To the editor:
Patients recovering from myelotoxic chemotherapy require antibiotic treatment to prevent infectious complications, because of their immunocompromised status. Antibiotics such as doxycylin and tetracyclin are known to inhibit matrix metalloproteinases (MMPs) including MMP-9.1-3 Recent studies revealed that hematopoietic recovery after administration of the myelotoxic agent 5-fluorouracil (5-FU) requires MMP-9, also known as gelatinase B.4 We therefore investigated whether antibiotics such as doxycycline might impair hematopoietic recovery after chemomyeloablation.
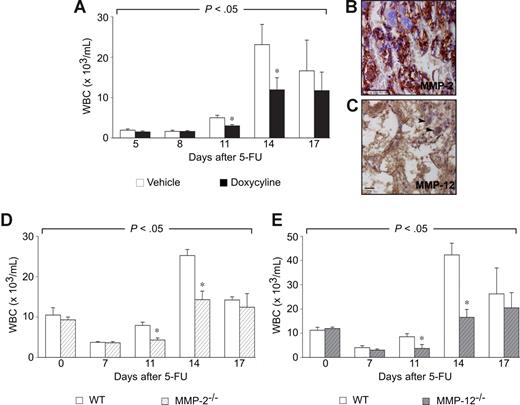
Mice were treated with doxycycline via the drinking water (30 mg/kg), as described previously.2 Compared with control mice, treatment with doxycycline impaired the recovery of white blood cells (WBCs) after 5-FU therapy (200 mg/kg intravenously; Figure 1A), similar to MMP-9 deficiency4 (data not shown). Doxycycline treatment did not affect WBC counts in steady-state conditions (data not shown).
MMP inhibition and hematopoietic recovery after 5-FU. (A) Compared with vehicle controls, WBC counts in C57Bl6 mice treated with 30 mg/kg doxycycline were impaired at different time points after 5-FU (200 mg/kg intravenously). P < .05 versus vehicle (n = 10; ANOVA). *P < .05 versus vehicle per time point. (B,C) Immunostaining on BM sections of WT mice for different MMPs at 7 days after 5-FU (200 mg/kg intravenously). MMP-2 (B, brown) was expressed in stroma cells/matrix and hematopoietic cells. MMP-12 (C, brown) was expressed in stroma cells/matrix and hematopoietic cells such as macrophages (black arrowheads). Magnification bars represent 50 μm. (D,E) Compared with their respective WT controls, WBC counts in MMP-2−/− (D) and MMP-12−/− mice (E) were impaired at different time points after 5-FU (200 mg/kg intravenously). P < .05 for each genotype versus WT (n = 8-15; ANOVA). *P < .05 versus WT per time point.
MMP inhibition and hematopoietic recovery after 5-FU. (A) Compared with vehicle controls, WBC counts in C57Bl6 mice treated with 30 mg/kg doxycycline were impaired at different time points after 5-FU (200 mg/kg intravenously). P < .05 versus vehicle (n = 10; ANOVA). *P < .05 versus vehicle per time point. (B,C) Immunostaining on BM sections of WT mice for different MMPs at 7 days after 5-FU (200 mg/kg intravenously). MMP-2 (B, brown) was expressed in stroma cells/matrix and hematopoietic cells. MMP-12 (C, brown) was expressed in stroma cells/matrix and hematopoietic cells such as macrophages (black arrowheads). Magnification bars represent 50 μm. (D,E) Compared with their respective WT controls, WBC counts in MMP-2−/− (D) and MMP-12−/− mice (E) were impaired at different time points after 5-FU (200 mg/kg intravenously). P < .05 for each genotype versus WT (n = 8-15; ANOVA). *P < .05 versus WT per time point.
Doxycycline is known to inhibit various other MMPs besides MMP-9, including MMP-2 (gelatinase A) and MMP-12 (metalloelastase), which, similar to MMP-9, is expressed predominantly by hematopoietic cells.5 However, the role of MMP-2 and MMP-12 in the hematopoietic recovery after chemomyeloablation remains unknown. To evaluate a possible role of MMP-2 and MMP-12, we first performed immunostaining on bone marrow (BM) sections of wild-type (WT) mice, and found that MMP-2 and MMP-12 were diffusely present throughout the BM cavity in stromal and hematopoietic cells, respectively, after 5-FU (Figure 1B,C).
