Abstract
Conflicting data have been reported on the risk for venous thrombosis in subjects with low free protein S levels. We performed a post-hoc analysis in a single-center retrospective thrombophilic family cohort, to define the optimal free protein S level that can identify subjects at risk for venous thrombosis. Relatives (1143) were analyzed. Relatives with venous thrombosis (mean age 39 years) had lower free protein S levels than relatives without venous thrombosis (P < .001), which was most pronounced in the lowest quartile. Only relatives with free protein S levels less than the 5th percentile (< 41 IU/dL) or less than the 2.5th percentile (< 33 IU/dL) were at higher risk of first venous thrombosis compared with the upper quartile (> 91 IU/dL); annual incidence 1.20% (95% confidence interval [CI], 0.72–1.87) and 1.81% (95% CI, 1.01–2.99), respectively; adjusted hazard ratios 5.6, (95% CI, 2.7–11.5) and 11.3 (95% CI, 5.4–23.6). Recurrence rates were 12.12% (95 CI, 5.23–23.88) and 12.73% (95% CI, 5.12–26.22) per year; adjusted hazard ratios were 3.0 (95% CI, 1.03–8.5) and 3.4 (95% CI, 1.1–10.3). In conclusion, free protein S level can identify young subjects at risk for venous thrombosis in thrombophilic families, although the cutoff level lies far below the normal range in healthy volunteers.
Introduction
Protein S is a vitamin K–dependent plasma glycoprotein that functions as a nonenzymatic cofactor of activated protein C in the inactivation of the procoagulant factors Va and VIIIa, and plays an important role in regulating thrombin generation.1 Approximately 60% of total protein S is bound to complement component C4b-binding protein.2 Until recently, it was believed that only free protein S has activated protein C cofactor activity. However, new evidence suggests that both bound and free protein S are cofactors for activated protein C.3 Three subtypes of protein S deficiency are recognized: type I, with decreased levels of both total and free protein S antigen (ie, a quantitative defect); type II, with total and free protein S antigen levels within their normal ranges, but decreased protein S activity (ie, a qualitative defect); and type III, with decreased free protein S and normal total protein S antigen levels.4 Whereas type I deficiency is an established risk factor for venous thrombosis,5,6 conflicting data have been reported on the risk of thrombosis associated with type III deficiency.6-14 A difference in risk between subjects with type I and type III deficiency, together with variation in the studied populations may explain this discrepancy. Another explanation could be that the cutoff level for protein S deficiency type III (ie, the lower limit of the normal range; in our laboratory < 65 IU/dL) is too high to identify subjects at risk for venous thrombosis.11 Moreover, studies that did not show an increased risk of venous thrombosis in association with free protein S deficiency may have been confounded by the inclusion of subjects with mild decrements in free protein S levels. The diagnostic criteria for type III protein S deficiency therefore likely need to be reconsidered.
We performed a retrospective study in a large series of families to assess the absolute risk of first venous thrombosis and its recurrence for different free protein S levels, to define an optimal cutoff level of free protein S to identify subjects at risk for venous thrombosis.
Methods
Data retrieval
We pooled data of individual subjects from 5 large retrospective family cohort studies with various thrombophilic index defects, which have been described previously.5,11,15-17 These studies had the same design and were performed by 3 university hospitals. The first 2 studies were single centered and performed in our hospital. It comprised first-degree relatives (ie, offspring, siblings, and/or parents) of consecutive patients (probands) with documented venous thrombosis and established hereditary deficiencies of either antithrombin, protein C, or protein S.5,11 As the number of antithrombin deficient probands was small, second-degree relatives (ie, grandparents and/or blood related uncles or aunts) with a deficient parent were also identified. They were enrolled between April 1999 and July 2004. Three studies were multicenter studies of first-degree relatives of consecutive patients with venous thrombosis or premature atherosclerosis (< 50 years of age) and the presence of either the prothrombin G20210A mutation, high levels of factor VIII at repeated measurements, or hyperhomocysteinemia.15-17 Enrollment started in May 1998 and was completed in July 2004. Probands were tested on multiple thrombophilic defects in all 5 studies. When multiple defects were demonstrated, probands were randomly assigned to one of the 5 study cohorts.5,11,15-17 As laboratory tests were locally performed, using different free protein S assays within the 3 centers and different calibration lines, we chose to only include the data obtained from subjects in our center. Approval was obtained from the institutional review board of University Medical Center Groningen.
