addition of detergent to wells only containing target cells. The percentage lysis obtained for each of 2 effector cell donors is represented in the bar graph. (C) Evaluation of membrane distribution of CD22 after treatment of Raji (top 2 panels) or Daudi (bottom 2 panels) with untreated (lane 1), e-mab (lane 2), v-mab (lane 3), 22-20 (lane 4), or 20-22 (lane 5). Cells were lysed in buffer containing 1% Triton X-100 and fractionated by sucrose density-gradient ultracentrifugation. The lipid rafts were collected from the interface of 5% and 30% sucrose, and the soluble fractions from the 40% sucrose at the bottom of the tubes. The lipid raft and soluble fractions were analyzed by anti-CD22 immunoblot. Vertical lines have been inserted to indicate a repositioned gel lane.
ADCC was assayed using freshly isolated peripheral blood mononuclear (effector) cells from 2 donors and Daudi (target) cells (Figure 5B), with h734, a humanized anti-indium-diethylenetriaminopenta-acetic acid IgG1 included as a nonspecific, isotype-matched control. V-mab showed potent ADCC whereas e-mab showed moderate but significant activity (P = .002) compared with h734. The 20-22, v-mab, and rituximab induced ADCC with a similar potency (P > .1). The 22-20 was more potent than e-mab (P = .001) but significantly less than v-mab (P < .001). In separate experiments, 20-14 showed similar ADCC as 20-22 and v-mab, whereas 734-20 had similar activity to 22-20 (not shown). These findings suggest that ADCC is governed primarily by CD20, which has a higher antigen density than CD22, and that constructs based on anti-CD20 IgG (v-mab, 20-22, and 20-14) mediate ADCC more efficiently.
Translocation of CD22/CD20 into lipid rafts
Binding of v-mab to lymphoma cells has been shown previously to induce the translocation of CD20 into lipid rafts by immunoblot analysis of the cell lysates in fractions isolated from sucrose density gradient centrifugation.12 Crosslinking of CD20 or CD22 by 22-20 or 20-22 results in enhanced translocation of CD22 into lipid rafts in both Daudi and Raji cells (Figure 5C), whereas e-mab treatment also resulted in moderately increased recruitment of CD22 into lipid rafts (lane 2), as reported previously.12 V-mab treatment had no effect on the submembrane distribution of CD22 (lane 3). Both 22-20 (lane 4) and 20-22 (lane 5) induced an extensive translocation of CD22 (as well as CD20) from the detergent-soluble membrane fraction into lipid rafts.
B-cell depletion
Anti-CD20 mAbs, such as rituximab and v-mab, are known to deplete normal B cells in patients. This critical property for treating patients with autoimmune diseases may not be essential for lymphoma therapy because e-mab is less B cell–depleting than rituximab or v-mab, but it is therapeutic in patients with CD22-positive lymphomas.31 Initially, we evaluated the ability of 22-20 and 20-22 to kill normal B and T cells in an ex vivo setting with human blood and found that they were much less efficient at killing B cells compared with v-mab or rituximab (Figure 6A). Both rituximab and v-mab significantly reduced the number of B cells down to approximately 15% of that in untreated blood (P < .001). The 22-20 and 20-20 did not significantly deplete B cells compared with untreated or blood treated with labetuzumab (P > .70). The 20-22-depleted B cells to 70% of that in untreated blood (P < .003), which was similar to the results for e-mab (80%, P = .054) and significantly less than those for v-mab or rituximab (P < .001). None of the agents tested resulted in depletion of T cells.
Preferential killing of NHL cells over normal B cells. (A) The effect of the indicated mAbs on peripheral blood lymphocytes from healthy volunteers was evaluated in vitro using flow cytometry. Decrease in the percentage of CD19+ (B cells) and CD3+ (T cells) present in the lymphocyte gate after a 2-day incubation of heparinized whole blood of a healthy volunteer with various mAbs. Error bars represent SD. (B) The effects of the indicated mAbs on peripheral blood B cells and Daudi (top) or Raji (bottom) lymphoma cells are indicated as the number of CD19+ events relative to untreated cell mixtures. B cells are derived as the CD19+ cells in the lymphocyte gate, whereas Daudi and Raji cells are located in the monocytes gate.
Preferential killing of NHL cells over normal B cells. (A) The effect of the indicated mAbs on peripheral blood lymphocytes from healthy volunteers was evaluated in vitro using flow cytometry. Decrease in the percentage of CD19+ (B cells) and CD3+ (T cells) present in the lymphocyte gate after a 2-day incubation of heparinized whole blood of a healthy volunteer with various mAbs. Error bars represent SD. (B) The effects of the indicated mAbs on peripheral blood B cells and Daudi (top) or Raji (bottom) lymphoma cells are indicated as the number of CD19+ events relative to untreated cell mixtures. B cells are derived as the CD19+ cells in the lymphocyte gate, whereas Daudi and Raji cells are located in the monocytes gate.
