Chronic granulomatous disease (CGD) is characterized by overexuberant inflammation and autoimmunity that are attributed to deficient anti-inflammatory signaling. Although regulation of these processes is complex, phosphatidylserine (PS)–dependent recognition and removal of apoptotic cells (efferocytosis) by phagocytes are potently anti-inflammatory. Since macrophage phenotype also plays a beneficial role in resolution of inflammation, we hypothesized that impaired efferocytosis in CGD due to macrophage skewing contributes to enhanced inflammation. Here we demonstrate that efferocytosis by macrophages from CGD (gp91phox−/−) mice was suppressed ex vivo and in vivo. Alternative activation with interleukin 4 (IL-4) normalized CGD macrophage efferocytosis, whereas classical activation by lipopolysaccharide (LPS) plus interferon γ (IFNγ) had no effect. Importantly, neutralization of IL-4 in wild-type macrophages reduced macrophage efferocytosis, demonstrating a central role for IL-4. This effect was shown to involve 12/15 lipoxygenase and activation of peroxisome-proliferator activated receptor γ (PPARγ). Finally, injection of PS (whose exposure is lacking on CGD apoptotic neutrophils) in vivo restored IL-4–dependent macrophage reprogramming and efferocytosis via a similar mechanism. Taken together, these findings support the hypothesis that impaired PS exposure on dying cells results in defective macrophage programming, with consequent efferocytic impairment and has important implications in understanding the underlying cause of enhanced inflammation in CGD.
Introduction
The recognition and removal of apoptotic inflammatory cells by tissue macrophages (MØs) and nonprofessional phagocytes, in a process called efferocytosis,1,2 is required for resolution of inflammation and is actively anti-inflammatory. Apoptotic cell recognition drives the production of anti-inflammatory mediators such as transforming growth factor β (TGFβ) that actively suppress production of inflammatory cytokines, chemokines, eicosanoids, and nitric oxide.3 Conversely, lack of recognition and efferocytosis of apoptosing cells is associated with both autoimmunity and enhanced inflammation.4 Apoptosing neutrophils in particular, if not recognized and cleared, release injurious intracellular constituents (eg, serine proteases), which further spur inflammation.4,5
Chronic granulomatous disease (CGD) involving multiple genetic variants of the phagocyte NADPH oxidase results from defective generation of superoxide anion and downstream reactive oxygen species (ROS).6 Although recurrent bacterial and fungal infections are attributed to the lack of a functional NADPH oxidase,6,7 the disease is also characterized by autoimmunity and overexuberant sterile inflammation, the mechanisms for which remain unknown.6,8,9 For instance, enhanced production or diminished degradation of chemokines, cytokines, and eicosanoids has been demonstrated.10,,–13 Further, the occurrence of sterile granulomas and enhanced recruitment of neutrophils after injection of phlogistic, but noninfectious, material into the skin of CGD patients14 and in CGD murine models (eg, killed pathogens or thioglycolate) demonstrate underlying defects in the control of inflammation.15 Notably, delayed apoptosis as well as deficient surface expression of phosphatidylserine (PS) have been described for CGD neutrophils and result in their deficient recognition ex vivo by normal macrophages.16,–18 Both delayed neutrophil apoptosis and deficient PS exposure result from the loss of NADPH oxidase function, although the precise mechanisms are not understood. Nonetheless, after a modest delay (of usually a few hours), neutrophil apoptosis ensues. These observations prompted us to hypothesize that additional defects in the recognition of apoptotic cells, specifically inefficient efferocytosis by MØs in CGD, might contribute to exaggerated inflammation and autoimmunity characteristic of this disease.
In support of this hypothesis, several MØ populations from the gp91phox−/− murine model of X-linked chronic granulomatous disease (X-CGD) were investigated and shown to be significantly impaired in their ability to efferocytose apoptotic cells both ex vivo and in vivo compared with wild-type (WT) MØs. Importantly, X-CGD MØs were classically activated, resulting in diminished efferocytosis. Further, a central role for interleukin 4 (IL-4) signaling through activation of 12/15 lipoxygenase (LO) and peroxisome-proliferator activated receptor γ (PPARγ) was demonstrated to be lacking in X-CGD (compared with WT). Following treatment with IL-4 signaling through this pathway restored efferocytosis. Finally, the requirement for phosphatidylserine (PS), a signal previously reported to be lacking on CGD neutrophils,16,–18 for endogenous production of IL-4 by MØs and their appropriate reprogramming was shown. Taken together, these studies demonstrate defects in MØ activation and efferocytic capacity that have previously not been reported and are hypothesized to contribute to overexuberant inflammation and autoimmunity in CGD.