Subjects
All relatives, identified by pedigree analysis, were 15 years of age or older and were contacted through the probands. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Physicians at our thrombosis outpatient clinic collected detailed information about previous episodes of venous thrombosis, exposure to exogenous risk factors for venous thrombosis, and anticoagulant treatment using a validated questionnaire,18 and by reviewing medical records. Clinical data were collected before laboratory testing. Relatives were tested for hereditary deficiencies of antithrombin, protein C and protein S type I, factor V Leiden, prothrombin G20210A, and high levels of factor VIII, regardless of their index defects. In addition, levels of free protein S were measured in most, but not all, relatives due to shortage of stored plasma. To avoid bias, relatives with protein S deficiency type I as well as all probands were excluded from the analyses.
Laboratory studies
Activity of antithrombin (Chromogenix, Mölndal, Sweden) and protein C (Behring, Marburg, Germany) were measured by chromogenic substrate assays, protein C, and protein S antigen levels by enzyme-linked immunosorbent assay (ELISA; DAKO, Glostrup, Denmark). Antithrombin deficiency was defined by decreased levels of antithrombin activity (< 70 IU/dL), protein C deficiency by decreased levels of either protein C antigen (< 65 IU/dL) and/or activity (< 65 IU/dL), and protein S deficiency type I by decreased total (< 65 IU/dL) and free protein S antigen levels (< 65 IU/dL), corresponding with plasma levels below the lower limit of their normal ranges. Strict criteria for inheritance of deficiencies were used.5 Free protein S antigen levels were measured after precipitation of protein S complexed with C4b-binding protein with polyethylene glycol.19 Normal values were obtained from a group of healthy volunteers, balanced for gender, without a history of venous thrombosis. The female volunteers did not use oral contraceptives and were not pregnant. Factor V Leiden and prothrombin G20210A were demonstrated by polymerase chain reactions (PCRs).20,21 Factor VIII:C levels were measured by one-stage clotting assays and were considered increased at levels above 150 IU/dL.16 If relatives were on treatment with acenocoumarol, a short-acting vitamin K antagonist, blood samples were taken after treatment had been interrupted for at least 2 weeks if possible; meanwhile, nadroparin was given subcutaneously.
Definitions
The first episode of venous thrombosis was considered established if deep vein thrombosis was confirmed by compression ultrasound or venography, and pulmonary embolism by ventilation/perfusion lung scanning, spiral computed tomography (CT) scanning or pulmonary angiography, or when the patient had received full-dose heparin and a vitamin K antagonist for at least 3 months without objective testing at a time when these techniques were not yet available. Superficial phlebitis was not classified as a thrombotic event. If recurrent deep vein thrombosis at the same site was suspected, but objective tests were not conclusive, it was considered ascertained if the patient revealed pronounced signs and symptoms of recurrence without preceding postthrombotic syndrome, or when pulmonary embolism was objectively demonstrated. Venous thrombosis was defined provoked if it occurred within 3 months after exposure to exogenous risk factors including surgery, trauma, immobilization for more than 7 days, pregnancy, postdelivery period, the use of oral contraceptives or hormonal replacement therapy, or malignancy. In the absence of these risk factors, venous thrombosis was classified idiopathic.
Statistical analysis
Cumulative distribution functions were constructed to visualize a possible relationship between free protein S levels and the occurrence of venous thrombosis. To find an optimal cutoff value of free protein S or a possible dose response relationship, we stratified free protein S levels into quartiles and levels lower than the 5th and 2.5th percentiles to calculate the absolute risk of both first and recurrent venous thrombosis in these subgroups. Observation time was defined as the period from the age of 15 years until the first thrombotic episode or the end of study. The absolute risk of recurrent venous thrombosis was calculated over the period from the end of anticoagulant treatment after the first episode of venous thrombosis until either the date of first recurrence or the end of study. Incidences and 95% confidence intervals (95% CIs) were calculated under the Poisson distribution assumption. Freedom of recurrent venous thrombosis was analyzed by the Kaplan-Meier method. Hazard ratios were calculated using a multivariable Cox regression model. As our study cohort consisted of subjects from thrombophilic families and were therefore prone to have multiple thrombophilic defects,5,22 we first adjusted for these defects with stepwise Cox regression, including antithrombin or protein C deficiency, factor V Leiden, prothrombin G20210A, and high factor VIII levels. To account for the nonrandomness of the relatives analyzed, outcome rates were also adjusted for clustering of events within families with Cox regression analysis and the robust sandwich method (in SAS 9.1). In this analysis, all relatives were identified by a family number, which included a code for the type of thrombophilic defect. Hazard ratios were also adjusted for age and sex.
