Abstract
The apoptotic and therapeutic activities of the histone deacetylase inhibitor (HDACi) vorinostat are blocked by overexpresssion of Bcl-2 or Bcl-XL. Herein, we used the small molecule inhibitor ABT-737 to restore sensitivity of Eμ-myc lymphomas overexpressing Bcl-2 or Bcl-XL to vorinostat and valproic acid (VPA). Combining low-dose ABT-737 with vorinostat or VPA resulted in synergistic apoptosis of these cells. ABT-737 was ineffective against Eμ-myc/Mcl-1 and Eμ-myc/A1 cells either as a single agent or in combination with HDACi. However, in contrast to the reported binding specificity data, Eμ-myc/Bcl-w lymphomas were insensitive to ABT-737 used alone or in combination with HDACi, indicating that the regulatory activity of ABT-737 is restricted to Bcl-2 and Bcl-XL. Eμ-myc lymphomas that expressed Bcl-2 throughout the tumorigenesis process were especially sensitive to ABT-737, while those forced to overexpress Mcl-1 were not. This supports the notion that tumor cells “addicted” to ABT-737 target proteins (ie, Bcl-2 or Bcl-XL) are likely to be the most sensitive target cell population. Our studies provide important preclinical data on the binding specificity of ABT-737 and its usefulness against primary hematologic malignancies when used as a single agent and in combination with HDACi.
Introduction
Histone deacetylase inhibitors (HDACis) are a new class of chemotherapeutic drugs that inhibit the enzymatic activity of HDACs, resulting in chromatin remodeling and altered gene transcription.1 These agents can induce tumor cell apoptosis, inhibit cell proliferation by blocking progression through the G1 or G2/M phases of the cell cycle, induce cellular differentiation, suppress angiogenesis, and modulate antitumor immunity.1 Using genetic mouse models of cancer, we and others have recently demonstrated a direct link between HDACi-mediated apoptosis and therapeutic efficacy,2,3 indicating that direct tumor cell killing by these agents plays an important role in mediating antitumor responses in vivo. We genetically manipulated primary Eμ-myc lymphoma cells to functionally inactivate either extrinsic apoptotic pathway signaling, by overexpression of the viral serpin CrmA or gene knockout of TRAIL, or the intrinsic apoptotic pathway, by overexpression of the prosurvival Bcl-2 proteins Bcl-2 or Bcl-XL, and tested for the ability of the HDACi vorinostat to kill these cells and mediate a therapeutic response.3 We found that disruption of death receptor signaling had no effect on the apoptotic and therapeutic activity of vorinostat. However, inhibition of mitochondrial membrane permeabilization and subsequent suppression of the intrinsic apoptotic pathway by overexpressed Bcl-2 or Bcl-XL completely inhibited vorinostat-induced apoptosis and abolished any therapeutic benefit. These data indicate that the clinical use of vorinostat and other HDACi as monotherapies may be limited to those tumors that do not overexpress prosurvival Bcl-2 proteins. However, we hypothesize that agents that inhibit the expression and/or function of prosurvival Bcl-2 family proteins may sensitize cells to HDACi-mediated apoptosis, providing a rationale for the clinical development of such combination approaches
The Bcl-2 family consists of 3 major subgroups: (1) Multidomain prosurvival proteins (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, A1) that share 4 Bcl-2 homology (BH) domains; (2) BH3-only proapoptotic proteins (Bid, Bim, Bik, Bmf, Noxa, Puma, Hrk, Bad) that contain only a 9- to 16-amino-acid region of BH3; (3) multidomain proapoptotic proteins (Bax, Bak, Bok) that share BH domains 1, 2, and 3.4 BH3-only proteins are “activated” by exogenous signals such as growth factor deprivation, irradiation, and chemotherapeutic drugs. These proteins can trigger the intrinsic apoptotic pathway by binding prosurvival Bcl-2 proteins, thereby relieving the inhibitory effect on Bax and Bak5,6 and/or by directly binding to and activating Bax and Bak.7,8
ABT-737 is a BH3-only mimetic compound developed to specifically inhibit the activity of prosurvival Bcl-2 family proteins.9 The binding specificity of ABT-737 was determined using competitive fluorescence polarization assays and recombinant proteins demonstrating that ABT-737 had “Bad-like” activity in that it preferentially bound Bcl-2, Bcl-XL, and Bcl-w, with inhibitory constants (Ki) less than or equal to 1 nM. In contrast, the affinity of ABT-737 for Mcl-1 and A1 was far lower (Ki > 1 μM).9 Similar biochemical assays using recombinant full-length or truncated prosurvival Bcl-2 proteins confirmed that ABT-737 had significantly greater affinity for Bcl-2, Bcl-XL, and Bcl-w than to Mcl-1 or A1.10,11 The ability of ABT-737 to inhibit the activity of Bcl-2 and Bcl-XL, but not Mcl-1 has been validated in cell-based assays.9–16 However, to our knowledge, the activity of ABT-737 against wild-type, nonrecombinant Bcl-w or A1 expressed in mammalian cells has yet to be determined.
While ABT-737 has single agent activity in vitro and in vivo, the destruction of platelets and resultant thrombocytopenia caused by the on-target effects of ABT-737 on Bcl-XL expressed in platelets raises concern about the possible detrimental side effects of the compound when used at high doses or for prolonged periods in the clinic.17,18 This, coupled with the fact that the antitumor activity of many chemotherapeutic drugs can be affected through overexpression of prosurvival Bcl-2 proteins,19 indicates that the utility of ABT-737 may be broadened clinically by combining it with other anticancer agents, especially in situations where Bcl-2, Bcl-XL, and Bcl-w are overexpressed.
Herein, we used genetically manipulated primary Eμ-myc B-cell lymphoma cells to investigate the specificity of ABT-737 for pro-survival Bcl-2 proteins and determine whether combining ABT-737 with the HDACi vorinostat or valproic acid (VPA) resulted in enhanced tumor cell apoptosis. Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and A1 all protected Eμ-myc lymphoma cells from HDACi-induced apoptosis, demonstrating for the first time that these prosurvival Bcl-2 family proteins could function equivalently to suppress the activity of structurally diverse HDACi. Consistent with the proposed binding specificity of ABT-737 determined by competitive fluorescence polarization assays, ABT-737 had single agent activity against Eμ-myc lymphomas overexpressing Bcl-2, Bcl-XL, and was ineffective against lymphomas overexpressing Mcl-1 and A1. However, we demonstrate that in contrast to previous reports indicating that ABT-737 could inhibit Bcl-w,9–11 ABT-737 had no effect against Eμ-myc lymphomas overexpressing Bcl-w, indicating that the target specificity of the compound may be more restricted than previously proposed. Consistent with this, sublethal doses of ABT-737 combined with vorinostat or VPA to synergistically kill Eμ-myc/Bcl-2 and Eμ-myc/Bcl-XL lymphomas, but cells overexpressing Bcl-w, Mcl-1, and A1 were resistant to this combination.