The finding that 22-20 and 20-22 were inefficient at killing normal B cells prompted us to investigate whether these agents can preferentially kill NHL cells over B cells in blood spiked with Daudi or Raji cells. Daudi killing was evaluated using blood from 3 donors. A representative experiment is shown in Figure 6B (top panel). The 22-20, 20-22, 20-20, v-mab, and rituximab all depleted Daudi to a similar extent (to 10% of untreated, P > .4 between reagents). The 22-20, 20-22, and 20-20 each depleted B cells to a similar extent (51%-57% of untreated, P > .25), which was significantly less than v-mab or rituximab (15%-23% of untreated, P < .002). In a similar experiment, Raji cells were depleted to a lesser extent by 22-20 (35% of untreated) than 20-22 (13% of untreated, P < .001), which was significantly less than v-mab and rituximab (1% of untreated, P < .001). As in the Daudi experiments, B cells were substantially depleted by v-mab and rituximab (15% of untreated), but not by 22-20 (90% of untreated) or 20-22 (70% of untreated). Although the results showed some variability among tumor cell lines as well as donors, 22-20 and 20-22 consistently showed a more preferential killing of NHL cells over normal B cells, compared with v-mab and rituximab.
PK analysis
Both 22-20 and 20-22 cleared from mouse blood faster than their parental IgGs (Table 3). In animals coinjected with 22-20 and e-mab, the T½ and mean residence time were more than 2-fold greater for e-mab. Likewise, 20-22 cleared more rapidly than v-mab, with an approximate 30% lower mean residence time. The 20-22 and 22-20 had similar biodistributions to v-mab and e-mab, respectively, indicating that the more rapid clearance of the bsAbs is not the result of increased uptake/elimination by any normal organs (Tables S1, S2). Analysis of pharmacokinetics serum samples with radiometric detection by SE-HPLC demonstrated that, although they are stable in serum, 22-20 and 20-22 slowly dissociate in vivo, presumably intracellularly (data not shown). Besides identifying the intact bsAb, SE-HPLC revealed the formation of increasing amounts of CH3-AD2-IgG over time. Radiolabeled Fab, F(ab)2, or other fragments were not detected in serum samples, as these are cleared rapidly. Negligible amounts (< 3%) of free isotope were detected in each sample. The half-lives of dissociation determined for 20-22 and 22-20 were 58 and 118 hours, respectively. This dissociation obviously contributes to the more rapid clearance of the bsAbs compared with IgG, yet other mechanisms may be involved.
In vivo efficacy
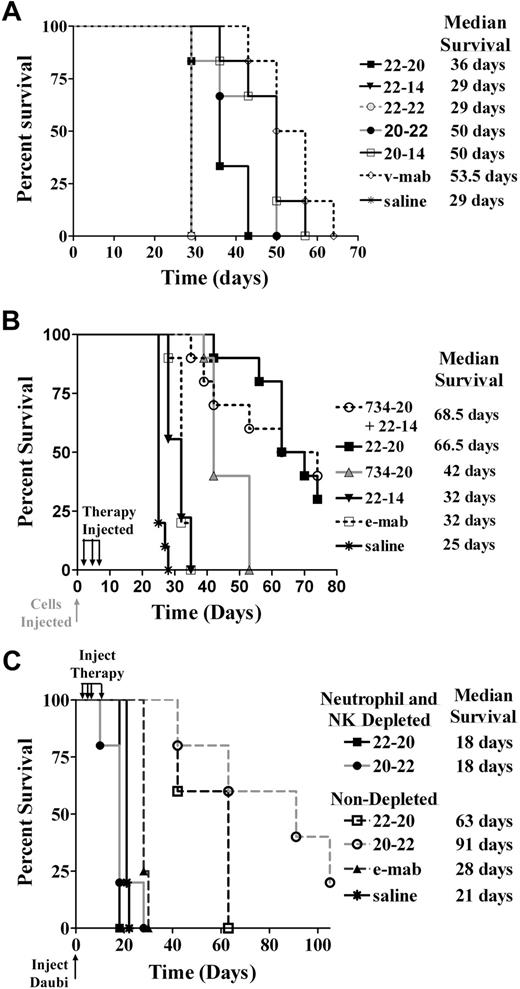
In the first study using a disseminated Burkitt lymphoma model, 22-20 and 20-22 each were administered as a single low dose (10 pmol) and compared with various controls (Figure 7A). The 22-20 significantly extended the median survival time (MST) compared with saline (36 vs 29 days, P = .005). The controls, 22-22, and 22-14 did not improve survival over saline (29 days, P = 1.0). Likewise, 20-22 treatment resulted in significantly longer MST in comparison with the saline control (MST = 50 days, P = .005). However, there was no significant difference between 20-22 and either v-mab (MST = 53.5, P = .082) or 20-14 (MST = 50 days, P = .554).