Methods
Animals
Male and female C57BL/6 and X-CGD (gp91phox−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and also used from a breeding colony housed at National Jewish Medical Research Center. All animals received care in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee and were maintained on food and water ad libitum. Bone marrow–derived MØs (BMDMØs), resident peritoneal MØs (RPMØs), thioglycolate-elicited and alveolar MØs (AMØs) were from mice between the ages of 8 and 16 weeks were age and sex matched for all experiments.
Reagents
Recombinant mouse IL-4 (10 ng/mL), IL-13 (10 ng/mL), and interferon γ (IFNγ; 100 ng/mL) were purchased from R&D Systems (Minneapolis, MN); LPS (100 ng/mL), from List Biologicals (Campbell, CA); GW9662 (5 μM), rosiglitazone (1 μM), and PD146176 (2.5 μM), from Sigma-Aldrich (St Louis, MO); and 12-HETE (6 μM), from Cayman (Ann Arbor, MI). IL-4–neutralizing antibody (500 ng/mL), anti–mouse IgG isotype control (500 ng/mL), anti–IL-4 (rat IgG1), anti-CD14 (rat IgG2a), anti-CD36 (rat IgG2a), and anti-CD45 (500 ng/106) to opsonize cells were from eBiosciences (San Diego, CA). Antibodies to MØ mannose receptor (MMR; goat IgG) and IL-4R (goat IgG) were purchased from R&D Systems; F4/80 (rat IgG2a) antibody, from Invitrogen (Carlsbad, CA); PPARγ (rabbit IgG), from Abcam (Cambridge, MA); and 12/15-LO (rabbit IgG), from Calbiochem (San Diego, CA); antibodies were used as described.
Isolation of MØs
Thioglycolate-elicited peritoneal MØs were obtained as described previously.19 Briefly, mice were injected intraperitoneally with 1.5 mL of 4% sterile and aged (1 month) solution of Brewer Thioglycolate Medium (Difco Laboratories, Detroit, MI). Five days after injection, mice were killed with CO2 and the peritoneal cavity was lavaged with 5 to 10 mL sterile HBSS (Cellgro, Kansas City, MO). Peritoneal cells were centrifuged at 516g for 10 minutes at 4°C, and plated at 3 × 105 cells/well in 24-well plates. RPMØs were isolated using 5 mL sterile HBSS to lavage the peritoneum after killing with CO2. Resident peritoneal cells were centrifuged at 516g for 10 minutes at 4°C, and plated at 5 × 105 cells/well under culture conditions described under “Cell culture.” Mouse bone marrow–derived MØs were prepared and cultured in Dulbecco modified Eagle medium (DMEM) containing 10% FBS and 10% L cell–conditioned medium as a source of macrophage colony-stimulating factor (M-CSF) for 5 days as previously described.20
Cell culture
RPMØs and thioglycolate MØs were cultured in DMEM supplemented with 10% heat-inactivated FBS (Atlanta Biologicals, Lawrenceville, GA), 2 mM l-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin in humidified 10% CO2 at 37°C. Human leukemia Jurkat T-cell line was obtained from ATCC (Manassas, VA) and cultured in RPMI (MediaTech, Herndon, VA) containing 10% heat-inactivated FBS supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich) in humidified 5% CO2 at 37°C.
Induction of apoptosis
Jurkat T cells were exposed to ultraviolet (UV) irradiation (254 nm) for 10 minutes and cultured for 2 hours in 5% CO2 at 37°C. Human neutrophils from healthy donors were obtained with approval of the institutional review board at the National Jewish Medical and Research Center. Using endotoxin-free reagents and plasticware, human neutrophils were isolated by the plasma Percoll method, as described previously,21 resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer with 0.05% bovine serum albumin (BSA) and UV irradiated for 5 minutes. Apoptosis was quantified by evaluation of nuclear morphology by light microscopy after incubation at 37°C for 2 to 2.5 hours. Jurkat T cells and neutrophils were used when approximately 50% apoptotic by this method. Thymocytes isolated from 4-week-old C57BL/6 mice were exposed to UV for 5 minutes in RPMI with 10% FBS and cultured in 5% CO2 at 37°C for 3 hours. Apoptosis of these cells was confirmed by Alexa Fluor 488–annexin V (Invitrogen) and propidium iodide (PI; Sigma-Aldrich) staining. Thymocytes were 50% to 70% apoptotic (annexin V positive, PI negative) by these methods. Paul Karl Moran (PKH26; 10 μM; Sigma-Aldrich) was used to label thymocytes for 15 minutes in a 37°C water bath. The reaction was stopped with 10% FBS, and cells were washed twice in RPMI and resuspended in phosphate-buffered saline (PBS; 20 × 106 cells/mL).
