Abstract
Pralatrexate is a novel antifolate, which shows increased antitumor activity in human tumor xenograft studies in mice compared with methotrexate. We investigated the effects of pralatrexate in a patient with adult T-cell lymphoma/leukemia with significant skin involvement. Atypical lymphocytes in epidermal Pautrier microabscesses were positive for HTLV-1. After the patient presented with leukemic conversion and with worsening of an erythematous generalized papular rash, he received one dose of pralatrexate. Within one week, his skin developed innumerable small erosions limited to the areas of the papular rash, sparing unaffected skin. Here we present in vivo evidence that pralatrexate-induced erosions in skin affected by adult T-cell lymphoma/leukemia are a manifestation of apoptosis of tumor cells infiltrating the epidermis and are not the result of cytotoxicity by pralatrexate on keratinocytes. This distinction is critical and may profoundly influence the clinical decision to continue pralatrexate treatment. Pralatrexate-induced skin erosions may indicate response to treatment.
Introduction
Pralatrexate, a 10-deazaaminopterin, is a novel inhibitor of dihydrofolate reductase. It is a rationally designed molecule with increased affinity for the reduced folate carrier type 1 (RFC-1), which transports reduced natural folates into rapidly proliferating cells. RFC-1 is highly expressed in embryonic and malignant tissues. In preclinical studies, pralatrexate has shown greater antitumor activity compared with methotrexate.1 Human tumor xenograft studies in mice (including human breast cancer and human lung cancer xenografts) demonstrated improved regression of tumors with pralatrexate treatment, whereas methotrexate treatment resulted only in delayed tumor growth without a sustained regression at comparable doses.2,3 Comparison of the efficacy of pralatrexate and methotrexate against several lymphoma cell lines (including diffuse large B-cell lymphoma, Burkitt lymphoma, transformed follicular lymphoma, T-cell lymphoma, and Hodgkin disease) showed a more than 10-fold increased in vitro cytotoxicity in pralatrexate-treated cells and improved regression of xenografts in nonobese diabetic/severe combined immunodeficiency mice.3 Notably, increased sensitivity to pralatrexate correlated with higher RFC-1 expression.
The ability of pralatrexate to induce apoptosis was shown in several lymphoma cell lines in vitro.4 Increased apoptosis was also demonstrated in vivo by caspase 3 labeling of tumor xenograft biopsies in mice.
However, direct evidence of tumor cell apoptosis has not been demonstrated in patients treated with pralatrexate. Clinical studies have demonstrated that pralatrexate has substantial activity in patients with T-cell lymphomas and non-small cell lung cancer.5,6 A phase 1 or 2 study of pralatrexate in 54 patients with B-cell or T-cell lymphomas demonstrated an overall response rate of 28%, with an overall response rate of 45% in patients with T-cell lymphoma6,7 (and O.A.O., unpublished data, May 2009). These responses were seen primarily in patients with refractory disease and, in general, lasted between 6 and 24 months. In this initial experience, in 2 patients with T-cell lymphoma, pralatrexate treatment was associated with skin erosions, so severe in one patient whose treatment had to be discontinued. The pathologic mechanisms underlying the formation of skin erosions in patients with cutaneous lymphoma treated with pralatrexate have not been elucidated. The dose-limiting toxicity of pralatrexate is severe stomatitis, which occurs more frequently in patients with elevated pretreatment levels of homocysteine and methylmalonic acid.6 This toxicity can be reduced by normalizing homocysteine and methylmalonic acid levels through supplementation of folic acid and vitamin B12. Thus, it is presently unknown whether the infrequently observed pralatrexate-induced skin erosions in patients with cutaneous lymphoma result from a direct toxic effect of pralatrexate on keratinocytes or from an apoptotic effect on atypical lymphocytes in the epidermis. This distinction is critical, as it could profoundly influence the decision-making regarding continuation of treatment.
We report here on a patient with adult T-cell lymphoma/leukemia (ATLL) treated with a single dose of pralatrexate that resulted in the development of innumerable skin erosions only in skin affected by ATLL. The skin erosions were the result of tumor cell lysis of HTLV-1–positive atypical lymphocytes in Pautrier microabscesses in the epidermis resulting from apoptosis. No apoptosis or signs of cytotoxicity were seen in adjacent keratinocytes. The erosions healed within a few days, and repeat exposure to pralatrexate did not cause any further skin erosions.
Methods
Skin biopsies and histologic preparation
Skin biopsies were fixed in formaldehyde and embedded in paraffin according to standard protocols. Hematoxylin and eosin staining was performed for histologic evaluation. Patient informed consent was obtained in accordance with Columbia University institutional guidelines and the Declaration of Helsinki.
