To the editor:
Recently in Blood, Schonthal and colleagues reported in an elegant study that green tea polyphenols interact directly with bortezomib and other boronic acid–based proteasome inhibitors and block their anticancer effects.1 This subject has significant practice implications. By reviewing the chemistry of reactions between boronic acids and polyphenols, in this letter we generalize why, at least in theory, polyphenol dietary supplements should be avoided in patients who take boronic acid–based chemotherapeutics.
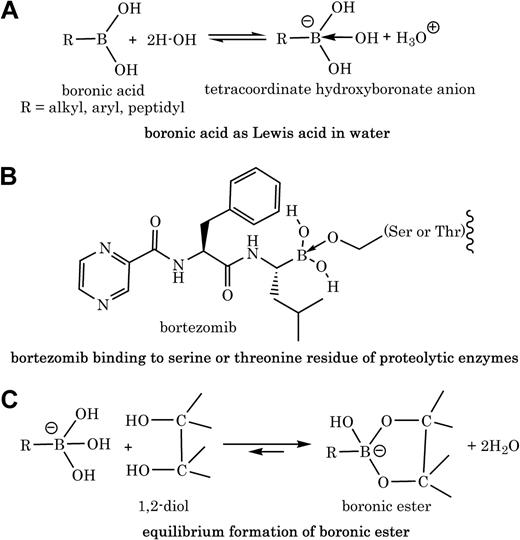
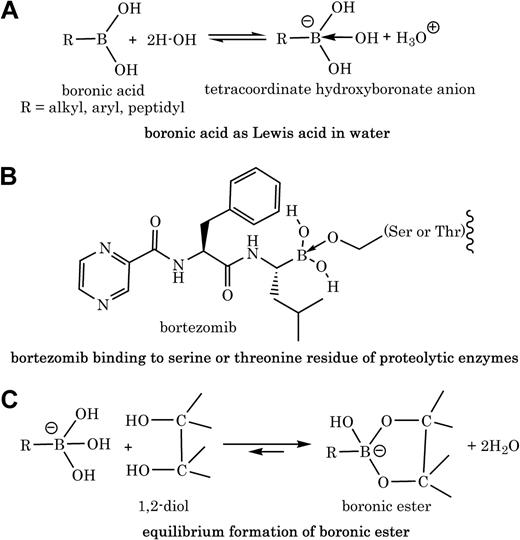
Boronic acids contain a trivalent boron to which is attached one organic substituent (ie, a carbon-boron bond in forms of alkyl, aryl, or peptidyl) and 2 hydroxyl groups (Figure 1A). This structure is consistent with the sp2 -hybridized boron with 6 valence electrons and possession of a low-energy vacant p-orbital that can accept 2 electrons from basic molecules. Thus, the acidic character of most boronic acids is usually that of a Lewis acid (ie, accepting electrons) and not that of Bronsted acid (ie, releasing proton; Figure 1A). These chemical properties translate well into biology, because the activity of peptidyl boronic acids like bortezomib as serine protease inhibitors or proteasome inhibitors is attributed to the availability of the empty p-orbital on boron, which accepts electrons from serine2 or threonine3 hydroxyl groups in the active sites of enzymes (Figure 1B).
Polyol molecules with vicinal (1,2 or 1,3) hydroxyl groups such as polyphenols react with boronic acids to form boronic esters.4 Various factors including solvent, pH, buffer, and ionic states affect the binding affinity between a boronic acid moiety and a diol. For example, it was shown that the tetracoordinate hydroxyboronate anion, in aqueous solution, is at least 103 to 104 times more reactive than the trigonal neutral boronic acid in forming esters with diols (Figure 1C).5 Schonthal and colleagues proved that there is a direct chemical interaction between bortezomib and green tea polyphenols; however, the provided “selected” peaks from the nuclear magnetic resonance spectra with no mass spectrometry data do not give sufficient information about the structures of products (ie, cyclic boronate ester adducts).
Regardless of the exact reaction outcomes, which can be the subject of the future research, we fully agree with Schonthal et al that patients undergoing bortezomib therapy must abstain from consuming green tea products. In addition, since this appears to be a class effect (ie, boronic acid–based drugs will likely bind polyphenols with similar structures), we suggest that other food products that contain high concentrations of polyphenols, such as berries, red wine, walnuts, peanuts, and pomegranates, should also be avoided by patients who are receiving bortezomib. In addition, future trials with bortezomib should require patients not to consume green tea or other sources of polyphenols while receiving treatment. As shown by Schonthal et al, newer non–boronic acid–based proteosome inhibitors are unlikely to have such reactions with polyphenols.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashkan Emadi, MD, PhD, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, The Bunting/Blaustein Cancer Research Bldg, 1650 Orleans St, Suite 191, Baltimore, MD 21231-1000; e-mail: aemadi1@jhmi.edu