Efferocytosis assay
Ex vivo efferocytosis assays were performed as previously described.22 Briefly, MØs cultured for 24 hours were washed with warm media, treated with various reagents at time and doses indicated, and washed twice before adding apoptotic Jurkat T cells or human neutrophils (resuspended in DMEM) at a ratio of 4:1 (target cell–phagocyte). Cells were cocultured for 60 minutes at 37°C in 5% CO2, washed 3 times with PBS, and stained with modified Wright Giemsa stain (Fisher Scientific, Pittsburgh, PA). Efferocytic index was calculated using the following formula: (number of apoptotic bodies/200 total MØs) × 100.19 A minimum of 200 MØs were counted blindly. Each condition was tested in duplicate using at least 4 mice per experiment and repeated at least 3 times or as indicated in figure legends.
In vivo efferocytosis assays in the peritoneum were performed by injecting 10 × 106 PKH-labeled apoptotic thymocytes intraperitoneally for 1 hour and lavages performed after mice were killed. Peritoneal cells were fixed, stained with anti-F4/80, and analyzed by flow cytometry. Percentage efferocytosis of F4/80-positive RPMØs was calculated as follows: (number of MØs with apoptotic thymocytes/total number of MØs) × 100. In vivo assays in the lung were performed as described previously.23 Briefly, apoptotic thymocytes, suspended in 50 μL PBS, were instilled intratracheally and 40 minutes later, whole lung bronchoalveolar lavages were performed. Cells were fixed and stained with modified Wright Giemsa (Fisher Scientific). Efferocytosis of AMØs was determined by visual inspection of samples and efferocytic index. A minimum of 400 AMØs were counted blindly.
Flow cytometric analysis
Cells were harvested, washed, and resuspended in Cytofix solution (BD Biosciences, Mountain View, CA) for 20 minutes, washed in PBS (0.1% BSA), blocked with anti–mouse Fc (CD16/32; eBiosciences) for 30 minutes, and stained with the following anti–mouse antibodies: Alexa Fluor 488 F4/80 (1:100), phycoerythrin (PE)–conjugated CD36 (1:25), Alexa Fluor 647 IL-4 (1:25), PE-CD14 (1:50), and unconjugated MMR (1:50) and IL-4R (1:50) antibodies or appropriate isotype controls for 1 hour at 4°C. Secondary conjugated antibodies were used for 30 minutes on ice. Cells were washed twice with PBS and analyzed using a BD FACScan flow cytometer (BD Biosciences, Mountain View, CA).
Preparation of liposomes
Small unilamellar liposomes made of pure 1-palmitoyl-2-oleoyl-sn-3-glycerophosphorylcholine (POPC) or POPC containing 1-palmitoyl-2-oleoyl-sn-3-glycerophospho-L-serine (POPS; 70:30 molar ratio; Avanti Polar Lipids, Alabaster, AL). Individual phospholipids, stored in chloroform-methanol (90:10), were added to glass tubes and dried under nitrogen. PBS was added, and the lipid mix vortexed and sonicated for approximately 15 minutes until clear. Liposomes (25 mg/mouse24 ) containing either 30 mol % PS with 70 mol % PC or 100 mol % PC in 1.0 mL PBS were injected intraperitoneally for 24 hours before further analysis.
Western blot analysis
Cell lysates (10 μg protein) were analyzed using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride (PVDF) membrane, blocked for 1 hour (PBS + 5% nonfat milk), and incubated with antibody (anti–mouse PPARγ or 12/15-LO, 1:1000) for 20 hours followed by addition of secondary antibody (1:1000) for half hour. Detection was performed using enhanced chemiluminescence (ECL) substrate (Amersham Biosciences, Piscataway, NJ) following the manufacturer's instructions.