Immunohistochemistry for activated caspase 3 and immunophenotyping of atypical lymphocytes
Immunohistochemistry was performed according to standard protocols. We used the following primary antibodies for immunohistochemistry: anticaspase 3 rabbit polyclonal antibody (Abcam, Cambridge, MA) at 1:25 dilution, anti-CD2 mouse monoclonal antibody (Vector Laboratories, Burlingame, CA) at 1:50 dilution, anti-CD5 mouse monoclonal antibody (Dako North America, Carpinteria, CA) at 1:40 dilution, anti-CD4 mouse monoclonal antibody (Biogenex, San Ramon, CA) at 1:60 dilution, anti-CD7 mouse monoclonal antibody (Vector Laboratories) at 1:40 dilution, and anti-CD8 mouse monoclonal antibody (Dako North America) at 1:50 dilution.
HTLV-1 in situ hybridization and immunohistochemistry
The in situ hybridization protocol has been previously described.8,9 The primers used recognized the HTLV-1 gag region (UP 5′-CTGCAGTACCTTTGCTCCTCCCTC-3′ and DW 5′-CCCGGGGGGGGGACGAGGCT-3′). Nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate was used as the chromogen with nuclear fast red as the counterstain. Serial sections were used to assure that the same groups of cells were examined. Negative controls for the HTLV-1 cDNA-based signal included the omission of the primers and the use of HTLV-2 primers (UP 5′-ATCTACCTCCACCATGTCCG-3′ and DW 5′-TCAGGGGAACAAGGGGAGCT-3′). The signal localized to the atypical mononuclear cells, which served as another indicator of the specificity of the reaction. For immunohistochemistry, we used an anti–HTLV-1 antibody that detects the p19 protein (Abcam) at a dilution of 1:250.
Results and discussion
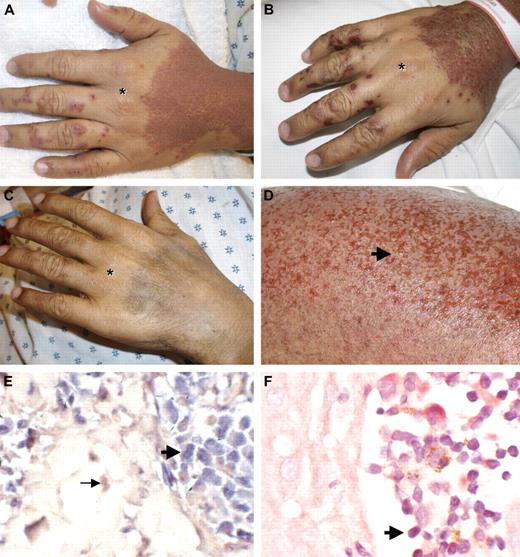
We report here the case of a patient with significant skin involvement with HTLV-1–associated ATLL who developed widespread skin erosions after receiving one dose of pralatrexate (270 mg/m2; Figure 1A,D). The patient is a 33-year-old man from the Dominican Republic who was diagnosed 20 months earlier with stage I HTLV-1–associated ATLL on lymph node biopsy (HTLV-1 antibody titer positive). Initially, he was treated with radiation to the affected lymph node. Fifteen months later, he experienced a systemic relapse of his disease with widespread adenopathy and bone marrow involvement (stage IVb). He received chemotherapy with 2 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) followed by 4 cycles of EPOCH (infusional CHOP with etoposide), resulting in significant interval resolution of his disease. However, within 2 months of completing therapy, he developed new left inguinal adenopathy. At the same time, he developed a rash on the forearms presenting with multiple small (1-2 mm) skin-colored papules. A skin biopsy of these papules showed Pautrier microabscesses with atypical cells in the epidermis (Figure 2A). Immunophenotyping demonstrated that these cells were atypical T cells, positive for CD4, CD5, and CD2 but negative for CD7 and CD8. T-cell receptor-β gene rearrangement studies confirmed clonality of the atypical lymphocytes. The patient was started on azidothymidine and interferon-α2b therapy. Two months later, he presented with generalized fatigue and a leukocytosis (34 000 white blood cell count with 57% atypical lymphocytes and clover cells on peripheral smears), consistent with leukemic conversion of his ATLL. His rash progressed; and within a few weeks, he developed widespread erythematous papules forming confluent plaques over his entire body surface, with areas of sparing at his hands and lower extremities. Pompholyx-like lesions were noted on his fingers (Figure 1A,B).