Survival curves showing therapeutic efficacy of 22-20 and 20-22 in a disseminated Burkitt lymphoma xenograft model. Female C.B. 17 SCID mice were administered 1.5 × 107 Daudi cells intravenously on day 0. (A) Therapy began on day 1 with groups of 6 mice receiving a single intraperitoneal injection (10 pmol) of 22-20, 20-22, v-mab, 22-22, 22-14, 20-14, or saline. (B) Groups of 10 mice were administered 10-μg doses of 22-20, 734-20, 22-14, or 10 μg of both 734-20 and 22-14 on days 1, 4, and 7. Additional groups received 4 μg of e-mab or saline. (C) Groups of 5 NK-cell/neutrophil-depleted or -nondepleted mice were administered intravenous injections of 230 μg of either 22-20 or 20-22 on days 1, 3, 5, and 9. Saline or 100 μg e-mab was administered to nondepleted mice.
Survival curves showing therapeutic efficacy of 22-20 and 20-22 in a disseminated Burkitt lymphoma xenograft model. Female C.B. 17 SCID mice were administered 1.5 × 107 Daudi cells intravenously on day 0. (A) Therapy began on day 1 with groups of 6 mice receiving a single intraperitoneal injection (10 pmol) of 22-20, 20-22, v-mab, 22-22, 22-14, 20-14, or saline. (B) Groups of 10 mice were administered 10-μg doses of 22-20, 734-20, 22-14, or 10 μg of both 734-20 and 22-14 on days 1, 4, and 7. Additional groups received 4 μg of e-mab or saline. (C) Groups of 5 NK-cell/neutrophil-depleted or -nondepleted mice were administered intravenous injections of 230 μg of either 22-20 or 20-22 on days 1, 3, 5, and 9. Saline or 100 μg e-mab was administered to nondepleted mice.
In the second study (Figure 7B), the efficacy of 22-20 was compared with e-mab, 22-14, 734-20, and a mixture of 22-14 and 734-20. All treatments significantly improved survival compared with saline controls (P < .001). The MST for mice treated with 22-20 (66.5 days) was significantly (P < .001) longer than those treated with e-mab (32 days), 22-14 (32 days), and 734-20 (42 days). Treatment with a combination of 22-14 and 734-20, which provides the same number of CD20 and CD22 binding groups with twice the number of Fc groups as 22-20, resulted in similar survival (MST = 68.5 days) as 22-20. There was no difference between e-mab and 22-14 treatment groups. The 734-20-treated animals had longer survival times than 22-14 and e-mab (P < .001). Thus, a single bsAb construct appeared to be at least as potent as mixtures of the parental mAbs.
In the third study, we examined the role of effector cells in the ability of either 22-20 or 20-22 to inhibit tumor growth in Daudi-inoculated mice (Figure 7C). In those animals depleted of natural killer (NK) cells and neutrophils, there was no difference between saline control and both groups of treated mice (MST = 21 and 18 days, respectively). In contrast, both 22-20 and 20-22 significantly increased animal survival in comparison with the saline control mice in the nondepleted animals (MST = 63 and 91 days for 22-20 and 20-22, respectively; P < .002). There was no significant difference in survival between the 22-20- and 20-22-treated mice.
Discussion
Our first recombinant anti-CD20/22 bsAbs, which were based on the IgG-(scFv)2 format, showed improved antitumor activity over the parental mAbs.12 These 2 bsAbs also retained ADCC, but not CDC, and suggested that bsAbs that are capable of simultaneously targeting both CD20 and CD22 may represent more potent agents for normal and malignant B-cell depletion in autoimmunity and NHL therapy, respectively. Because binding affinity of the anti-CD22 scFv groups was compromised, we turned to the DNL method to link 1 IgG with 4 Fab fragments, resulting in 22-20 and 20-22. Competitive ELISA and cell-binding/dissociation experiments demonstrated that all 6 arms of 22-20 and 20-22 can bind simultaneously and with similar affinity to the parental mAbs. These homogeneous defined structures are highly stable on storage and in human sera, and in our hands have higher productivity levels than scFv bsAb constructs.