Cytokines
RPMØs were cultured for 24 hours, washed with warm medium and culture supernatants 6 hours later were analyzed using Luminex 200 (Luminex, Austin, TX) according to the manufacturer's instructions. Briefly, 50 μL of sample was incubated with antibody-coated capture beads overnight at 4°C. Beads were washed and incubated with biotin-labeled antihuman cytokine antibodies for 2 hours at room temperature (RT) followed by incubation with streptavidin-phycoerythrin for 30 minutes. Samples were analyzed using Luminex and Beadview software (version 1.0; Upstate Cell Signaling Solutions, Temecula, CA). Standard curves of recombinant mouse cytokines were used to determine concentrations.
Statistical analysis
Statistical analysis and P value calculations were conducted using analysis of variance (ANOVA; JMP statistical program; SAS Institute, Cary, NC). The Dunnett and Tukey-Kramer tests were used for single and multiple comparisons, respectively.
Results
X-CGD MØs exhibit impaired efferocytosis ex vivo and in vivo
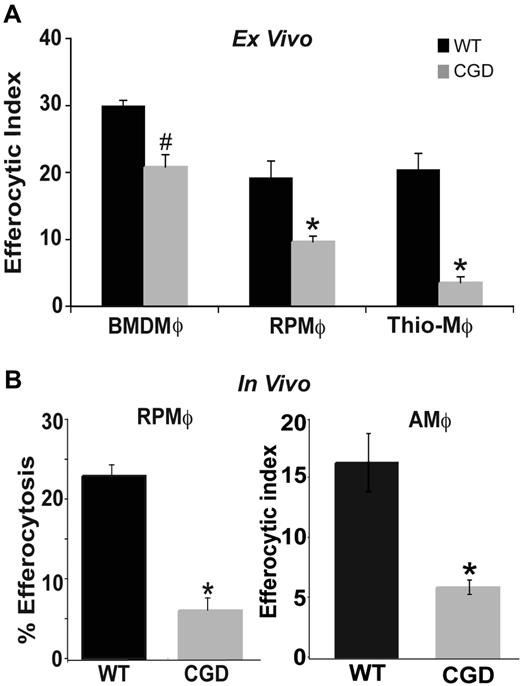
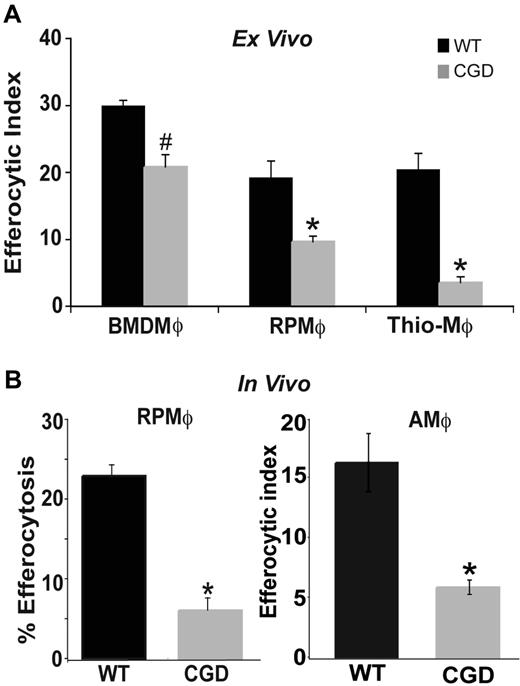
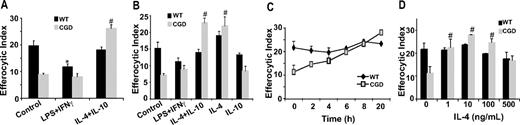
Resolution of inflammation requires that recruited inflammatory cells undergo apoptosis and are cleared by phagocytes such as MØs. Conversely, deficiency in this clearance results in disordered inflammation and autoimmunity.10,–12 To test whether MØs from gp91phox−/− X-CGD mice have impaired efferocytosis, several MØ populations were isolated and examined. RPMØs from WT and X-CGD mice were incubated with apoptotic Jurkat T cells ex vivo to assess apoptotic cell clearance. MØs from X-CGD mice showed decreased efferocytic ability compared with MØs from WT C57BL/6 mice (Figure 1A). Identical results were obtained when apoptotic human neutrophils were used as targets (data not shown). Inflammatory peritoneal MØs collected at 5 days after thioglycolate-induced sterile peritonitis also demonstrated a similar defect (Figure 1A). To rule out the possibility that this impairment was unique to the local environment of the peritoneum, bone marrow–derived MØs (BMDMØs) from WT and X-CGD mice were tested and showed decreased uptake, although somewhat less than that demonstrated by the other MØ populations (Figure 1A; “Discussion”). To determine whether the defect was also evident in vivo, efferocytosis was examined 1 hour after the injection of PKH-labeled apoptotic thymocytes into the peritoneal cavity of WT and X-CGD mice. The peritoneum was lavaged and the percentage of MØs that engulfed apoptotic thymocytes determined by flow cytometry. Defective uptake by X-CGD resident peritoneal MØs (RPMØs) was evident in vivo (Figure 1B). Similarly, apoptotic thymocytes were instilled intratracheally into the lungs of WT and X-CGD mice, and in vivo efferocytosis was determined microscopically after bronchoalveolar lavage. Alveolar MØs (AMØs) from X-CGD showed a similar defect (Figure 1B). Since diminished efferocytosis appeared to be a consistent feature of X-CGD MØs, further studies using peritoneal MØs were performed to determine the mechanism.