We further demonstrate that Eμ-myc lymphomas that develop in the presence of overexpressed Bcl-2, but not Mcl-1, were hypersensitive in vitro to ABT-737 compared with established Eμ-myc lymphomas where Bcl-2 was expressed subsequent to cell transformation. This model of “oncogene addiction” strongly indicates that those tumors that arise as a consequence of deregulated expression or Bcl-2 or Bcl-XL might be highly susceptible to ABT-737. Moreover, we show that quiescent Eμ-myc/Bcl-2 lymphomas were sensitive to ABT-737 indicating that noncycling tumor cells that have a very slow rate of proliferation20 may be effectively targeted by ABT-737. Finally, we demonstrate that ABT-737 killed Eμ-myc cells overexpressing Bcl-2 in vivo, resulting in a statistically significant decrease in tumor burden. Consistent with our in vitro data, lymphoma cells overexpressing Bcl-w or Mcl-1 remained insensitive to ABT-737 when grown in mice. Importantly, we demonstrate that a combination of vorinostat and ABT-737 was more effective in reducing tumor burden in mice with Eμ-myc/Bcl-2 lymphomas, compared with either agent alone. Taken together, these studies reveal the more selective target specificity of ABT-737 and demonstrate for the first time strong synergy between HDACi and ABT-737 in tumor cells that overexpress Bcl-2 or Bcl-XL.
Methods
Eμ-myc lymphomas, cell culture, and reagents
Eμ-myc lymphomas were isolated from Eμ-myc transgenic mice by harvesting enlarged brachioaxial and mesenteric lymph nodes. A cell suspension was prepared, filtered through nylon mesh, and then stored in liquid N2. Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were engineered by retroviral transduction of freshly isolated lymphoma cells. For this purpose, retrovirus-containing supernatant was produced by transfecting packaging cells with murine stem cell virus-internal ribosome entry site–green fluorescent protein plasmid (MSCV-IRES-GFP), MSCV-IRES-GFP/Bcl-2, MSCV-IRES-GFP/Bcl-XL, MSCV-IRES-GFP/Flag-Bcl-w, MSCV-IRES-GFP/Bcl-w, MSCV-IRES-GFP/humanBcl-w, MSCV-IRES-GFP/Flag-Mcl-1, and MSCV-IRES-GFP/Flag-A1 using standard calcium phosphate transfection methods. Viral supernatant was used to transduce primary lymphoma cells in RetroNectin (TaKaRa Bio, Shiga, Japan)–precoated 6-well plates (Becton Dickinson, Franklin Lakes, NJ). After 48 hours, GFP-positive cells were isolated by flow cytometry–mediated cell sorting and reinjected into C57BL/6 recipients for amplification.
Eμ-myc lymphomas were cultured in tissue-culture grade 6-well plates (Greiner Bio-One, Monroe, NC) in the high-glucose version of Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, penicillin (100 U/mL)/streptomycin (100 mg/mL), 0.1 mM L-asparagine, and 50 mM 2-mercaptoethanol. The production of FLR-Eμ-myc/Bcl-2, FLR-Eμ-myc/Bcl-w, and FLR-Eμ-myc/Mcl-1 lymphomas by transducing fetal liver progenitor cells with MSCV-IRES-GFP, MSCV-IRES-GFP/Bcl-2, MSCV-IRES-GFP/Bcl-w, and MSCV-IRES-GFP/Flag-Mcl-1 and analysis of tumor latency was performed as described.21
Vorinostat was kindly provided by Merck (Whitehouse Station, NJ), ABT-737 was kindly provided by Abbott Laboratories (Abbott Park, IL), and VPA was purchased from Sigma-Aldrich (St Louis, MO). For in vitro experiments, stock solutions of vorinostat and ABT-737 (10 mM and 50 mM, respectively) were prepared by dissolution in dimethyl sulfoxide (DMSO). For in vivo experiments, vorinostat was dissolved in DMSO to give a stock of 100 mg/mL, while ABT-737 was dissolved in DMSO at 500 mg/mL and then diluted 1:25 in a solution of 30% 1,2 propanediol, 5% Tween 80, and 65% D5W (5% dextrose, pH 1.0). This 10 mg/mL ABT-737 solution was sonicated, and the pH adjusted to 4.2 to 4.3. All stock solutions were stored at −20°C.
Western blot
Western blot assays using whole cell lysates were performed as previously described3 using primary antibodies against mouse Bcl-2 (BD Bio-Sciences, San Jose, CA), mouse Bcl-XL (Santa Cruz Biotechnology, Santa Cruz, CA), Flag epitope (Sigma-Aldrich), Bcl-w (Millipore, Billerica, MA), Mcl-1 (Rockland, Gilbertsville, PA), α-tubulin and β-actin (both Sigma-Aldrich).
In vitro cell-death analysis
Eμ-myc lymphoma cells (5 × 105 cells/mL) were incubated in the presence of the indicated compounds for 20 hours in 1 mL cell culture media in 24-well plates (Greiner Bio-One). Viability of cells was measured by propidium iodide (PI) uptake, cell-cycle analysis, or tetramethylrhodamine ethyl ester (TMRE) staining as described.3 Clonogenic assays were performed as described.22
In vivo assays
C57BL/6 mice were injected intravenously with 1 to 6 × 105 Eμ-myc lymphoma cells, and mice with established tumors were injected with 25, 75, or 100 mg/kg ABT-737, 200 mg/kg vorinostat, or a combination of the 2 agents by intraperitoneal injection. Control mice were injected intraperitoneally with DMSO or the vehicle for ABT-737. At various time points, peripheral blood was collected into tubes containing 10 mM EDTA (ethylenediaminetetraacetic acid), diluted in phosphate-buffered saline (PBS), and white blood cell (WBC) and platelet numbers were calculated (Advia 120 Hematology System; Siemens Healthcare Diagnostics, Deerfield, IL). At each time point in repetitive dosing experiments, mean WBC counts were compared using a 2-tailed Mann-Whitney t test. Approval was obtained from the Peter MacCallum Cancer Centre Animal Experimentation institutional review board for these studies.
Results
Prosurvival Bcl-2 proteins confer resistance to HDACi-induced apoptosis
We have previously demonstrated that overexpression of Bcl-2 or Bcl-XL in established human tumor cell lines23,24 and primary Eμ-myc lymphomas3 confers resistance to HDACi-induced apoptosis in vitro and suppresses the therapeutic activity of vorinostat in vivo.3 To determine whether other prosurvival Bcl-2 proteins could also suppress the apoptotic activities of HDACi, we developed populations of tumor cells differing only in their expression of prosurvival Bcl-2 family proteins by retroviral transduction of lymphoma cells isolated from Eμ-myc transgenic mice. A control population was generated by transduction of cells with empty retroviral vector.