Continuous variables were expressed as mean values and standard deviations; categorical data as counts and percentages. Differences between groups were evaluated by the Student t test or Mann-Whitney U test, depending on the normality of data for continuous variables and by Fisher exact test for categorical variables. A 2-tailed P value of less than .05 indicated statistical significance.
Statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
Results
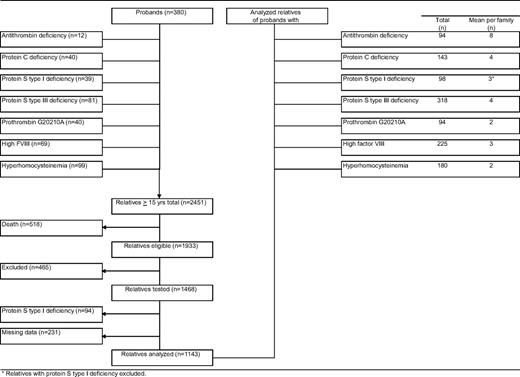
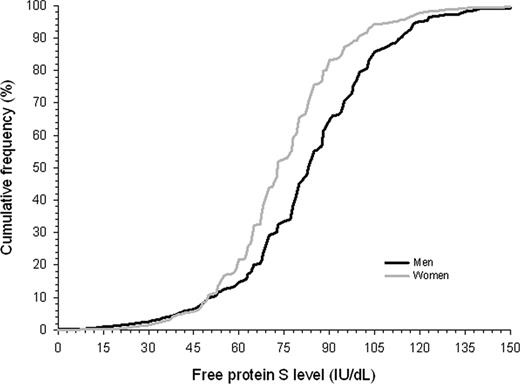
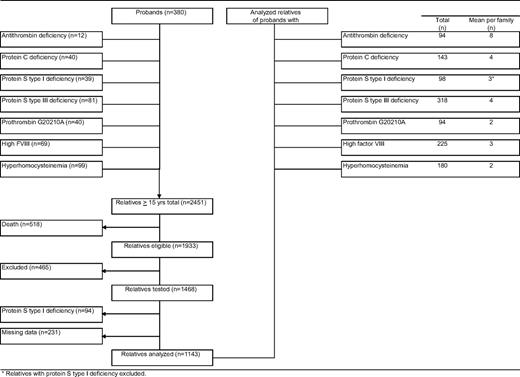
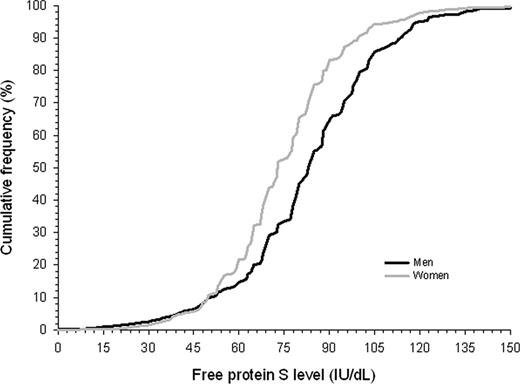
Our study cohort comprised 2451 relatives aged 15 years or older, of 380 probands (Figure 1). Of relatives, 518 (21%) had died before the start of the study. Another 465 relatives did not participate because of various reasons, including refusal, inability to give informed consent, or residence outside The Netherlands (exclusion rate 24%). A total of 94 relatives were excluded because of protein S deficiency type I, and 231 relatives could not be evaluated because of missing laboratory data. Forty-seven percent were male (Table 1). Mean plus or minus standard deviation (SD) age at enrollment was 45 plus or minus 17 years. Mean observation period was 29 plus or minus 16 years. Mean free protein S level was 80 plus or minus 20 IU/dL; 83 plus or minus 26 IU/dL in men and 75 plus or minus 24 IU/dL in women (P < .001). Free protein S levels less than 50 IU/dL were equally distributed in men and women (Figure 2). Venous thrombosis had occurred in 96 relatives (8%). Mean age at onset of the first episode of venous thrombosis was 39 plus or minus 15 years. Median calendar date of onset of first venous thrombosis was August 1991 (range, January 1955–June 2004).