Efferocytosis by X-CGD MØs is impaired ex vivo and in vivo. (A) BMDMØs were cultured for 6 days; RPMØs and thioelicited MØs were plated for 24 hours and efferocytosis assays performed as described in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 5 experiments. (B) In vivo efferocytosis assays in the peritoneum and lung were performed as in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 5 experiments. #P ≤ .05; *P ≤ .005 compared with respective WT.
Efferocytosis by X-CGD MØs is impaired ex vivo and in vivo. (A) BMDMØs were cultured for 6 days; RPMØs and thioelicited MØs were plated for 24 hours and efferocytosis assays performed as described in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 5 experiments. (B) In vivo efferocytosis assays in the peritoneum and lung were performed as in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 5 experiments. #P ≤ .05; *P ≤ .005 compared with respective WT.
Alternative activation of X-CGD MØs restores efferocytosis
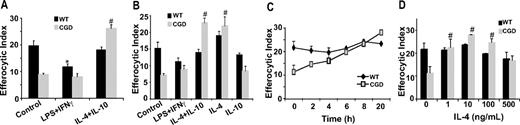
It is well established ex vivo that environmental factors activate MØs differentially resulting in distinct patterns of chemokines, surface markers, and metabolic enzymes and the ability of MØs to recognize and engulf apoptotic cells. Although overly simplified, classically activated (M1) MØs are induced by proinflammatory mediators such as IFNγ plus LPS, and produce proinflammatory cytokines (tumor necrosis factor α [TNFα], IL-6, IL-12). Alternatively activated (M2) MØs are generated by exposure to anti-inflammatory cytokines such as IL-10, IL-4, and IL-13 and various studies conducted in vitro demonstrate that M2 MØs have enhanced endocytic capacities over M1 MØs.25,,,,–30 Accordingly, we hypothesized that CGD MØs may be M1 skewed in vivo accounting for impaired efferocytosis. Classical activation (IFNγ + LPS) of X-CGD RPMØs ex vivo had no appreciable effect on efferocytosis, whereas alternative activation with IL-10 plus IL-4 significantly increased efferocytosis in comparison to untreated control X-CGD cells (Figure 2A), and to levels comparable with untreated WT MØs. Alternative activation of WT MØs had no effect on efferocytosis, whereas classical activation resulted in decreased uptake. Similar results were observed after classical and alternative activation ex vivo of WT and X-CGD thioglycolate-elicited MØs (data not shown). These data suggest that X-CGD peritoneal MØs are classically activated in vivo, accounting for their efferocytic impairment, and that this defect can be corrected after alternative activation ex vivo. To identify whether IL-4 or IL-10, or both, was required for enhanced efferocytosis after alternative activation, RPMØs were treated with either cytokine or the combination, and efferocytosis examined. Treatment with IL-4 but not IL-10 corrected the impaired efferocytosis of X-CGD MØs, while having no appreciable effect on WT cells (Figure 2B). A time course with IL-4 treatment revealed that a dose of 10 ng/mL enhanced efferocytosis within 6 to 8 hours (Figure 2C). Assays for dose dependency after 24-hour treatment with IL-4 indicated that a dose as low as 1 ng/mL was sufficient (Figure 2D). Further experiments to explore the mechanism of IL-4–mediated enhancement of X-CGD efferocytosis were performed using RPMØs.