We next studied the hematopoietic recovery after 5-FU in mice lacking MMP-2 (MMP-2−/−) or MMP-12 (MMP-12−/−). Because the genetic background of these MMP-deficient lines is not identical, we used these mice not to compare their myeloid recovery quantitatively side-by-side, but to identify qualitatively which of these MMPs participated in hematopoietic recovery after 5-FU. As sensitivity to 5-FU is dependent on the genetic background, we first determined, in pilot experiments, a sublethal dose of 5-FU for each respective background (200 mg/kg intravenously; not shown). Irrespective of their genetic background, WT mice from all control strains experienced leukopenia at 7 and 11 days after 5-FU, followed by rebound leukocytosis on days 14 and 17, with complete normalization of WBC counts after 2 to 3 weeks (Figure 1D,E). In contrast, WBC recovery after 5-FU was impaired in the absence of MMP-2 or MMP-12 (P < .05 by analysis of variance [ANOVA]; Figure 1D,E). The defect in hematopoietic recovery of 5-FU–treated MMP-2−/− and MMP-12−/− mice was specific, as their response to the mobilizing agent G-CSF was normal (M.T. and P.C., unpublished observations, 2009). These data suggest that doxycycline impaired hematopoietic recovery through in-hibition of these MMPs, though we cannot exclude the possibility that doxycycline might act through MMP-independent pathways as well.
Hence, our data warrant caution in the indiscriminate use of antibiotics such as doxycycline after myeloablation, as they may impair hematopoietic recovery through broad-spectrum MMP inhibition.
Methods.
WT mice and mice lacking MMP-2 (gift from S. Itohara, Institute of Physical and Chemical Research, Brain Science Institute, Wako, Japan; 100% C57Bl6) and MMP-12 (gift from S. Shapiro, Harvard Medical School and Massachusetts General Hospital, Boston, MA; 50% C57Bl6 × 50% Swiss) were used. For all experiments, age-, sex- and strain-matched littermate mice were used. Mice were maintained in high-efficiency particulate air (HEPA)–filtered individually ventilated cage (IVC) units. All experiments were performed according to the guidelines for care and use of laboratory animals approved by the institutional ethical animal care committee. Mice were injected with an intravenous bolus of 5-FU (200 mg/kg; Fluroblastin; Pfizer SA/NV, Brussels, Belgium). Peripheral blood was repetitively sampled by retro-orbital puncture under light anesthesia, and full blood counts (ethylenediaminetetraacetic acid [EDTA]–buffered) were determined on a hemocytometer (Cell-Dyn 1300; Abbott, Abbott Park, IL). Doxycycline was administered via drinking water protected from light (30 mg/kg), as described.2
Mice were killed by cervical dislocation. The femurs were removed, fixed in 2% paraformaldehyde in phosphate-buffered saline for 24 hours, and decalcified in 0.5 M EDTA solution for 8 days. After dehydration and paraffin embedding, 10-μm longitudinal sections were prepared on Superfrost Plus slides (Thermo Scientific, Braunsweig, Germany). Immunohistochemistry was performed using antibodies against MMP-2 (Santa Cruz Biotechnology, Santa Cruz, CA) and MMP-12 (R&D Systems, Minneapolis, MN). Specificity for MMP staining was performed using deficient mice (not shown). Corresponding secondary antibodies labeled with horseradish peroxidase or biotin for signal amplification via tyramide signal amplification (TSA; PerkinElmer, Waltham, MA) or via Vectastain ABC kit (Vector Laboratories, Burlingame, CA) were used. For light microscopy, sections were developed with 3,3′-diaminobenzidine (DAB; Sigma-Aldrich, Bornem, Belgium) as a chromogen substrate and counterstained with Harris hematoxylin. Analysis was performed on a Zeiss Axioplan2 connected to a 3CCD video camera (DXC-93OP; Sony, Londerzeel, Belgium), and KS300 software (Zeiss, Zaventem, Belgium).
We used SPSS version 11.0 for statistical calculations. Unless stated otherwise, data (mean ± SEM) were statistically analyzed by an unpaired Student t test. To determine the genotypic differences in WBC counts after 5-FU, an ANOVA for repeated measurements was used, complemented with t test to identify statistically significant genotypic differences at each individual time point. A P value less than .05 was considered statistically significant.
Acknowledgments: The authors thank A. Carton, M. De Mol, L. Frederix, B. Hermans, A. Manderveld, K. Maris, S. Meynen, J. Souffreau, S. Terclavers, B. Van Hoef, P. Van Wesemael, B. Vanwetswinkel, and J. Verelst for assistance.
This work was supported by grants from the Fund for Scientific Research Flanders (FWO; G.0121.02 and G.0209.07), the Belgian Science Policy (project no. IAP-P5/02), and an unrestricted Bristol Myers-Squibb grant (P.C.). M.T. was a research fellow of the Flanders Institute for the Promotion of Innovation by Science and Technology (IWT) and is now cofunded by the FWO.
Authorship
Contribution: M.T. designed and performed experiments, analyzed data, and participated in discussion and manuscript writing; L.M. provided advice with histologic stainings; C.V. participated in discussion and manuscript writing; and P.C. designed and analyzed data, and participated in discussion and manuscript writing, and scientific direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Carmeliet, Vesalius Research Center, VIB, KULeuven, Campus Gasthuisberg, Herestraat 49, Box 912, B-3000, Leuven, Belgium; e-mail: peter.carmeliet@med.kuleuven.be.