Pralatrexate induces skin erosions limited to areas affected by ATLL. (A) ATLL skin lesions with confluent erythematous papules of the dorsal hands with areas of sparing over knuckles (*). Pompholyx-like lesions on digits. Pictures were taken 3 days after the first dose of pralatrexate was given. (B) Skin erosions within affected areas 6 days after the first dose of pralatrexate was administered. (C) Resolution of rash with postinflammatory hyperpigmentation 3 months later. (D) Higher magnification of skin erosions (arrow) on the right leg 6 days after the first dose of pralatrexate. (E) In situ hybridization for HTLV-1 shows positive signal within atypical lymphocytes in the epidermis (bold arrow), whereas red counterstain is observed in dermal fibroblast (→; original magnification ×40). (F) Immunohistochemistry for HTLV-1 shows positive signal in atypical lymphocytes in Pautrier microabscesses ( ; original magnification ×40).
; original magnification ×40).
Pralatrexate induces skin erosions limited to areas affected by ATLL. (A) ATLL skin lesions with confluent erythematous papules of the dorsal hands with areas of sparing over knuckles (*). Pompholyx-like lesions on digits. Pictures were taken 3 days after the first dose of pralatrexate was given. (B) Skin erosions within affected areas 6 days after the first dose of pralatrexate was administered. (C) Resolution of rash with postinflammatory hyperpigmentation 3 months later. (D) Higher magnification of skin erosions (arrow) on the right leg 6 days after the first dose of pralatrexate. (E) In situ hybridization for HTLV-1 shows positive signal within atypical lymphocytes in the epidermis (bold arrow), whereas red counterstain is observed in dermal fibroblast (→; original magnification ×40). (F) Immunohistochemistry for HTLV-1 shows positive signal in atypical lymphocytes in Pautrier microabscesses ( ; original magnification ×40).
; original magnification ×40).
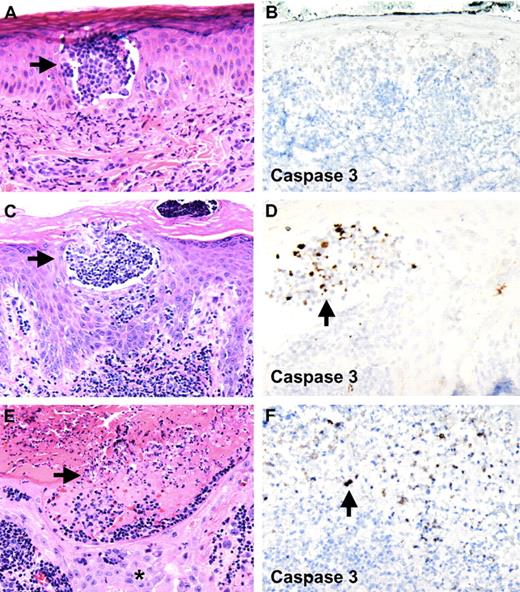
Pralatrexate induces tumor cell lysis within epidermal Pautrier microabscesses without affecting keratinocytes. Apoptosis of atypical lymphocytes is demonstrated with staining for activated caspase 3. (A,B) First skin biopsy of papule 2 months before initiation of pralatrexate therapy. Pautrier microabscess in epidermis is seen. No staining for activated caspase 3 is observed (original magnifications: A, ×10; B, ×10). (C,D) Skin biopsy 3 days after the first dose of pralatrexate was given shows atypical cells within epidermal Pautrier microabscesses undergoing apoptosis, whereas no activated caspase 3 staining is observed in adjacent keratinocytes (original magnifications: C, ×10; D, ×20). (E,F) Skin biopsy of skin erosion 6 days after pralatrexate therapy was initiated. Necrosis and cellular debris are seen within epidermal Pautrier microabscesses accompanied by positive staining for activated caspase 3. No necrosis is seen in adjacent keratinocytes (*, original magnifications: E, ×10; F, ×20). Slides were viewed with a Zeiss Axio Scope light microscope using N-ACHROPLAN objectives (10×/0.25, 20×/0.45, and 40×/0.65), and images were acquired with an AxioCam HR camera (Carl Zeiss AG). Images were processed using Image-Pro Plus version 5.0 (Media Cybernetics).
Pralatrexate induces tumor cell lysis within epidermal Pautrier microabscesses without affecting keratinocytes. Apoptosis of atypical lymphocytes is demonstrated with staining for activated caspase 3. (A,B) First skin biopsy of papule 2 months before initiation of pralatrexate therapy. Pautrier microabscess in epidermis is seen. No staining for activated caspase 3 is observed (original magnifications: A, ×10; B, ×10). (C,D) Skin biopsy 3 days after the first dose of pralatrexate was given shows atypical cells within epidermal Pautrier microabscesses undergoing apoptosis, whereas no activated caspase 3 staining is observed in adjacent keratinocytes (original magnifications: C, ×10; D, ×20). (E,F) Skin biopsy of skin erosion 6 days after pralatrexate therapy was initiated. Necrosis and cellular debris are seen within epidermal Pautrier microabscesses accompanied by positive staining for activated caspase 3. No necrosis is seen in adjacent keratinocytes (*, original magnifications: E, ×10; F, ×20). Slides were viewed with a Zeiss Axio Scope light microscope using N-ACHROPLAN objectives (10×/0.25, 20×/0.45, and 40×/0.65), and images were acquired with an AxioCam HR camera (Carl Zeiss AG). Images were processed using Image-Pro Plus version 5.0 (Media Cybernetics).