IL-4 reverses impaired efferocytosis of X-CGD MØs. RPMØs were plated for 24 hours and treated with 100 ng/mL each of LPS and IFNγ or 10 ng/mL IL-4 and/or IL-10 for 20 hours (A,B), or 10 ng/mL IL-4 for the times indicated (C), or with indicated doses of IL-4 for 20 hours (D). Efferocytosis assays were performed as described in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 5 experiments. *P value less than or equal to .005 compared with WT control; #, P value less than or equal to .005 compared with X-CGD control. Significant differences demonstrated for RPMØs in Figure 1 were maintained but not designated for clarity.
IL-4 reverses impaired efferocytosis of X-CGD MØs. RPMØs were plated for 24 hours and treated with 100 ng/mL each of LPS and IFNγ or 10 ng/mL IL-4 and/or IL-10 for 20 hours (A,B), or 10 ng/mL IL-4 for the times indicated (C), or with indicated doses of IL-4 for 20 hours (D). Efferocytosis assays were performed as described in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 5 experiments. *P value less than or equal to .005 compared with WT control; #, P value less than or equal to .005 compared with X-CGD control. Significant differences demonstrated for RPMØs in Figure 1 were maintained but not designated for clarity.
Endogenous IL-4 is required for efficient efferocytosis by WT MØs
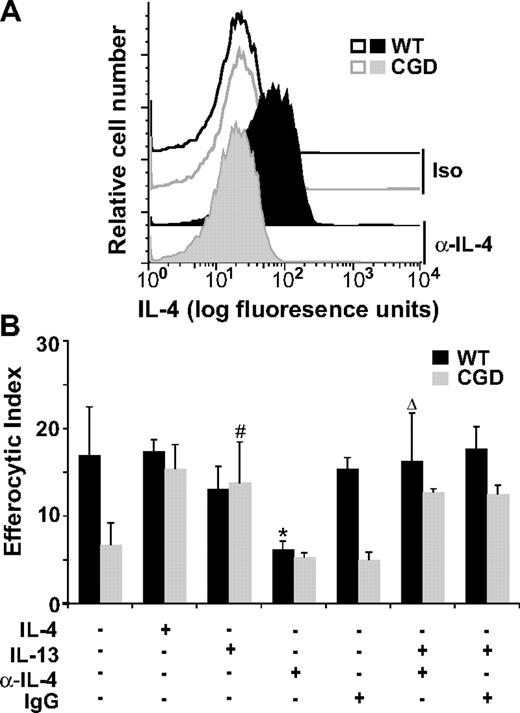
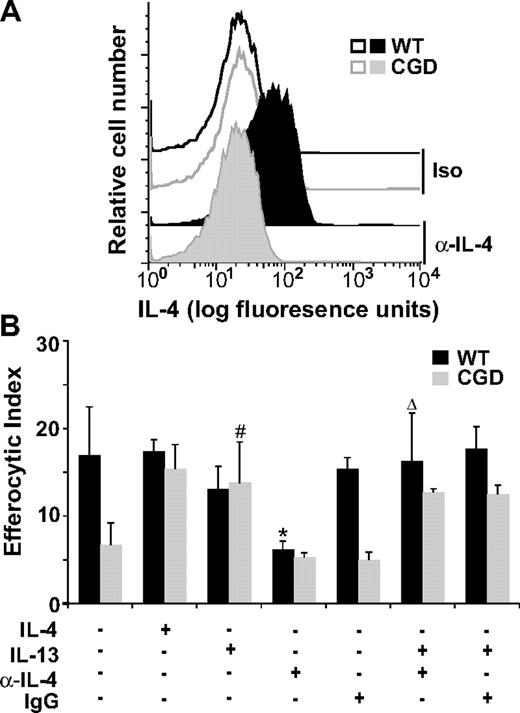
Since IL-4 was effective in reversing the efferocytic impairment of X-CGD MØs while having little effect on WT MØs, it was important to determine whether endogenous levels of IL-4 were different between WT and X-CGD MØs. Although the levels of IL-4 secreted by cultured RPMØs were below the detection limit, freshly lavaged X-CGD RPMØs, relative to WT, exhibited reduced levels of intracellular IL-4 as determined by flow cytometry (Figure 3A). Importantly, when WT MØs were cultured overnight with an IL-4–neutralizing antibody (or isotype control) before the addition of apoptotic cells, it was found that neutralization of IL-4 significantly impaired efferocytosis to levels comparable with X-CGD (Figure 3B). IL-13, known to often parallel the effects of IL-4, also restored efferocytosis of X-CGD MØs (Figure 3B). Further, the efferocytic impairment induced by the neutralizing IL-4 antibody in WT MØs was reversible by IL-13 (Figure 3B). These data demonstrate that (1) endogenous production of IL-4 in WT but not X-CGD RPMØs critically supports efferocytosis, and (2) treatment with IL-4 (or IL-13 as a substitute for IL-4) reverses efferocytic impairment of X-CGD MØs.