Cumulative distribution function of free protein S levels in male and female relatives of probands with a thrombophilic defect.
Cumulative distribution function of free protein S levels in male and female relatives of probands with a thrombophilic defect.
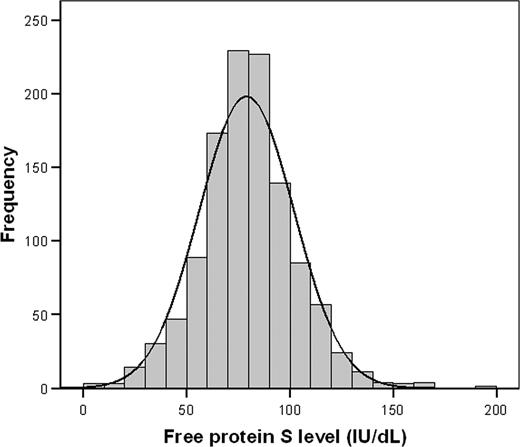
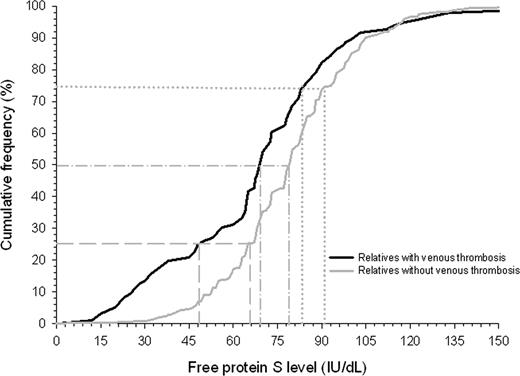
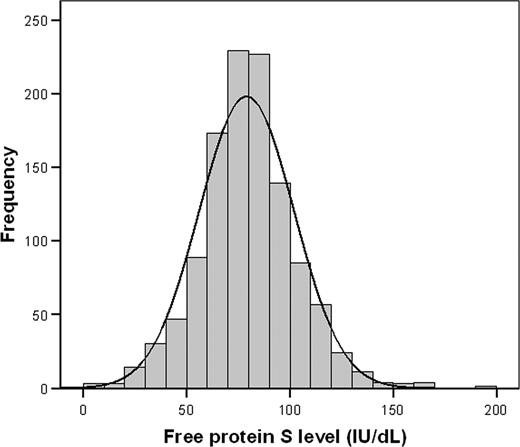
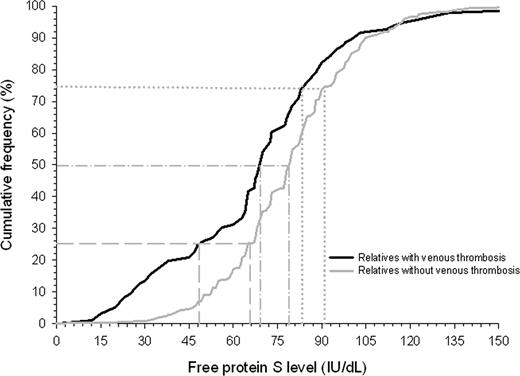
A histogram showed a normal distribution of free protein S levels in the study cohort, with a left shift from the normal mean (ie, 100 IU/dL; Figure 3). Relatives with venous thrombotic events had lower free protein S levels than relatives without venous thrombosis (P < .001), which was most pronounced in the lowest quartile (Figure 4). Annual incidences of first venous thrombosis in relatives with free protein S levels more than the 75th percentile, between the 50th and 75th percentile, the 25th and 50th percentile, the 5th and 25th percentile, less than the 5th percentile, and less than the 2.5th percentile were 0.20%, 0.21%, 0.31%, 0.26%, 1.20%, and 1.81%, respectively (Table 2). Concomitance of thrombophilic defects were observed in 87% to 100% of relatives with an event within the 6 analyzed groups. Only relatives with free protein S levels below the 5th percentile (< 41 IU/dL) or below the 2.5th percentile (< 33 IU/dL) were at higher risk of first venous thrombosis compared with relatives with free protein S levels in the upper quartile; crude hazard ratio 5.7 (95% CI, 2.9–10.1) and 10.8 (95% CI, 5.2–22.1), respectively. Adjusted for age, sex, and clustering of events in families, hazard ratios were 5.6 (95% CI, 2.7–11.5) and 11.3 (95% CI, 5.4–23.6), respectively. To account for possible misclassification, as not all events were confirmed by objective techniques because these were not available at the time of onset, we excluded relatives with clinically diagnosed first venous thrombosis (n = 35) from our cohort and repeated the analysis that adjusted for age, sex, and clustering within families. Adjusted hazard ratios for first venous thrombosis in relatives with free protein S levels, between the 50th and 75th percentile, the 25th and 50th percentile, the 5th and 25th percentile, less than the 5th percentile, and less than the 2.5th percentile compared with the upper quartile were 0.8 (95% CI, 0.3–2.1), 1.4 (95% CI, 0.6–3.1), 0.9 (95% CI, 0.3–2.5), 5.3 (95% CI, 2.1–13.6), and 11.6 (95% CI, 4.3–31.2).
Distribution of free protein S levels in relatives of probands with a thrombophilic defect.