The 22-20 and 20-22 showed different attributes, related to whether an anti-CD20 or anti-CD22 IgG was present, although the 4 complementary Fab fragments also contributed thereto, as summarized in Table 4. Because v-mab has been shown to differentiate from rituximab by having a significantly slower off-rate in all lymphoma lines tested,31 it was predictable that 20-22 showed a similar off-rate to parental v-mab and also a significantly slower off-rate than rituximab, but surprisingly not as slow as 22-20. This indicates that the 4 anti-CD20 Fab fragments contributed to the increased binding duration of 22-20 with an anti-CD22 IgG, compared with 20-22 having 4 anti-CD22 Fabs fused to anti-CD20 IgG. The distinguishing internalization property of e-mab was retained by the 20-22 form with 4 anti-CD22 Fab fragments, but not the 22-20 with the e-mab IgG fused to 4 anti-CD20 Fab fragments, indicating that the anti-CD20 Fabs inhibited the internalization of the anti-CD22 IgG.
Both bsAbs showed potent antiproliferation and apoptosis effects against B-lymphoma cells in vitro without the second-antibody crosslinking required for the parental mAbs. To show apoptotic and proliferation-inhibition effects, v-mab needs to be crosslinked, whereas e-mab must be immobilized as well as crosslinked.30 The 22-20 and 20-22 constructs could inhibit lymphoma cells without immobilization or crosslinking, which did not enhance their activity. Comparatively, in Daudi cells, 22-20 and 20-22 showed similar antiproliferation activities, which were approximately 50-fold higher than the combination of both parental mAbs. In Raji and Ramos tumor cells, 22-20 was 8- to 10-fold more potent than 20-22, which was in turn 8- to 10-fold more potent than the combination of both parental mAbs. Thus, 22-20 was 100-fold more potent than the parental mAbs given in combination. These constructs appear to inhibit lymphoma proliferation in vitro by signaling induced by 3 distinct binding events: (1) low potency bivalent (and possibly monovalent) binding of CD20, which is only detectable at more than 10 nM concentrations; (2) high potency multivalent (≥ 3) CD20 binding, which is effective at less than 0.1 nM concentrations9 ; and (3) intermediate potency with CD20/CD22 crosslinking. The high potency activity of multivalent CD20 binding was demonstrated previously with constructs comprising 3, 4, and 6 CD20-binding Fabs,9 and again here with 22-20, 734-20, and hypercrosslinked v-mab. The intermediate potency of CD20/CD22 crosslinking was evident with 20-22, which was more potent than v-mab and 20-14 but less potent than any of the constructs comprising 4 Fabs of v-mab. Although the action of 22-20 probably involves CD20/CD22 crosslinking, this effect is apparently overpowered by the signal induced by multivalent CD20 binding because 22-20 and 734-20 showed similarly high potency.
The apoptosis assays indicated that treatment with 22-20 or 20-22 resulted in more cells in early apoptosis compared with v-mab or e-mab, or their combination, and that these effects were caspase-independent.
The 22-20 and 20-22 induced strong homotypic adhesion in lymphoma cells, whereas under the same conditions the parental v-mab and e-mab were ineffective. This was also observed with other forms of anti-CD20/22 bsAbs (data not shown) as well as multivalent anti-CD20 constructs,9 indicating that the ability of an agent to crosslink CD20 and CD22 or to bind more than 2 CD20 molecules leads to homotypic adhesion, which may contribute to the enhanced in vitro cytotoxicity.
The 22-20 and 20-22 caused a spike in intracellular Ca2+ only on crosslinking with second antibody, and the magnitude was substantially less than that for v-mab. Calcium mobilization induced by crosslinking of bound anti-CD20 mAbs is thought to be involved in the induction of CD20-mediated apoptosis, which can be attenuated with Ca2+ chelators.32 It is of note that, unlike v-mab, 22-20 and 20-22 can induce apoptosis and inhibit proliferation of B-lymphoma cell lines in vitro without crosslinking, suggesting that a unique signaling mechanism is involved that does not require calcium mobilization.
These effects also translated to interesting differences with regard to their relative potency for killing normal human B cells versus Daudi or Raji Burkitt lymphoma cells in vitro, whereas 22-20 and 20-22 showed a higher therapeutic ratio (% killing of malignant vs % normal B cells), compared with v-mab and rituximab. Neither of the DNL bsAbs showed CDC activity, in contrast to v-mab. The ADCC of 22-20 was less than 20-22 or v-mab, but more than e-mab, indicating surprisingly that this function can be enhanced when 4 anti-CD20 Fab fragments are fused to the anti-CD22 IgG which, by itself, has more moderate ADCC than anti-CD20 mAbs.30
Lipid-raft trafficking of CD20 was shown for all forms, 22-20, 20-22, and v-mab, but trafficking of CD22 was more prominent for 22-20 and 20-22 than for e-mab, presumably because of crosslinking of CD20 and CD22, indicating that 22-20 may be a more potent CD22-binding mAb than e-mab.