Intracellular IL-4 is reduced in X-CGD RPMØs and is required for efficient efferocytosis. (A) Freshly isolated F4/80-positive RPMØs were analyzed for intracellular IL-4 (compared with isotype control) by flow cytometry; histograms are representative of N = 8. (B) RPMØs were plated for 24 hours and treated with 10 ng/mL IL-4 or IL-13 and/or 500 ng/mL neutralizing antibody to IL-4 or isotype control antibody for 20 hours before performing efferocytosis assays. Data represent means plus or minus SEM; N = 5 experiments. *P ≤ .005 compared with untreated WT; #P ≤ .005 compared with untreated X-CGD; and Δ, P value less than or equal to .005 compared with α-IL-4–treated WT. Significant differences demonstrated in Figures 1 and 2 were maintained but not designated for clarity.
Intracellular IL-4 is reduced in X-CGD RPMØs and is required for efficient efferocytosis. (A) Freshly isolated F4/80-positive RPMØs were analyzed for intracellular IL-4 (compared with isotype control) by flow cytometry; histograms are representative of N = 8. (B) RPMØs were plated for 24 hours and treated with 10 ng/mL IL-4 or IL-13 and/or 500 ng/mL neutralizing antibody to IL-4 or isotype control antibody for 20 hours before performing efferocytosis assays. Data represent means plus or minus SEM; N = 5 experiments. *P ≤ .005 compared with untreated WT; #P ≤ .005 compared with untreated X-CGD; and Δ, P value less than or equal to .005 compared with α-IL-4–treated WT. Significant differences demonstrated in Figures 1 and 2 were maintained but not designated for clarity.
X-CGD RPMØs constitutively produce proinflammatory cytokines and are classically activated
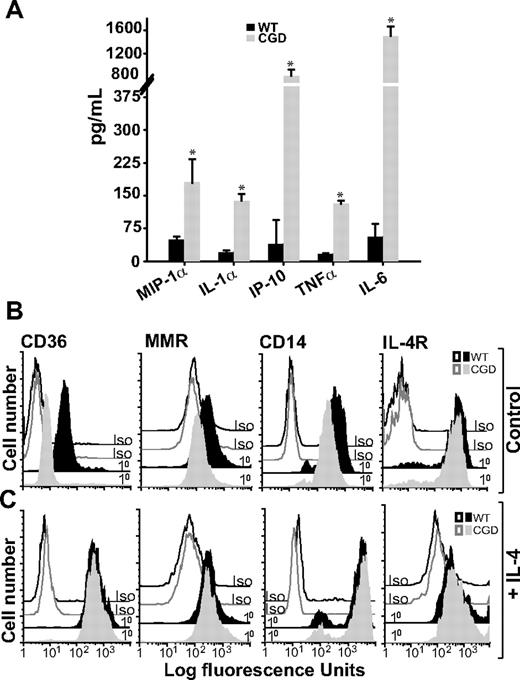
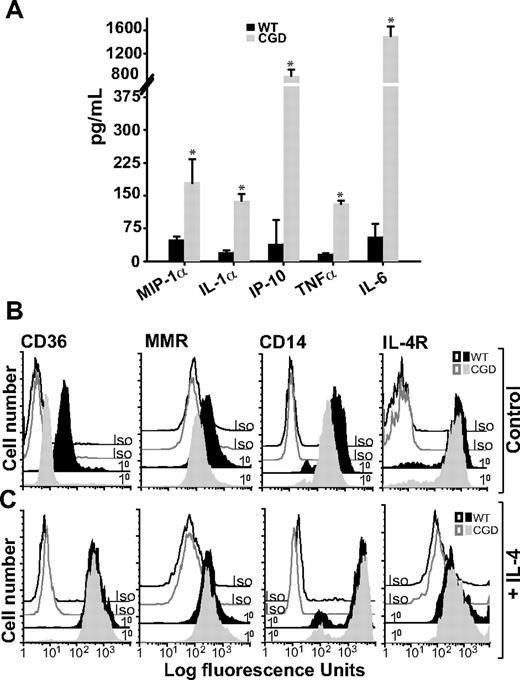
Given that deliberate classical activation did not further diminish efferocytosis by X-CGD MØs, whereas it significantly decreased efferocytosis in WT MØs (Figure 2), it was hypothesized that X-CGD MØs were classically activated in vivo, consistent with previous reports of enhanced proinflammatory mediator production associated with CGD.10,–12 RPMØs from X-CGD and WT mice were harvested and plated ex vivo, and cytokines were measured in culture supernatants 6 hours later. Higher levels of proinflammatory cytokines, macrophage-inflammatory protein 1α (MIP1α), IL-1α, IFN-γ–inducible protein 10 (IP-10), TNFα, and IL-6, were observed in the supernatants from unstimulated X-CGD MØs compared with WT (Figure 4A). Cytokines associated with alternative activation (IL-4, IL-10, IL-13, and M-CSF) were at or below the level of detection (data not shown).