After confirming the overexpression of prosurvival Bcl-2-family proteins in each test population of tumor cells by western blot (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), control and test tumor cells were exposed in vitro to varying concentrations of the structurally different HDACis vorinostat and VPA, for 20 to 24 hours and then assessed for (1) loss of plasma membrane integrity by PI uptake; (2) loss of mitochondrial outer membrane potential (MOMP) by a decrease in TMRE staining; and (3) DNA fragmentation by cell-cycle analysis. Control tumor cells were sensitive to vorinostat and VPA in a concentration-dependent manner (Figures 1A,B, S1C). In contrast, test tumor cells overexpressing any one of the prosurvival Bcl-2 proteins were relatively resistant to vorinostat and VPA. Similar results were observed using a second, independently derived, set of test and control tumor cells generated from another Eμ-myc transgenic mouse (Figures S1B, S2), demonstrating that the responses observed were primarily due to the level of prosurvival protein expression, and not a consequence of random mutations arising during development or expansion of the test tumor cells. Taken together, these results support our claim that HDACi-induced apoptosis in Eμ-myc lymphoma cells occurs via the intrinsic apoptotic pathway. We therefore hypothesized that inhibitors of prosurvival Bcl-2 proteins would restore sensitivity to HDACi in tumor cells overexpressing these apoptosis inhibitory molecules.
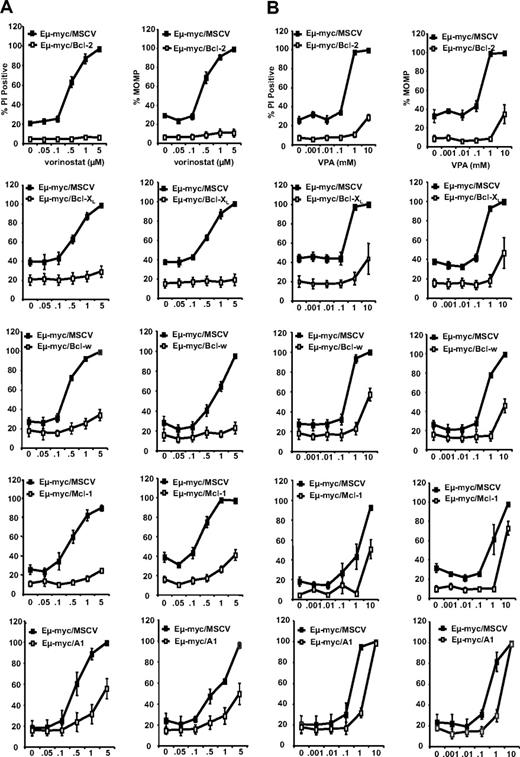
Prosurvival Bcl-2 proteins inhibit HDACi-induced apoptosis. 4242 Eμ-myc/MSCV, Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated for 24 hours with increasing concentrations of (A) vorinostat or (B) VPA. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments.
Prosurvival Bcl-2 proteins inhibit HDACi-induced apoptosis. 4242 Eμ-myc/MSCV, Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated for 24 hours with increasing concentrations of (A) vorinostat or (B) VPA. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments.
ABT-737 induces apoptosis in tumor cells overexpressing Bcl-2 or Bcl-XL, but is ineffective as an inhibitor of Bcl-w, Mcl-1, or A1
To test our hypothesis, we decided to coincubate our test and control tumor cells with the HDACis vorinostat or VPA and the small molecule ABT-737, which reportedly has a high affinity for Bcl-2, Bcl-XL, and Bcl-w, but not for Mcl-1 or A1.9–11 First, however, we determined the sensitivity of tumor cells overexpressing Bcl-2 family proteins to ABT-737 alone. Control cells and tumors overexpressing Bcl-2 were exposed in vitro to varying concentrations of ABT-737 or its less potent enantiomer (ABT-737e) for 20 to 24 hours and then assessed for cell viability as before. Tumor cells overexpressing Bcl-2 were sensitive to as little as 0.1 μM ABT-737 as assessed by increased uptake of PI and loss of MOMP (Figure 2A,B), and an increase in DNA fragmentation (see Figure S6C). At 1 μM ABT-737, more than 60% of these tumor cells had lost MOMP and plasma membrane integrity (Figure 2B). In contrast, control lymphomas were not sensitive to apoptosis mediated by ABT-737 until doses as high as 10 and 100 μM were used, even though these cells showed a higher basal percentage of apoptotic cells when grown in the absence of any ABT-737 (Figure 2A). Similar results were obtained using 2 additional sets of matched control and Bcl-2–overexpressing lymphomas (Figure S3).
Eμ-myc lymphomas overexpressing Bcl-2 and Bcl-XL are sensitive to ABT-737–induced apoptosis. (A) 4242 Eμ-myc/MSCV and Eμ-myc/Bcl-2 lymphomas were treated with increasing concentrations of ABT-737 (top panel) or ABT-737e (bottom panel). Outer cell membrane disruption was assessed by uptake of PI. (B) 4242 Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated for 24 hours with increasing concentrations of ABT-737 or ABT-737e. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments.
Eμ-myc lymphomas overexpressing Bcl-2 and Bcl-XL are sensitive to ABT-737–induced apoptosis. (A) 4242 Eμ-myc/MSCV and Eμ-myc/Bcl-2 lymphomas were treated with increasing concentrations of ABT-737 (top panel) or ABT-737e (bottom panel). Outer cell membrane disruption was assessed by uptake of PI. (B) 4242 Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated for 24 hours with increasing concentrations of ABT-737 or ABT-737e. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments.
Tumor cells overexpressing Bcl-XL were also sensitive to apoptosis induced by ABT-737 and were relatively resistant to ABT-737e (Figure 2B). In contrast, tumor cells overexpressing Mcl-1 or A1 were resistant to both ABT-737 and ABT-737e except at the highest dose used (100 μM; Figure 2B). Unexpectedly, tumor cells overexpressing Bcl-w had a similar pattern of insensitivity to ABT-737 as tumor cells overexpressing Mcl-1 or A1 (Figure 2B). As before, similar results were observed using a second, independently derived, set of test and control tumor cells generated from another Eμ-myc transgenic mouse (Figure S4A). These data were further confirmed after analysis of a third set of matched control and test lymphomas overexpressing Bcl-2 or Bcl-w (Figure S4B) that demonstrated again that ABT-737 was ineffective against lymphomas that overexpressed Bcl-w.
Our findings that ABT-737 had specificity for Bcl-2 and Bcl-XL, but not Bcl-w, were counter to the biochemical data previously published.9–11 We first ensured that the sequence of the DNA fragment used to generate the retroviral vector that resulted in overexpression of Bcl-w in our tumor cells was identical to the published sequence of murine Bcl-w, which is translated to an amino acid sequence that differs from human Bcl-w at only 2 residues (Figure S5A). Neither of these is located in the BH domains forming the BH3 binding groove of Bcl-w (Figure S5B), indicating that it is unlikely that these 2 amino acid changes would confer functional differences between the human and mouse Bcl-w proteins. We next tested whether the FLAG epitope positioned at the amino-terminus of Bcl-w that we expressed in our lymphoma cells might affect the activity of ABT-737. The presence of the FLAG epitope did not appear to affect the ability of Bcl-w to confer resistance to the HDACi vorinostat and VPA (Figure 1), or more conventional agents, such as etoposide (data not shown). However, to rule out the possibility that the additional amino acids had affected the binding affinity of ABT-737 for Bcl-w, we generated another set of Bcl-w–overexpressing test tumor cells using a retroviral vector that resulted in expression of a nontagged, wild-type Bcl-w protein. When tested with varying concentrations of ABT-737 or its less potent enantiomer for 20 to 24 hours, these cells had the same pattern of insensitivity to ABT-737 as the tumor cells overexpressing FLAG-tagged Bcl-w protein (Figure S6A). As overexpression of Mcl-1 may confer resistance to ABT-737 in cells that express Bcl-2,10,11 we checked, by western blotting, the expression level of Mcl-1 in control tumor cells and test tumor cells overexpressing both nontagged or FLAG-tagged Bcl-w, or Bcl-2. All 4 lymphomas showed comparable levels of endogenous Mcl-1 expression (Figure S6B). Finally, we produced Eμ-myc lymphomas overexpressing human Bcl-w and demonstrated that these cells were also refractory to apoptosis mediated by ABT-737 (Figure S6C).