Pk analyses showed that the circulating half-life of the bsAbs in mice was 2- to 3-fold shorter than that of the parental mAbs. Biodistibution studies in mice showed that 20-22 and 22-20 had similar tissue uptake to v-mab and e-mab, respectively, indicating that the bsAbs are not cleared more rapidly than their parental mAbs because of increased uptake in normal tissues. SE-HPLC analysis of Pk serum samples showed a slow dissociation of both 20-22 and 22-20 in vivo, which probably contributes to their increased rate of clearance. Because the bsAbs do not dissociate in serum, this presumably occurs inside cells. The in vivo efficacy of the bsAbs is not compromised even though they are larger molecules, dissociate over time, and are eliminated from circulation faster than the parental antibodies. This suggests that more frequent or higher dosing may be indicated to achieve the most optimal antitumor effects in vivo. The 22-20 was found to be significantly more potent than either anti-CD22 bivalent, tetravalent, or hexavalent forms, presumably because it contains 4 anti-CD20 Fabs joined with the anti-CD22 IgG, e-mab. Epratuzumab or other anti-CD22 constructs tested showed minimal antilymphoma activity in this Daudi model, consistent with prior results not showing activity for e-mab in preclinical models, although it is clearly active as a monotherapy and in combination with rituximab in NHL patients.27-29,33 Treatment with 22-20 as a single agent was equivalent to combination therapy with 22-14 and 734-20, even though the combination used double the amount of total antibody and Fc fragments. Thus, the bsAb constructs appeared to be at least as potent in vivo as their parental mAbs when used in combination, even when the former had shorter half-lives, suggesting that higher and/or more frequent doses could be even more potent in controlling the proliferation of lymphoma cells. Compared with each other, 20-22 showed a higher potency over 22-20 in vivo, yet the activity of both was abrogated by depleting NK cells and neutrophils. The loss of efficacy in NK/neutrophil-depleted mice could potentially be attributed to either lack of ADCC or the absence of Fc receptor-mediated crosslinking by effector cells. It is noteworthy that crosslinking did not affect the growth-inhibition activity of the bsAbs in vitro. In addition, the finding that in vivo efficacy is abrogated in depleted mice for 22-20 as well as 20-20,9 which exhibit multivalent CD20 binding without exogenous crosslinking, argues against the importance of Fc receptor–mediated crosslinking and instead that ADCC was responsible for cell killing in this model.
Taking into consideration that both 22-20 and 20-22 had a higher therapeutic index in vitro in terms of relative killing of lymphoma versus normal B cells than their parental mAbs, it is intriguing to speculate that these hexavalent, bispecific anti-CD20/CD22 forms may be a more potent class of antilymphoma therapeutic antibodies for clinical use. Therefore, we are also studying whether multivalent bsAbs against other cancer targets also can show different and improved therapeutic properties over the parental bivalent antibody forms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Diane Nordstrom, Diana Pilas, Yang Wang, Roberto Arrojo, Preeti Trisal, Maria Zalath, Anju Nair, Victoria Shi, and John Kopinski for excellent technical assistance, and Dr Francisco Hernandez-Ilizaliturri for the protocol of depleting NK cells and neutrophils in SCID mice.
This work was supported in part by the National Cancer Institute (grant P01-CA103985), National Institutes of Health (D.M.G.).
National Institutes of Health
Authorship
Contribution: E.A.R., D.M.G., and C.-H.C. designed research, analyzed data, and wrote the paper; and T.M.C. and R.S. performed research, collected and analyzed data, and revised the paper.
Conflict-of-interest disclosure: E.A.R., D.M.G., T.M.C., and C.-H.C. have employment, stock, and/or stock options with Immunomedics Inc (Morris Plains, NJ). R.S. declares no competing financial interests.
Correspondence: Edmund A. Rossi, Immunomedics Inc, 300 American Rd, Morris Plains, NJ 07950; e-mail: erossi@immunomedics.com; or David M. Goldenberg, Garden State Cancer Center, CMMI, 520 Belleville Ave, Belleville, NJ 07910; e-mail: dmg.gscancer@att.net.
References
Author notes
Presented in part at the 48th Annual Meeting of the American Society of Hematology, Orlando, FL, December 9-12, 2006.