The patient was enrolled in a phase 2 study of pralatrexate administered (270 mg/m2) on an every-other-week basis. He also received folic acid and vitamin B12 supplementations to reduce the occurrence of severe stomatitis. Within 10 days of receiving his first dose of pralatrexate, his white blood cell count decreased to 16 400 with 8% atypical lymphocytes (from 31 900 with 57% atypical lymphocytes). Adverse effects included intermittent episodes of thrombocytopenia and stomatitis. Three days after the first dose of pralatrexate, the patient reported increased pruritus. Skin biopsies were obtained from the papular eruption on the back and from the pompholyx-like lesions on the digits. Both skin biopsies showed Pautrier microabscesses in the epidermis filled with atypical lymphocytes (Figure 2C). In addition, atypical lymphocytes were seen in the dermis. We performed in situ hybridization studies and immunohistochemistry for HTLV-1 on these biopsies, confirming a positive signal in the atypical T cells (Figure 1E,F). The HTLV-1-positive cells in the Pautrier microabscesses showed enlarged nuclei, a high nuclear/cytoplasmic ratio, and nuclear atypia, consistent with atypical T cells of ATLL. Within one week, the patient's rash progressed to develop innumerable pinpoint-sized erosions, strictly limited to areas affected by the papular rash (Figure 1B,D). Two skin biopsies were obtained from these erosions. Histologic examination revealed that epidermal Pautrier microabscesses showed extensive cellular debris, with normal-appearing adjacent keratinocytes (Figure 2E). These findings suggest that pralatrexate therapy induced selective apoptosis of atypical tumor cells without affecting adjacent normal keratinocytes.
To test this hypothesis, we performed immunohistochemical labeling for activated caspase 3, a marker for cells undergoing apoptosis, on all skin biopsies obtained from this patient. The first skin biopsy of his rash, obtained 2 months before pralatrexate therapy, was completely negative for caspase 3 staining (Figure 2B). Skin biopsies of his rash obtained 3 days after pralatrexate treatment was initiated showed a large number of cells staining positive for caspase 3 (Figure 2D). These cells were found mainly within Pautrier microabscesses and not in adjacent epidermis. Caspase 3 staining on sections obtained from skin biopsies of the erosions (6 days after pralatrexate treatment) showed numerous positive cells within areas of cellular debris in Pautrier microabscesses, again without labeling adjacent keratinocytes (Figure 2F). No skin biopsy showed evidence for a drug hypersensitivity reaction, such as erythema multiforme, fixed-drug eruption, or toxic epidermal necrolysis.
The erosions healed within a few days and did not recur with subsequent cycles of pralatrexate therapy. Indeed, complete resolution of the rash was observed, and only postinflammatory hyperpigmentation persisted at previously affected skin areas (Figure 1C).
These findings are consistent with pralatrexate-induced tumor cell apoptosis that leads to erosions at areas of Pautrier microabscesses. Skin erosions in patients with cutaneous lymphoma may reflect response to pralatrexate treatment and may not be a sign of a hypersensitivity reaction or toxicity to this novel medication.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The patient received pralatrexate as part of a clinical study supported in part by ALLOS Therapeutics (Westminster, CO). HTLV-1 immunohistochemistry materials were provided by Dr Christopher Roberts and Ms Kathleen Sergott (Ventana Medical Systems, Tucson, AZ).
Authorship
Contribution: A.G.M. contributed to the dermatologic diagnosis and management of the patient, planned the experiments and performed the skin biopsies, analyzed and assembled the data, histology, and photographs, and wrote the manuscript; M.E.G. contributed to the dermatologic diagnosis and management of the patient; D.N.S. and S.H. performed histopathologic diagnosis; G.J.N. performed HTLV-1 studies; and B.M.-C., E.N., M.P., O.A.O., and J.M.Z. managed pralatrexate therapy as part of a clinical study and contributed to the accumulation of clinical data and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Owen A. O'Connor, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY 10032; e-mail: oo2130@columbia.edu; or Alexander G. Marneros, Cutaneous Biology Research Center, Massachusetts General Hospital, Bldg 149, 13th St, Charlestown, MA 02129; e-mail: alexander_marneros@yahoo.com.