To ensure that the insensitivity of tumor cells overexpressing Bcl-w, Mcl-1, or A1 to ABT-737 was not merely due to a delay in ABT-737-induced apoptosis, we performed colony assays on our set of control and test tumor cells. Tumor cells were exposed to 1 μM of ABT-737 for 22 to 24 hours and seeded into agar, and the number of colonies arising counted 6 days later. Consistent with our dose response assays (Figure 2), the number of colonies (< 40% relative to untreated cells) arising from ABT-737–treated tumor cells overexpressing Bcl-2 and Bcl-XL was significantly decreased in comparison to ABT-737–treated control cells, or tumor cells overexpressing Mcl-1, A1, and Bcl-w (> 90% relative to untreated cells; Figure 3).
ABT-737 decreases the clonogenic potential of Eμ-myc lymphomas overexpressing Bcl-2 and Bcl-XL. 4242 Eμ-myc/MSCV, Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated for 24 hours with 1.0 μM ABT-737 or an equivalent volume of DMSO (the ABT-737 diluent). Cells were plated in soft agar and clonogenic growth was assessed 6 days later. Results shown are the mean and SE from at least 3 separate experiments.
ABT-737 decreases the clonogenic potential of Eμ-myc lymphomas overexpressing Bcl-2 and Bcl-XL. 4242 Eμ-myc/MSCV, Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated for 24 hours with 1.0 μM ABT-737 or an equivalent volume of DMSO (the ABT-737 diluent). Cells were plated in soft agar and clonogenic growth was assessed 6 days later. Results shown are the mean and SE from at least 3 separate experiments.
In summary, these results demonstrate that ABT-737 is effective at inducing apoptosis in tumor cells overexpressing Bcl-2 or Bcl-XL, but is ineffective as an inhibitor of Bcl-w, Mcl-1, or A1.
HDACi and ABT-737 induce synergistic apoptosis in tumor cells overexpressing Bcl-2 or Bcl-XL lymphomas
Our results thus far indicated that apoptosis mediated by vorinostat and VPA was inhibited by all prosurvival Bcl-2 family proteins and ABT-737 could specifically inhibit the activity of Bcl-2 and Bcl-XL but does not affect Bcl-w, Mcl-1, and A1. We therefore proposed that a combination of HDACi and ABT-737 would be effective in Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL lymphomas, and we wished to determine whether these compounds could induce synergistic apoptosis in these cells. ABT-737–sensitive tumor cells (ie, those overexpressing Bcl-2 or Bcl-XL) were exposed in vitro for 20 to 24 hours to varying concentrations of vorinostat or VPA in the presence 0.5 μM ABT-737 or ABT-737e. Tumor cells overexpressing Bcl-w, Mcl-1, and A1 were treated similarly, except 1 μM ABT-737 or ABT-737e was used. As before, tumor cells overexpressing any of the prosurvival proteins were relatively insensitive to 0.5 to 5 μM vorinostat, 0.1 to 1.0 mM VPA, and 0.5 or 1 μM ABT-737 when used alone (Figures 4, S7A). However, when vorinostat and ABT-737 (Figure 4) or VPA and ABT-737 (Figure S7A) were combined, tumor cells overexpressing Bcl-2 or Bcl-XL showed a decrease in viability as assessed by increased uptake of PI and loss of MOMP, and an increase in DNA fragmentation (sub G1 cells; Figures 4, S7B). For tumor cells overexpressing Bcl-2 in particular, the response to the combination was comparable to that of control cells treated only with vorinostat or VPA (Figures 4, S7B). As expected from our single-agent tests (Figures 1,Figure 2–3), tumor cells overexpressing Mcl-1, A1, or Bcl-w were resistant to 0.5 to 5 μM vorinostat and 0.1 to 1.0 mM VPA, despite the presence of 1 μM ABT-737 (Figures 4, S7).
Vorinostat and ABT-737 induce synergistic apoptosis in Eμ-myc lymphomas overexpressing Bcl-2 and Bcl-XL. 4242 Eμ-myc/MSCV, Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated with increasing concentrations of vorinostat (0, 0.5, 1.0, or 5.0 μM) in the presence or absence of ABT-737 or ABT-737e. ABT-737 and ABT-737e were used at a concentration of 0.5 μM for Eμ-myc/Bcl-2 and Eμ-myc/Bcl-XL lymphomas, and at 1.0 μM for Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 cells. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments.
Vorinostat and ABT-737 induce synergistic apoptosis in Eμ-myc lymphomas overexpressing Bcl-2 and Bcl-XL. 4242 Eμ-myc/MSCV, Eμ-myc/Bcl-2, Eμ-myc/Bcl-XL, Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 lymphomas were treated with increasing concentrations of vorinostat (0, 0.5, 1.0, or 5.0 μM) in the presence or absence of ABT-737 or ABT-737e. ABT-737 and ABT-737e were used at a concentration of 0.5 μM for Eμ-myc/Bcl-2 and Eμ-myc/Bcl-XL lymphomas, and at 1.0 μM for Eμ-myc/Bcl-w, Eμ-myc/Mcl-1, and Eμ-myc/A1 cells. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments.
These results, therefore, indicate that HDACi and ABT-737 can function synergistically to kill tumor cells overexpressing Bcl-2 or Bcl-XL. Moreover, we have confirmed our previous observations that ABT-737 specifically inhibits only Bcl-2 or Bcl-XL and has no effect on Mcl-1, A1, or surprisingly, Bcl-w.
ABT-737 is effective in noncycling tumor cells that overexpress Bcl-2
We demonstrated that overexpression of Bcl-2 or Bcl-XL in established Eμ-myc lymphomas led to increased sensitivity to ABT-737 compared with that observed using parental Eμ-myc cells. This model might represent a situation where increased expression of prosurvival Bcl-2 proteins in established tumors occurs after additional cell stress (ie, after exposure to chemotherapeutic drugs) or due to selection of a small pool (or clone) of cells that overexpress such apoptosis inhibitory proteins in response to apoptotic stimuli. We were interested to determine whether Eμ-myc lymphoma cells that develop in the presence of overexpressed Bcl-2 may be hypersensitive to ABT-737 as these cells may be more “addicted” to the presence of functional Bcl-2. To assess this, myc-positive fetal liver hemopoietic progenitor cells from Eμ-myc transgenic mice were transduced with control retrovirus (MSCV) or retroviral vectors overexpressing Bcl-2, Bcl-w, and Mcl-1. Transduced fetal liver cells were injected into irradiated recipient mice and GFP+/Myc+ tumor cells were obtained from the mice that subsequently develop lymphoma. Tumors derived via this method were termed FLR (fetal liver reconstituted) lymphomas, and this system serves as an “oncogene addiction” model in which tumorigenesis occurs in an environment where both myc and a pro-survival Bcl-2 family protein are coexpressed throughout the cellular transformation process.
