Abstract
Acute myeloid leukemia (AML) patients with internal tandem duplication (ITD) mutations in the Fms-like tyrosine-3 (FLT3) gene have a dismal prognosis. Here we report compassionate-use results with the multikinase and FLT3-ITD inhibitor sorafenib for the treatment of relapsed or refractory FLT3-ITD–positive AML. Sorafenib induced clinically meaningful and very rapid responses in all 6 patients treated either before (n = 2), after (n = 3), or both before and after (n = 1) allogeneic stem cell transplantation (allo-SCT). Sorafenib-induced remissions facilitated allo-SCT in 2 of the 3 refractory patients. Two of the 4 patients who were treated after allo-SCT survived 216 and 221 days, respectively, whereas the other 2 remain in ongoing complete molecular remission. Sorafenib response was associated with an inhibition of the antiapoptotic FLT3-ITD target Stat-5 in vivo. Together, sorafenib monotherapy before or after allo-SCT has remarkable clinical activity in poor risk FLT3-ITD–positive AML and deserves further evaluation in prospective clinical trials.
Introduction
Acute myeloid leukemia (AML) is the most frequent acute leukemia of adults and has an unfavorable prognosis.1 In 20% to 30% of the AML patients, mutations in the Flt3-receptor tyrosine kinase (Flt3-RTK) occur, leading to internal tandem duplications in the juxtamembrane domain of the receptor (FLT3-ITD).2,3 FLT3-ITD dictates a particularly poor clinical outcome.4-8 Therefore, several specific FLT3 inhibitors have been developed and evaluated in clinical trials.9 However, their overall clinical efficacy in AML must so far be considered as minor.9
Sorafenib (Nexavar, formerly BAY 43-9006; Bayer HealthCare) has been approved for the treatment of metastatic renal cancer and advanced hepatocellular carcinoma.10 It inhibits the serine threonine kinase RAF-1, but also the FLT3-RTK and FLT3-ITD,11 suggesting that may have a role also in AML.12 Sorafenib was recently tested in a phase 1 clinical trial on 16 patients with AML and was found to be particularly active in 6 of the 7 Flt3-ITD–positive patients.11 However, treatment duration was short (21-70 days), and no durable responses were reported. Notably, a complete molecular remission has recently been reported in a patient relapsing after stem cell transplantation (SCT).13 We here show, in a cohort of 6 FLT3-ITD–positive AML patients, that single-agent sorafenib given on a compassionate-use basis led to notable long-term responses in refractory and relapsed disease before and after allogeneic SCT (allo-SCT).
Methods
Patients, treatment, and objective
From November 2007 to November 2008, refractory or relapsed Flt3-ITD–positive AML patients (n = 6) before or after allo-SCT were treated after informed consent at the University of Marburg with sorafenib on a compassionate-use basis. Treatment was performed in the absence of alternative therapeutic options outside a clinical trial. The initial dose was 2 × 400 mg orally daily and was adjusted in case of cytopenia, suspected toxicity, or resistance (dose range, 200-800 mg daily).
Response
Treatment response was monitored and remission was defined according to standard criteria14 : (1) complete remission (CR): marrow blasts less than 5%, neutrophils more than 109/L, platelets more than 100 × 109/L for at least 4 weeks; (2) bone marrow response (BMR): marrow blasts reduction by more than 50% from start of sorafenib without hematologic recovery; (3) hematologic response (HR): disappearance of blasts from the peripheral blood; and (4) complete molecular response (CMR): CR plus molecular negativity for FLT3-ITD by polymerase chain reaction.
Intracellular p-STAT-5 staining
Intracellular staining with phycoerythrin-conjugated antiphospho-STAT-5 (Y694) was performed as previously described15 after informed consent was obtained in accordance with the Declaration of Helsinki, according to a vote of the local ethics committee of the University of Marburg.
Results and discussion
Sorafenib monotherapy in relapsed FLT3-ITD–positive AML after allo-SCT
Four patients (median age, 50 years; range, 42-62 years) with relapsed FLT3-ITD–positive AML after allo-SCT were treated with sorafenib on a compassionate-use basis. The median time from allo-SCT to relapse was 192 days (range, 87-322 days). All patients had a normal karyotype (treatment and response details, Table 1).
Patient 1 was a 62-year-old man with AML-M4 (FLT3-ITD ratio, 11.4). After daunorubicin/cytarabine (DA) induction therapy and one consolidation cycle, he underwent allo-SCT in first CR but relapsed. He obtained a rapid HR and BMR under sorafenib. Because of leucopenia, sorafenib was repeatedly paused and the sorafenib dose level was reduced. The patient died of AML progression that was resistant to sorafenib on day 216 (Table 1).
