Platelet activation is an essential event in hemostasis, but platelet activation in atherosclerotic vessels can lead to vessel occlusion. If this occurs in the heart or brain, it can lead to serious disability or even death. As such, the development of effective but selective antithrombotic therapies is important.
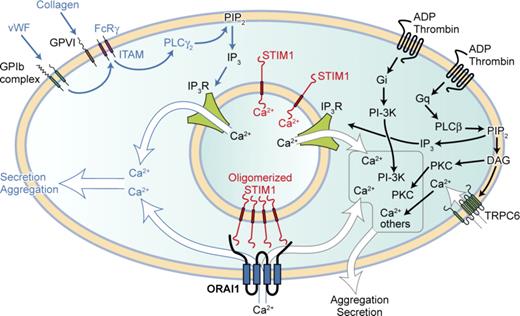
Cytosolic Ca2+ elevation has long been known to be important for platelet activation and arises due to agonist-mediated increase of phospholipase C activity, resulting in the formation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 causes rapid release of Ca2+ from intracellular stores (known as the dense tubular system in platelets) via activation of the IP3 receptors.1 This release of Ca2+ results in depletion of the stores and the influx of Ca2+ by a process known as store-operated Ca2+ entry (SOCE). Although SOCE has been studied for more than 20 years, only recently has the link between store depletion and the Ca2+ entry channel including its identity been defined. Stromal interaction molecule 1 (STIM1) is a type 1A transmembrane protein that is located primarily in the stores. STIM1 senses the Ca2+ content of intracellular stores through the presence of an EF-hand domain that binds Ca2+ and protrudes into the lumen of the store.2,3 Upon store depletion, Ca2+ is released from the EF-hand domain, and STIM1 activates the plasma membrane SOCE channel. Functionally, STIM1 has been shown to be essential in SOCE function in every cell examined including platelets.4 The discovery of Orai1 as the SOCE channel in T cells via genome wide screens and its functional importance in a subset of patients with severe combined immunodeficiency disorder (SCID)5,6 has been equally exciting.
Orai1 (also known as CRACM1) belongs to a family of channels that have 4 putative transmembrane domains and contain the pore-forming subunits of SOCE channels. To test the role of Orai1 in platelet function in vivo, Braun et al generated Orai1−/− mice by use of gene-trap cassette technology.7 However, most of the mice homozygous for Orai1-null mutation died after birth, and the surviving pups had growth retardation. So the authors generated platelets deficient in Orai1 by transplantating Orai1−/− bone marrow cells into lethally irradiated mice. Subsequently, these mice had normal platelet counts and expression of platelet surface receptors, thus Orai1 is not needed for normal platelet development. However, when examined in Ca2+ signaling tests, Orai1−/− platelets showed an almost complete absence of cation entry after store depletion by thapsigargin and much reduced Ca2+ entry when stimulated by agonists. Its function was not compensated by other members of the Orai family (of which Orai2 and Orai3 were detected) or by other candidate channels. This allowed the authors to test the relevance of SOCE-mediated Ca2+ entry in a range of platelet function tests. They found that in vitro platelet aggregation responses, αIIbβ3 activation, and P-selectin exposure were normal to all agonists except low-dose collagen and other GPVI-acting ligands. Thus, Orai1-mediated Ca2+ entry is particularly important for GPVI-ITAM–mediated cell activation but not for agonists acting at G-protein–linked receptors, such as thrombin (see figure). In addition, Orai1-deficient platelets failed to form 3-dimensional thrombi on collagen-coated surfaces under medium to high shear rates and in 2 in vivo models. When tested in the model FeCl3 induced injury model, where thrombin generation is the main stimulus for thrombus formation, Orai1−/− chimeras formed thrombi similar to control chimeras.