We found that Bcl-2, Bcl-w, and Mcl-1 function equivalently to greatly accelerate myc-mediated lymphomagenesis (Figure 5A). FLR lymphomas harvested from these mice were shown to overexpress the appropriate prosurvival Bcl-2 family protein (Figures 5B, S8). Bcl-2– and Mcl-1–overexpressing cells were cultured with ABT-737 or ABT-737e ex vivo and apoptosis was assessed. Consistent with our results using established Eμ-myc lymphomas, FLR lymphomas overexpressing Bcl-2 were more sensitive to ABT-737 than control FLR tumors (Figure S8). Importantly, FLR lymphomas overexpressing Bcl-2 were significantly more sensitive to ABT-737 compared with FLR lymphomas overexpressing Mcl-1 (Figure 5C). Of note, FLR lymphomas overexpressing Bcl-2 were greater than 10-fold more sensitive to ABT-737 than were lymphomas in which Bcl-2 was overexpressed subsequent to the tumorigenic process (Figures 2B, 5C). Moreover, we noted that FLR lymphomas overexpressing Bcl-2 grown ex vivo did not proliferate when cultured for up to 3 days (data not shown) and appeared to be arrested in the G1 phase of the cell cycle (Figure 5D). This demonstrated that ABT-737 effectively killed Bcl-2–overexpressing tumor cells even if the cells were quiescent.
Eμ-myc lymphomas forced to overexpress Bcl-2 throughout lymphomagenesis are hypersensitive to ABT-737. (A) Myc-positive fetal liver progenitor cells were transduced with MSCV, MSCV-Bcl-2, MSCV-Bcl-w, and MSCV-Mcl-1 retrovirus and injected into irradiated recipient PTPRCA mice (MSCV [n=54[,MSCV-Bcl-2 [n=18[, MSCV-Bcl-w [n=8[, and MSCVMcl-1 [n=8[). GFP-positive cells were injected into irradiated recipient PTPRCA mice (MSCV [n = 54], MSCV-Bcl-2 [n = 18], MSCV-Bcl-w [n = 8], and MSCV-Mcl-1 [n = 8]). Lymphomagenesis was monitored and Kaplan-Meier survival curves were obtained. Lymphoma cells harvested from the mice at sacrifice, designated FLR-Eμ-myc lymphomas, were stored for further use. (B) Western blot was performed using whole cell lysates from representative FLR-Eμ-myc/Bcl-2 and FLR-Eμ-myc/Mcl-1 cells (both derived from pool no. 12 of fetal liver cells) and antibodies against Mcl-1, Bcl-2, and α-tubulin. (C) FLR-Eμ-myc/Bcl-2 (top panels) and FLR-Eμ-myc/Mcl-1 cells (bottom panels) were treated with increasing concentrations of ABT-737 or ABT-737e. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments. (D) The cell-cycle profile of FL-Eμ-myc/Bcl-2 cells cultured ex vivo was obtained by analyzing the DNA content of cells by fluorescence-activated cell sorting (FACS) after incubation in a hypotonic solution containing PI.
Eμ-myc lymphomas forced to overexpress Bcl-2 throughout lymphomagenesis are hypersensitive to ABT-737. (A) Myc-positive fetal liver progenitor cells were transduced with MSCV, MSCV-Bcl-2, MSCV-Bcl-w, and MSCV-Mcl-1 retrovirus and injected into irradiated recipient PTPRCA mice (MSCV [n=54[,MSCV-Bcl-2 [n=18[, MSCV-Bcl-w [n=8[, and MSCVMcl-1 [n=8[). GFP-positive cells were injected into irradiated recipient PTPRCA mice (MSCV [n = 54], MSCV-Bcl-2 [n = 18], MSCV-Bcl-w [n = 8], and MSCV-Mcl-1 [n = 8]). Lymphomagenesis was monitored and Kaplan-Meier survival curves were obtained. Lymphoma cells harvested from the mice at sacrifice, designated FLR-Eμ-myc lymphomas, were stored for further use. (B) Western blot was performed using whole cell lysates from representative FLR-Eμ-myc/Bcl-2 and FLR-Eμ-myc/Mcl-1 cells (both derived from pool no. 12 of fetal liver cells) and antibodies against Mcl-1, Bcl-2, and α-tubulin. (C) FLR-Eμ-myc/Bcl-2 (top panels) and FLR-Eμ-myc/Mcl-1 cells (bottom panels) were treated with increasing concentrations of ABT-737 or ABT-737e. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments. (D) The cell-cycle profile of FL-Eμ-myc/Bcl-2 cells cultured ex vivo was obtained by analyzing the DNA content of cells by fluorescence-activated cell sorting (FACS) after incubation in a hypotonic solution containing PI.
ABT-737 selectively kills lymphomas overexpressing Bcl-2 in vivo and synergizes with vorinostat in mice bearing FLR lymphomas overexpressing Bcl-2
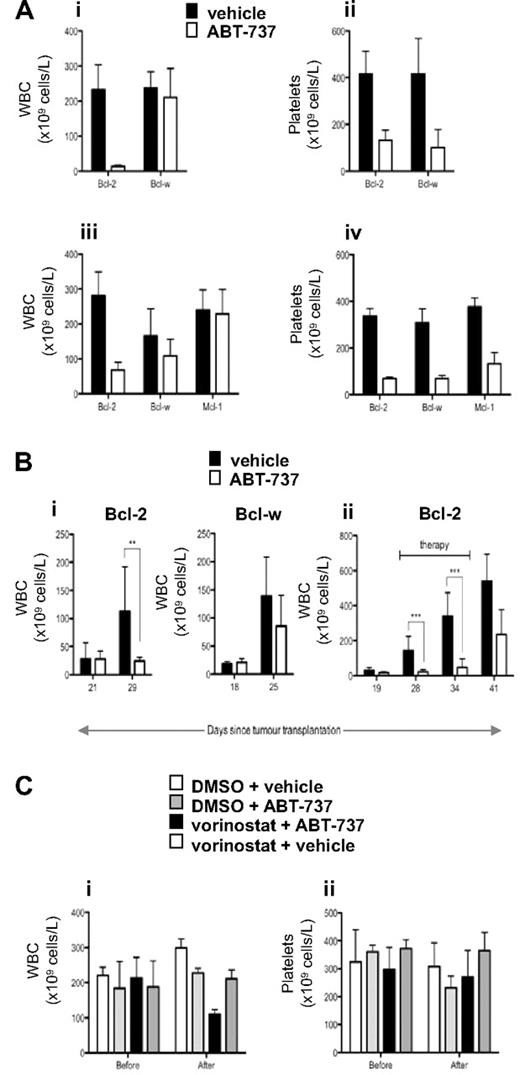
Our in vitro data demonstrated that ABT-737 selectively killed tumor cells overexpressing Bcl-2 or Bcl-XL and at lower doses could sensitize these cells to vorinostat-induced apoptosis. To determine whether these effects could be recapitulated in vivo, we treated mice bearing established FLR lymphomas overexpressing Bcl-2, Bcl-w, or Mcl-1 with ABT-737. As demonstrated in Figure 6A, treatment of mice bearing FLR lymphomas overexpressing Bcl-2 with a single dose of 75 or 100 mg/kg ABT-737 resulted in a decrease in tumor burden 12 hours after administration of the compound. At the 100 mg/kg dose, WBC levels were restored to physiologic levels (Figure 6A). In contrast, treatment of FLR lymphomas overexpressing Bcl-w or Mcl-1 had no significant effect on WBC numbers (Figure 6A). The activity of ABT-737 at the doses used in these experiments was demonstrated by the dramatic reduction in platelet numbers in the treated tumor-bearing mice (Figure 6A), which is consistent with previous studies demonstrating that ABT-737 directly induces apoptosis of platelets in vivo.17,18 To further demonstrate the in vivo effects of ABT-737 used at a relatively high dose as a single agent, mice bearing FLR lymphomas overexpressing Bcl-2 or Bcl-w were treated daily for 1 week with 100 mg/kg ABT-737. As shown in Figure 6Bi, the tumor burden in mice bearing FLR lymphomas overexpressing Bcl-2 was significantly reduced after treatment with ABT-737 for 7 days. In contrast, ABT-737 had no effect on the WBC counts in mice with established FLR lymphomas overexpressing Bcl-w (Figure 6Bi). Extended daily treatment of mice bearing FLR lymphomas overexpressing Bcl-2 with ABT-737 resulted in a sustained suppression of tumor load, however after removal of the agent the WBC counts elevated and the mice became leukemic (Figure 6Bii).