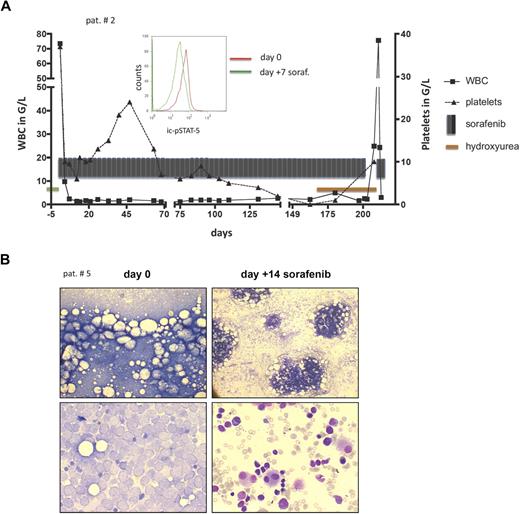
Patient 2 was a 42-year-old woman with normal karyotype AML-M4 (FLT3-ITD ratio, 1.15). After 2 DA-induction cycles, she underwent allo-SCT in first CR and relapsed despite experiencing graft-versus-host disease (GVHD; intestine and skin). With sorafenib at 400 mg orally twice a day, she obtained a rapid and sustained HR and BMR (Figure 1A; Table 1). Sorafenib inhibited the antiapoptotic FLT3-ITD target STAT-5 in vivo (Figure 1A). Concomitantly to an increasing donor chimerism (from 47% to 91%), GVHD reoccurred, indicating that sorafenib did not compromise a graft-versus-leukemia response. The patient died on day 221 of sorafenib but remained essentially sorafenib-sensitive (Figure 1A).
Sorafenib treatment response. (A) Patient 2: A fast response to sorafenib was associated with a dephosphorylation of the antiapoptotic FLT3-ITD target STAT-5, measured intracellularly in the blastic population by fluorescence-activated cell sorter (middle panel histogram): the left shift of the green curve documents a decrease in intracellular STAT-5 phosphorylation 7 days after commencing sorafenib compared with baseline (day 0, red line). Sorafenib was withdrawn on day 204 of sorafenib and hydroxyurea commenced because of a newly diagnosed cerebral mass. AML was resistant to hydroxyurea (peripheral white blood cell count increase to 75.6 × 109/L), but reexposure to sorafenib again led to an instant response (white blood cell count decline to 1.3 × 109/L). (B) Patient 5: Bone marrow light microscopy to evaluate bone marrow response (patient 5) before allo-SCT. Giemsa staining shows blast infiltration before (left panels) and extensive blast clearance 14 days after commencing sorafenib (right panels). Low and high magnification of bone marrow smear (top and bottom panels, respectively). Images were viewed with an Olympus BH-2 microscope using Giemsa stain; left panel, 10×/0.3 numeric aperture (NA); right panel, 60×/1.4 NA oil objective. Images were taken with a Sony Exwave HAD camera using DHS Bilddatenbank software (Dietermann & Heuser Solution GmbH, Greifenstein-Beilstein, Germany).
Sorafenib treatment response. (A) Patient 2: A fast response to sorafenib was associated with a dephosphorylation of the antiapoptotic FLT3-ITD target STAT-5, measured intracellularly in the blastic population by fluorescence-activated cell sorter (middle panel histogram): the left shift of the green curve documents a decrease in intracellular STAT-5 phosphorylation 7 days after commencing sorafenib compared with baseline (day 0, red line). Sorafenib was withdrawn on day 204 of sorafenib and hydroxyurea commenced because of a newly diagnosed cerebral mass. AML was resistant to hydroxyurea (peripheral white blood cell count increase to 75.6 × 109/L), but reexposure to sorafenib again led to an instant response (white blood cell count decline to 1.3 × 109/L). (B) Patient 5: Bone marrow light microscopy to evaluate bone marrow response (patient 5) before allo-SCT. Giemsa staining shows blast infiltration before (left panels) and extensive blast clearance 14 days after commencing sorafenib (right panels). Low and high magnification of bone marrow smear (top and bottom panels, respectively). Images were viewed with an Olympus BH-2 microscope using Giemsa stain; left panel, 10×/0.3 numeric aperture (NA); right panel, 60×/1.4 NA oil objective. Images were taken with a Sony Exwave HAD camera using DHS Bilddatenbank software (Dietermann & Heuser Solution GmbH, Greifenstein-Beilstein, Germany).
Patient 3 was a 46-year-old woman with FLT3-ITD–positive AML-M2 (FLT3-ITD ratio, 4.2). After 2 DA-induction cycles, she underwent allo-SCT in first CR but relapsed (Table 1). She also had experienced chronic GVHD of the skin, joints, and polyserositis requiring immune suppressive therapy with steroids. The relapse was treated with sorafenib 400 mg orally twice a day; and within 2 months, a still ongoing CMR was achieved.