STIM1-Orai1–mediated Ca2+ entry is particularly important for platelet agonists acting via the ITAM motif and PLCγ2. All stimulatory platelet agonists elevate cytosolic Ca2+ as part of their activation mechanism. Two major routes exists: one utilizing the ITAM motif present on the FcRγ chain leading to activation of PLCγ2, the other utilized by G-protein–linked receptors activating PLCβ. Both produce inositol-1,4,5-trisphophate (IP3), which releases Ca2+ from intracellular stores via the IP3Rs. Store depletion causes Ca2+ to come off STIM1, allowing STIM1 to oligomerize and activate Orai1. Ca2+ entry via this pathway is particularly important for platelet activation during thrombus formation in vivo where ITAM engagement is necessary. For platelet activation via G-protein–linked receptors, the more rapid Ca2+ release from stores (and additionally Ca2+ entry from Orai1 and non-Orai1 channels such as TRPC6) may synergize with other messengers (such as PKC and PI-3K) to effect cell activation. Professional illustration by Kenneth X. Probst.
STIM1-Orai1–mediated Ca2+ entry is particularly important for platelet agonists acting via the ITAM motif and PLCγ2. All stimulatory platelet agonists elevate cytosolic Ca2+ as part of their activation mechanism. Two major routes exists: one utilizing the ITAM motif present on the FcRγ chain leading to activation of PLCγ2, the other utilized by G-protein–linked receptors activating PLCβ. Both produce inositol-1,4,5-trisphophate (IP3), which releases Ca2+ from intracellular stores via the IP3Rs. Store depletion causes Ca2+ to come off STIM1, allowing STIM1 to oligomerize and activate Orai1. Ca2+ entry via this pathway is particularly important for platelet activation during thrombus formation in vivo where ITAM engagement is necessary. For platelet activation via G-protein–linked receptors, the more rapid Ca2+ release from stores (and additionally Ca2+ entry from Orai1 and non-Orai1 channels such as TRPC6) may synergize with other messengers (such as PKC and PI-3K) to effect cell activation. Professional illustration by Kenneth X. Probst.
Particularly interesting are studies in a model of ischemic stroke, as this is currently an area of limited therapeutic options, and drugs targeted at αIIbβ3 receptors have serious bleeding complications.8 Here, thrombus formation is known to be dependent on functional GP1b and to a lesser extent on GPVI. Chimeras with Orai1-deficient platelets showed a 70% reduction in infarct volumes, had better neurologic and motor function, importantly with less intracranial bleeding complications. Coupled with this, tail bleeding times in Orai1−/− chimeras were only slightly prolonged compared with controls. The Orai1-deficient platelets showed similar characteristics to platelets deficient in STIM14 and therefore confirm the importance of this pathway in GPVI-ITAM signaling axis. Where STIM1−/− platelets differ is that STIM1 appears to have an additional function related to store refilling, thus STIM1−/− platelets show reduced Ca2+ release upon activation by all agents.
The Braun et al study does raise the question of whether Ca2+ entry is required at all for platelet responses to agonists acting on G-protein–linked receptors where phospholipase Cβ is activated, in contrast to GPVI-ITAM signaling where PLCγ is activated. The answer may lie with the rapidity of responses seen with agonists acting on G-protein–linked receptors, particularly in Ca2+ release from stores and the likelihood that cytosolic Ca2+ may synergize with other signals generated from activation of these receptors, such as Gi (see figure). Further confirmation that the SOCE channel is Orai1 comes in a recent report where knock-in of a mutant Orai1R93W (identified as responsible for the SCID defect) in platelets results in impaired Ca2+ entry, a defect in aggregation and secretion mediated by low concentrations of agonist and impaired procoagulant responses.9 However, the mutated Orai1R93W platelets were able to form stable thrombi on collagen-coated surfaces, and the reason for this difference needs to be evaluated.
Taken together, these studies strongly suggest that the SOCE pathway in platelets is critically important in arterial occlusive thrombi formation, though not so in primary hemostasis. Our future challenge lies in whether we can dissect these 2 processes sufficiently in order to develop safer antithrombotic drugs. Further studies of the Ca2+ entry pathways may yield more answers.
Conflict-of-interest disclosure: The author is supported by the British Heart Foundation. ■