In vivo effect of ABT-737 in mice bearing lymphomas overexpressing Bcl-2, Mcl-1, and Bcl-w. (A) Eμ-myc FLR tumor cells overexpressing Bcl-2, Bcl-w, or Mcl-1 were injected intravenously into C57BL/6 mice, and tumors allowed to develop over 22 to 36 days. When the tumor burden was high (average WBC count > 200 × 109 cells/L), mice were divided into groups (n ≥ 3, except for the Bcl-2 diluent group in iii and iv, where n = 2) matched for both WBC and platelet count, and treated by intraperitoneal injection of (i,ii) 100 mg/kg ABT-737 or vehicle; (iii,iv) 75 mg/kg ABT-737 or vehicle. Peripheral blood was collected 12 hours after treatment, and WBC and platelet numbers were determined. The Bcl-2– and Bcl-w–overexpressing tumors in i and ii were derived from the same pool (10) of Eμ-myc fetal liver cells. The Bcl-2– and Mcl-1–overexpressing tumors in iii and iv were also derived from the same pool (12) of Eμ-myc fetal liver cells. The Bcl-w–overexpressing FLR lymphomas tested in iii and iv were from another pool (11) of Eμ-myc fetal liver cells. Mean and standard deviation is shown. (Bi) Eμ-myc FLR tumor cells overexpressing Bcl-2 or Bcl-w (derived from Eμ-myc fetal liver pool 10) were injected intravenously into C57BL/6 mice, and tumors allowed to develop over time until WBC counts were greater than 50 × 109cells/L. ABT-737 (100 mg/kg) or vehicle was administered intraperitoneally daily, and WBC numbers were counted after 7 days of therapy. **P < .01. (ii) Eμ-myc FLR tumor cells overexpressing Bcl-2 (derived from Eμ-myc fetal liver pool 2) were injected intravenously into C57BL/6 mice, and tumors allowed to develop over 19 days until the mice became leukemic (WBC count > 13 × 106 cells/mL). From days 20 through 37, mice were injected intraperitoneally with ABT-737 (100 mg/kg) or vehicle daily and WBC counts were recorded over 2 weeks after therapy. ***P < .001. (C) Eμ-myc FLR tumor cells overexpressing Bcl-2 (derived from Eμ-myc fetal liver pool 12) were injected intravenously into C57BL/6 mice, and tumors allowed to develop over time until WBC counts were greater than 200 × 109 cells/L. Mice were injected intraperitoneally with the diluent for vorinostat (DMSO; n = 3) and the diluent for ABT-737 (vehicle; n = 3), ABT-737 (25 mg/kg) and DMSO (n = 3), vorinostat (200 mg/kg) and vehicle (n = 3), or vorinostat (200 mg/kg) and ABT-737 (25 mg/kg, n = 4). Peripheral blood was collected 12 hours before and 12 hours after treatment, and WBC and platelet numbers were determined. Mean and SD are shown.
In vivo effect of ABT-737 in mice bearing lymphomas overexpressing Bcl-2, Mcl-1, and Bcl-w. (A) Eμ-myc FLR tumor cells overexpressing Bcl-2, Bcl-w, or Mcl-1 were injected intravenously into C57BL/6 mice, and tumors allowed to develop over 22 to 36 days. When the tumor burden was high (average WBC count > 200 × 109 cells/L), mice were divided into groups (n ≥ 3, except for the Bcl-2 diluent group in iii and iv, where n = 2) matched for both WBC and platelet count, and treated by intraperitoneal injection of (i,ii) 100 mg/kg ABT-737 or vehicle; (iii,iv) 75 mg/kg ABT-737 or vehicle. Peripheral blood was collected 12 hours after treatment, and WBC and platelet numbers were determined. The Bcl-2– and Bcl-w–overexpressing tumors in i and ii were derived from the same pool (10) of Eμ-myc fetal liver cells. The Bcl-2– and Mcl-1–overexpressing tumors in iii and iv were also derived from the same pool (12) of Eμ-myc fetal liver cells. The Bcl-w–overexpressing FLR lymphomas tested in iii and iv were from another pool (11) of Eμ-myc fetal liver cells. Mean and standard deviation is shown. (Bi) Eμ-myc FLR tumor cells overexpressing Bcl-2 or Bcl-w (derived from Eμ-myc fetal liver pool 10) were injected intravenously into C57BL/6 mice, and tumors allowed to develop over time until WBC counts were greater than 50 × 109cells/L. ABT-737 (100 mg/kg) or vehicle was administered intraperitoneally daily, and WBC numbers were counted after 7 days of therapy. **P < .01. (ii) Eμ-myc FLR tumor cells overexpressing Bcl-2 (derived from Eμ-myc fetal liver pool 2) were injected intravenously into C57BL/6 mice, and tumors allowed to develop over 19 days until the mice became leukemic (WBC count > 13 × 106 cells/mL). From days 20 through 37, mice were injected intraperitoneally with ABT-737 (100 mg/kg) or vehicle daily and WBC counts were recorded over 2 weeks after therapy. ***P < .001. (C) Eμ-myc FLR tumor cells overexpressing Bcl-2 (derived from Eμ-myc fetal liver pool 12) were injected intravenously into C57BL/6 mice, and tumors allowed to develop over time until WBC counts were greater than 200 × 109 cells/L. Mice were injected intraperitoneally with the diluent for vorinostat (DMSO; n = 3) and the diluent for ABT-737 (vehicle; n = 3), ABT-737 (25 mg/kg) and DMSO (n = 3), vorinostat (200 mg/kg) and vehicle (n = 3), or vorinostat (200 mg/kg) and ABT-737 (25 mg/kg, n = 4). Peripheral blood was collected 12 hours before and 12 hours after treatment, and WBC and platelet numbers were determined. Mean and SD are shown.