Distribution of free protein S levels in relatives of probands with a thrombophilic defect.
Cumulative distribution function of free protein S levels in relatives of probands with a thrombophilic defect. Gray dashed, dashed dotted, and dotted lines display free protein S level for relatives with and without venous thrombosis at cumulative frequency of 25%, 50%, and 75%, respectively.
Cumulative distribution function of free protein S levels in relatives of probands with a thrombophilic defect. Gray dashed, dashed dotted, and dotted lines display free protein S level for relatives with and without venous thrombosis at cumulative frequency of 25%, 50%, and 75%, respectively.
Cumulative recurrence rates in relatives (not on continuing anticoagulant treatment) with free protein S levels in the aforementioned subgroups were 4.13%, 2.07%, 1.66%, 2.19%, 12.12%, and 12.73% (Table 3), respectively. Relatives with free protein S levels lower than the 5th or 2.5th percentile had an adjusted 3.0-fold (95% CI, 1.03–8.5) and 3.4-fold (95% CI, 1.1–10.3) higher risk of recurrence compared with relatives with free protein S levels in the upper quartile.
Discussion
Most clinical studies on protein S type III deficiency refer to laboratory reference values obtained from healthy volunteers (which in our laboratory is < 65 IU/dL).6-14 Our study showed that this cutoff level is too high to identify subjects at risk for venous thrombosis due to protein S type III deficiency. Rather, we identified a threshold level, both for first venous thrombosis and its recurrence, at free protein S levels lower than 41 IU/dL (5th percentile). Using this cutoff point increased the absolute risk of first venous thrombosis in our study population from 0.20% per year to 1.20% per year and for recurrence from 4.13% per year to 12.12% per year. These risks are high compared with the general population, with an annual incidence of first venous thrombosis of 0.1% to 0.3% and annual recurrence rate of 3% to 5%.23-26 It should be noted, however, that relatives with first venous thrombosis were relatively young (mean age 39 years vs 62 years in the general population),27 which emphasizes that thrombophilic defects are risk factors for venous thrombosis at young age.22 It also explains why first episodes of venous thrombosis associated with malignancy were scarce in our study. Although numbers were small, it is tempting to speculate that subjects with venous thrombosis and free protein S levels in the lower 5th percentile could benefit from prolonged anticoagulant treatment, given the high rate of recurrence.