Sorafenib facilitates allo-SCT in chemotherapy-refractory FLT3-ITD–positive AML
Three patients were rescued with sorafenib before allo-SCT. The median age of these patients was 40 years (range, 38-57 years).
Patient 4 was a 56-year-old man with FLT3-ITD–positive (FLT3-ITD ratio, 1.16) AML, who relapsed after one cytarabine consolidation cycle. The patient's clinical status was poor. Under 400 mg sorafenib, twice a day, he instantly achieved a HR (Table 1) and thrombopoiesis recovered in part (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Sorafenib inhibited STAT-5 activation in AML blasts in vivo, confirming in vivo the presumed mode of action of sorafenib via inhibiting antiapoptotic targets of FLT3-ITD (Figure S1).
Patient 5 was a 40-year-old woman with AML-M5a (FLT3-ITD ratio, 0.8) experiencing primary induction failure. Sorafenib was commenced; and within 14 days of treatment, a fast HR and BMR were seen (Figure 1B). The patient underwent allo-SCT using a dose-reduced conditioning regimen.16 She achieved a CR but relapsed again on day 111 after allo-SCT. Sorafenib was restarted at 400 mg twice a day, and a still ongoing CMR was obtained (Table 1).
Patient 6 was a 38-year-old man with primary chemotherapy–refractory AML-M2. He had the lowest FLT3-ITD ratio in this cohort (0.39). A donor was not available, and sorafenib was commenced at 400 mg twice a day on an outpatient basis. An HR was achieved (Table 1). The patient relapsed on day 71 of sorafenib; but at this time, allo-SCT could be performed. The patient remained in CR at last visit.
Here we support and significantly extend initial findings on the efficacy of sorafenib monotherapy in AML.11,13 Apparently, sorafenib has a consistent activity in relapsed and refractory FLT3-ITD–positive AML. With a median treatment duration of 158 days after allo-SCT (range, 90-221 days), only 2 patients developed sorafenib drug resistance, whereas 2 patients obtained an ongoing CMR. Based on these observations and the limited literature on sorafenib monotherapy in AML, it is tempting to speculate that sorafenib may be most effective when applied in the context of an allo-SCT because it has the potential to reduce leukemic burden, without compromising the restoration of a graft-versus-leukemia response (patients 2, 3, and 5). This is similar to the successful scenario seen with preemptive imatinib treatment after allo-SCT in BCR/ABL+ acute lymphatic leukemia (Ph+ ALL).17
However, sorafenib could also be a valuable compound to bridge the time to allo-SCT. It spared chemotherapy toxicity while still exerting antileukemic activity (patients 5, 6). Equally important in this regard, circumventing ineffective cycles of chemotherapy before allo-SCT was shown to translate into better overall survival in high-risk AML.16,18 Notably, sorafenib did not adversely affect engraftment in patients 5 and 6. However, long-term sorafenib treatment was associated with neutropenia and thrombopenia in many patients. This is not typically seen during sorafenib treatment of renal or hepatic cancer10 but may be explained by the compromised normal bone marrow reservoir in relapsed AML after allo-SCT.
Taken together, the role of sorafenib monotherapy in FLT3-ITD–positive AML deserves evaluation in clinical trials. Based on its ability to induce CMRs in relapsed patients after allo-SCT, a prophylactic treatment strategy after allo-SCT may hold the promise to markedly improve the poor outcome of FLT3-ITD–positive AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Atta (University of Frankfurt) and Drs Ritter and Wolf (Kassel Hospital) for excellent cooperation in treating patients reported in this study.
This work was supported by the Deutsche José Carreras Leukämie-Stiftung eV (DJCLS 06/01v; A.B.), the Deutsche Forschungsgemeinschaft (KFO210, BU 1321/3-1, NE 310/14-1; A.B., A.N.), and by the University Medical Center Giessen and Marburg (a research grant; A.B.).
Authorship
Contribution: A.B., A.N., and S.M. designed the research and analyzed the data; A.B. wrote the paper; S.M., Y.W., M.W., S.T., and A.C. performed the in vitro research; A.B., E.W., S.M., and A.N. treated the patients; and M.W., E.E., and M.E. contributed vital reagents and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Burchert, Philipps Universität Marburg, Universitätsklinikum Giessen und Marburg, Standort Marburg, Klinik für Hämatologie, Onkologie und Immunologie, 35043 Marburg, Germany; e-mail: burchert@staff.uni-marburg.de; or Andreas Neubauer, Philipps Universität Marburg, Universitätsklinikum Giessen und Marburg, Standort Marburg, Klinik für Hämatologie, Onkologie und Immunologie, 35043 Marburg, Germany; e-mail: neubauer@staff.uni-marburg.de.