To demonstrate in vivo synergy using the combination of ABT737 and vorinostat, mice bearing FLR lymphomas overexpressing Bcl-2 were treated vorinostat or ABT-737 alone at doses that had little or no effect on tumor load (Figure 6Ci). However, a combination of vorinostat and ABT-737 at these doses resulted in a significant decrease in WBC numbers. Importantly, and in contrast to the data shown in Figure 6A, these doses of vorinostat or ABT-737, used alone or in combination had little or no effect on the platelet counts in the treated mice (Figure 6Cii). These data demonstrate that ABT-737 and vorinostat can synergistically kill Bcl-2–overexpressing tumor cells in vivo at doses that cause no demonstrable side effects.
Discussion
Recent evidence using preclinical mouse models of cancer suggests that the therapeutic effects of HDACi are dependent on their ability to mediate apoptosis.2,3 We have shown that the HDACi vorinostat induced tumor cell apoptosis via activation of the intrinsic apoptotic pathway, and overexpression of Bcl-2 or Bcl-XL inhibited the apoptotic and therapeutic activities of the compound.3 We therefore hypothesized that a combination of vorinostat and an inhibitor of Bcl-2 and/or Bcl-XL would be effective in killing those tumors that are resistant to vorinostat due to overexpression of these prosurvival proteins. Herein, we used ABT-737, a small molecule inhibitor of prosurvival Bcl-2 proteins with putative specificity for Bcl-2, Bcl-XL, and Bcl-w9–16 to test our hypothesis.
Using established primary Eμ-myc lymphoma cells induced to overexpress Bcl-2, Bcl-XL, Bcl-w, Mcl-1, or A1, we found all 5 prosurvival Bcl-2 proteins could confer resistance to 2 structurally different HDACis, vorinostat and VPA. Enforced expression of Bcl-2 and Bcl-XL, but not Bcl-w, Mcl-1, or A1 sensitized Eμ-myc lymphoma cells to death induced by ABT-737 used as a single agent. Accordingly, ABT-737 could synergize with vorinostat or VPA in vitro to kill tumors overexpressing Bcl-2 and Bcl-XL, but not those lymphoma cells overexpressing Bcl-w, Mcl-1, or A1. Moreover, we found that Eμ-myc lymphomas that develop in the presence of overexpressed Bcl-2 were hypersensitive to apoptosis mediated by ABT-737, despite being arrested in G1. Importantly, we demonstrated that our in vitro data could be recapitulated in mice bearing lymphoma cells overexpressing Bcl-2, Mcl-1, or Bcl-w. Tumor burden was dramatically reduced in mice with FLR Eμ-myc/Bcl-2 cells using a single relatively high dose of ABT-737 that caused a concomitant reduction in platelet numbers in the peripheral blood. In contrast, no such therapeutic effect was seen in ABT-737–treated mice bearing tumors overexpressing Mcl-1 or Bcl-w. Finally, we demonstrated that ABT-737 and vorinostat could cooperate in vivo to reduce the tumor burden of mice bearing lymphoma cells overexpressing Bcl-2, at doses that did not cause a demonstrable decrease in platelet numbers.
Chemoresistance that arises after initial rounds of therapy is frequently associated with overexpression of prosurvival Bcl-2 family proteins.19 Such “acquired resistance” may result from genetic alterations precipitated by exposure to genotoxic agents and/or from drug-induced selection of resistant clones. Our data indicate that regardless of the mechanism responsible, tumors with acquired addiction to Bcl-2 or Bcl-XL that, therefore, develop sustained resistance to conventional chemotherapeutic drugs and agents such as HDACis, may be prime targets for compounds like ABT-737. Such agents may, therefore, be particularly useful as second-line therapies after more conventional first-line treatment with cytotoxic agents. Undoubtedly, not all tumor cells overexpressing Bcl-2 or Bcl-XL will be sensitive to ABT-737 or similar compounds. For example, loss of expression or function of certain BH3-only proteins, or key sensitizers of “oncogenic stress” such as p53, that function upstream of the Bcl-2 family proteins could suppress the intrinsic pathway apoptotic signal. Accordingly, these cells would be rendered less sensitive to ABT-737 as a single agent as these cells may not be “primed” to die once the prosurvival signal provided by Bcl-2 or Bcl-XL is released.25 In these circumstances, the combination of ABT-737 and an HDACi could be effective since HDACis have been shown to rapidly increase the expression or otherwise activate multiple BH3-only proteins including Bmf, Bim, Bid, Puma, Noxa, and Bad,1,26–28 and can function in the absence of wild-type p53.3 Indeed, we have recent data indicating that pretreatment of Eμ-myc/Bcl-2 and Eμ-myc/Bcl-XL cells with vorinostat for 12 to 16 hours at nonapoptotic doses sufficiently “primes” cells for rapid (4-8 hours) and robust (> 60% apoptosis) cell death using low concentrations (< 0.5 μM) of ABT-737 (data not shown). Furthermore, we found that a combination of vorinostat and ABT-737 at doses that alone had no effect on tumor load in vivo, effectively reduced the number of FLR lymphoma cells overexpressing Bcl-2 present in the peripheral blood. These preclinical proof-of-principal experiments show that the combination of HDACi and ABT-737 may be a therapeutically attractive approach.
Our data also demonstrate that ABT-737 may have a more selective target specificity profile for Bcl-2 family proteins than originally thought. The affinity of ABT-737 for Bcl-2, Bcl-XL, and Bcl-w had been previously determined using competitive binding assays that used recombinant proteins and peptides representing BH3-only domains.9–11 It is possible that the biochemical binding assays used to define the target specificity of ABT-737 may not have reflected the physiologic situation. Indeed, the hydrophobic groove within Bcl-w that docks with the BH3 domain of BH3-only proteins has been shown to be occupied by an α-helix located within its own C-terminal tail in vivo.29,30 This feature serves to regulate access of BH3 domains to the hydrophobic groove of Bcl-w, and this could similarly prevent access to small molecule BH3 mimetics such as ABT-737. Indeed, consistent with our own data, it was recently shown that elevated expression of Bcl-w mRNA was a feature of primary acute lymphoblastic leukemias resistant to ABT-263, a structural analog of ABT-737.31
Eμ-myc lymphomas that developed in the presence of overexpressed Bcl-2 were highly “addicted” to the prosurvival protein, as these cells were at least 10 times more sensitive to ABT-737 than were established lymphomas that had enforced expression of Bcl-2 after cellular transformation. Accordingly, diseases such as follicular lymphoma, which develops as a result of deregulated expression pf Bcl-2 caused by a t(14;18) chromosomal translocation, will be prime candidates for single agent therapy with ABT-737. Our ex vivo studies using FLR lymphomas overexpressing Bcl-2 provided a final important piece of information to our study, in that these cells did not proliferate in culture yet were highly sensitive to ABT-737. This may be important in the context of the utilization of ABT-737 to treat hematologic malignancies such as chronic lymphocytic leukemia that often overexpress Bcl-2 but have slow rates of proliferation and solid tumors that often contain a mix of highly proliferative and quiescent tumor cells.32
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank David Huang, Andreas Strasser, Clare Scott, Jerry Adams, Suzanne Cory, and Alan Harris from the Walter and Eliza Hall Institute for helpful advice, Eu-myc mice, and cDNAs for Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and A1. We thank Drs Saul Rosenberg, Steve Elmore, Alex Shoemaker, and Victoria Richon for helpful advice and providing reagents. Vorinostat was kindly provided by Merck, and ABT-737 was kindly provided by Abbott Laboratories.