Free protein S levels in our study (mean 80 IU/dL) showed a left shift compared with the normal population (mean 100 IU/dL), which is likely a result of including thrombophilic families, although we did not exclude from analysis women who were on oral contraceptives. However, more than 60% of patients with venous thrombosis have at least one thrombophilic defect,28 whereas mostly relatives of patients with thrombophilic defects are seeking advice concerning screening and risk management,29 whereas in The Netherlands, more than 40% of fertile women use oral contraceptives.30 Therefore, the results of this study probably concern young subjects that are screened in daily practice.
It is noteworthy that low free protein S levels are seen in patients with systemic lupus erythematosus,31 the nephrotic syndrome,32 or HIV infection,33 conditions that are all associated with a higher risk for venous thrombosis. It is not likely that our results were influenced by these conditions according to the rare disease assumption. Nevertheless, low free protein S levels probably involve both genetic and acquired (transient) factors.34 As genotype-phenotype associations on free protein S levels was not performed in all our subjects, this may be a limitation since it would have enabled us a more accurate classification.
Some methodologic aspects of our study warrant comment. First, as events were not always confirmed by objective techniques, our absolute risk estimates may have been overestimated by misclassification. We assume, however, that misclassification would occur equally over all the free protein S strata, and therefore would not change our relative risk estimates. Moreover, relative risk estimates did not change when we excluded relatives with clinically diagnosed first venous thrombosis. Second, referral bias may have been introduced by the university hospital setting. However, this was probably reduced by testing consecutive patients with thrombosis. Third, oral contraceptive use and hormonal replacement therapy decrease free protein S levels35 and are known risk factors for venous thrombosis as well.36 Adjustment for these possible confounders was difficult as we only had information on used oral contraceptives or hormonal replacement therapy at time of venous thrombosis or at time of blood sampling. When low free protein S levels are regarded as a risk factor for venous thrombosis, adjustment for estrogen use with regression analysis is not appropriate, considering that estrogens may be part of the causal pathway. Therefore, the data were reanalyzed, excluding women who were on oral contraceptives or hormonal replacement therapy at time of enrollment or at onset of venous thrombosis. This approach did not change our main outcomes. Fourth, as expected, because we enrolled relatives of probands with a thrombophilic defect, concomitance of thrombophilic defects in relatives with venous thrombosis was observed more frequently than in the normal population (ie, approximately 60%, not including free protein S deficiency).28 However, low free protein S levels remained a risk factor for venous thrombosis after we adjusted for concomitance of separate thrombophilic defects and for clustering of events within families. Fifth, by pooling data from several studies, a very large sample size was obtained, which is a strength of the study. Including more than 1000 subjects allowed us to not only establish a relationship between decreased free protein S and the risk of venous thrombosis, but to also be able to stratify this risk according to the level of free protein S. This pooled analysis only looked at subjects who were tested at a single hospital, thereby allowing a single reference range to be established and eliminating inter-laboratory assay variability. Finally, although we obtained a study population of a large size by pooling different family studies, not all relatives were tested for free protein S levels. Because we did a post-hoc analysis of data, we are not able to retrieve this missing data. Bias, however, seems unlikely as free protein S levels were normally distributed in our study cohort.
In conclusion, our study demonstrates that decreased free protein S levels are a risk factor for venous thrombosis in subjects at a young age in thrombophilic families. However, the laboratory cutoff level lies far below the lower limit of the normal range in healthy volunteers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was partly funded by grant 99.187 from the Dutch Heart Foundation. The funding organization is a public institution and had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript.
Authorship
Contribution: W.M.L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; J.v.d.M. provided the study concept and design and supervised the study; W.M.L., R.M., and M.K.t.K. were responsible for the acquisition of the data; W.M.L., R.M., M.K.t.K., N.J.G.M.V., A.B.M., and J.v.d.M. provided the analysis and interpretation of the data; W.M.L. drafted the manuscript; W.M.L., R.M., M.K.t.K., N.J.G.M.V., A.B.M., and J.v.d.M. provided critical revision of the manuscript for important intellectual content; W.M.L., M.K.t.K., N.J.G.M.V., and J.v.d.M. were responsible for statistical analysis; J.v.d.M. obtained funding; and W.M.L., R.M., M.K.t.K., and A.B.M. provided administrative, technical, or material support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Willem Lijfering, Division of Haemostasis, Thrombosis and Rheology, Department of Hematology, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: w.lijfering@int.umcg.nl.