R.W.J. is a Pfizer Australia Research Fellow and is supported by a NHMRC (Canberra, Australia) Program Grant 251608, the Cancer Council Victoria (Melbourne, Australia), the Leukemia Foundation of Australia (Box Hill, Australia), and the Bennelong Foundation (Melbourne, Australia).
Authorship
Contribution: K.F.W. performed research and analyzed data; A.E.A. performed research, analyzed data, and wrote the paper; L.A.C. performed research and contributed vital new reagents; A.W., K.-M.B., and C.C. performed research; M.J.P. contributed vital new reagents; A.N. and R.K.L. performed research and contributed vital new reagents; and R.W.J. analyzed data and wrote the paper.
Conflict-of-interest disclosure: R.W.J. receives a collaborative research grant from Merck for research involving vorinostat. The other authors declare no competing financial interests.
Correspondence: Ricky Johnstone, Cancer Immunology Program, The Peter MacCallum Cancer Centre, Trescowthick Research Laboratories, St Andrews Place, East Melbourne 3002 Victoria, Australia; e-mail: ricky.johnstone@petermac.org.
References
Author notes
*K.F.W. and A.E.A. contributed equally to this work.




![Figure 5. Eμ-myc lymphomas forced to overexpress Bcl-2 throughout lymphomagenesis are hypersensitive to ABT-737. (A) Myc-positive fetal liver progenitor cells were transduced with MSCV, MSCV-Bcl-2, MSCV-Bcl-w, and MSCV-Mcl-1 retrovirus and injected into irradiated recipient PTPRCA mice (MSCV [n=54[,MSCV-Bcl-2 [n=18[, MSCV-Bcl-w [n=8[, and MSCVMcl-1 [n=8[). GFP-positive cells were injected into irradiated recipient PTPRCA mice (MSCV [n = 54], MSCV-Bcl-2 [n = 18], MSCV-Bcl-w [n = 8], and MSCV-Mcl-1 [n = 8]). Lymphomagenesis was monitored and Kaplan-Meier survival curves were obtained. Lymphoma cells harvested from the mice at sacrifice, designated FLR-Eμ-myc lymphomas, were stored for further use. (B) Western blot was performed using whole cell lysates from representative FLR-Eμ-myc/Bcl-2 and FLR-Eμ-myc/Mcl-1 cells (both derived from pool no. 12 of fetal liver cells) and antibodies against Mcl-1, Bcl-2, and α-tubulin. (C) FLR-Eμ-myc/Bcl-2 (top panels) and FLR-Eμ-myc/Mcl-1 cells (bottom panels) were treated with increasing concentrations of ABT-737 or ABT-737e. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments. (D) The cell-cycle profile of FL-Eμ-myc/Bcl-2 cells cultured ex vivo was obtained by analyzing the DNA content of cells by fluorescence-activated cell sorting (FACS) after incubation in a hypotonic solution containing PI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/9/10.1182_blood-2008-05-156851/6/m_zh80090931580005.jpeg?Expires=1765124340&Signature=1N6JUlaQguAc8Kbv4E2cbCh1MT3xZ5jKn9IPq4PmRRQOOZrN3WI6lyJg8M-j0AR5FuisrvQ2LBhQZ4Dmhxs6eERoTfqn5bgr6axABSEWY5-hqkc2FiZP5WYySXZueTeQGYqNivBIBsQOc6byE92lh0wWGqwNT-AJQWMzehT4BeauzOhAIgjmsOLbuhWHifd5PgsSh3IVPFOQQWKIrjzNi0HDSF1jIEHRlaOcTX8b6iDvvnlgMYsJ~dHe1crRm5WDqE3QTaKMRliYdsgc1qLJnRN8q4UAEYuTXd42b~KZFh-2-QcDNg~mGOuAdzOINjXrYWfzs2An19g4TiFq~torLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 5. Eμ-myc lymphomas forced to overexpress Bcl-2 throughout lymphomagenesis are hypersensitive to ABT-737. (A) Myc-positive fetal liver progenitor cells were transduced with MSCV, MSCV-Bcl-2, MSCV-Bcl-w, and MSCV-Mcl-1 retrovirus and injected into irradiated recipient PTPRCA mice (MSCV [n=54[,MSCV-Bcl-2 [n=18[, MSCV-Bcl-w [n=8[, and MSCVMcl-1 [n=8[). GFP-positive cells were injected into irradiated recipient PTPRCA mice (MSCV [n = 54], MSCV-Bcl-2 [n = 18], MSCV-Bcl-w [n = 8], and MSCV-Mcl-1 [n = 8]). Lymphomagenesis was monitored and Kaplan-Meier survival curves were obtained. Lymphoma cells harvested from the mice at sacrifice, designated FLR-Eμ-myc lymphomas, were stored for further use. (B) Western blot was performed using whole cell lysates from representative FLR-Eμ-myc/Bcl-2 and FLR-Eμ-myc/Mcl-1 cells (both derived from pool no. 12 of fetal liver cells) and antibodies against Mcl-1, Bcl-2, and α-tubulin. (C) FLR-Eμ-myc/Bcl-2 (top panels) and FLR-Eμ-myc/Mcl-1 cells (bottom panels) were treated with increasing concentrations of ABT-737 or ABT-737e. Cell membrane disruption (left panels) and MOMP (right panels) were assessed by PI and TMRE staining, respectively. Results shown are the mean and SE from at least 3 separate experiments. (D) The cell-cycle profile of FL-Eμ-myc/Bcl-2 cells cultured ex vivo was obtained by analyzing the DNA content of cells by fluorescence-activated cell sorting (FACS) after incubation in a hypotonic solution containing PI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/9/10.1182_blood-2008-05-156851/6/m_zh80090931580005.jpeg?Expires=1765407715&Signature=XMqs2FwBlY~B1x-BIegMItnr3ZVL84OKth~Gq5hCT2baja70bSBxFtbZh5g01ugCGHl~cphMRvkiIEu2vT76h9Uy2MrMfrpwGH8UW03TAPNaRcjN28hwwNd~fkhqrWb2krJJFIuHNI2ZpfZqj5qYwXE5yVZuSbKBLUnkzuRSFKa7DMYbyYH1aXkN0yb~fBUjG0rR871dNwgN97u6vL8tm6OmteABajE9XN5RwMhy4IFyCZrKFNxTpJD-XS2hriyEkUcUNrjxqM5TBtEiGSgVehPJR6exhWH5Zp6j7fb0aHgyUx~kbz18po9eSh-INX8k-DTz7VPSBvZFXAe6etrZbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