Unstimulated X-CGD RPMØs produce proinflammatory cytokines and lack alternative activation markers until treated with IL-4. (A) RPMØs were plated for 24 hours, medium was changed, and 6 hours later cytokines were measured in culture supernatants as in “Cytokines.” Data represent means plus or minus SEM; N = 3 experiments. * indicates P value less than or equal to .005 compared with WT. RPMØs were plated for 2 hours and then treated either with medium alone (B) or 10 ng/mL IL-4 for 20 hours (C). Cells were analyzed by flow cytometry for alternative activation markers (CD36, MMR, and CD14) and IL-4R; histograms are representative of N = 3 experiments.
Unstimulated X-CGD RPMØs produce proinflammatory cytokines and lack alternative activation markers until treated with IL-4. (A) RPMØs were plated for 24 hours, medium was changed, and 6 hours later cytokines were measured in culture supernatants as in “Cytokines.” Data represent means plus or minus SEM; N = 3 experiments. * indicates P value less than or equal to .005 compared with WT. RPMØs were plated for 2 hours and then treated either with medium alone (B) or 10 ng/mL IL-4 for 20 hours (C). Cells were analyzed by flow cytometry for alternative activation markers (CD36, MMR, and CD14) and IL-4R; histograms are representative of N = 3 experiments.
On the other hand, markers of alternative activation were deficient in X-CGD compared with WT RPMØs. Reduced MMR, CD36, and CD14 (the latter markers implicated in the recognition of apoptotic cells31,32 ) were observed on X-CGD RPMØs compared with WT MØs (Figure 4B). Taken together, these data suggest that X-CGD RPMØs are classically activated. Importantly, treatment of X-CGD RPMØs with IL-4 resulted in acquisition of an alternatively activated receptor repertoire comparable with WT RPMØs (Figure 4C). IL-4R expression on X-CGD RPMØs was not different from WT RPMØs at either baseline or after stimulation with IL-4.
IL-4 enhances efferocytosis of X-CGD RPMØs via 12/15-lipoxygenase–dependent PPARγ activation
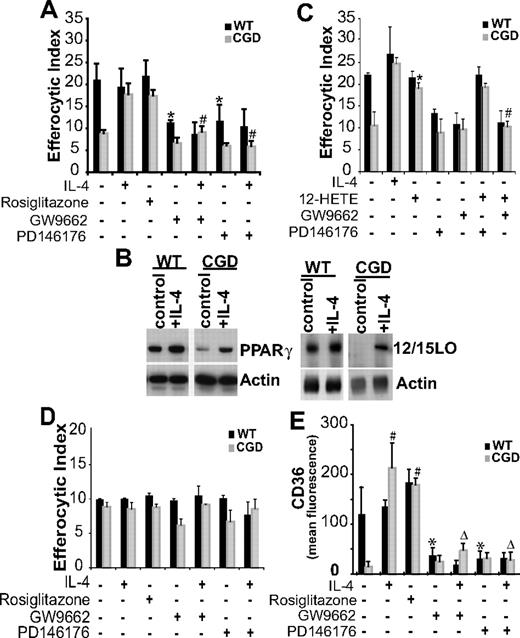
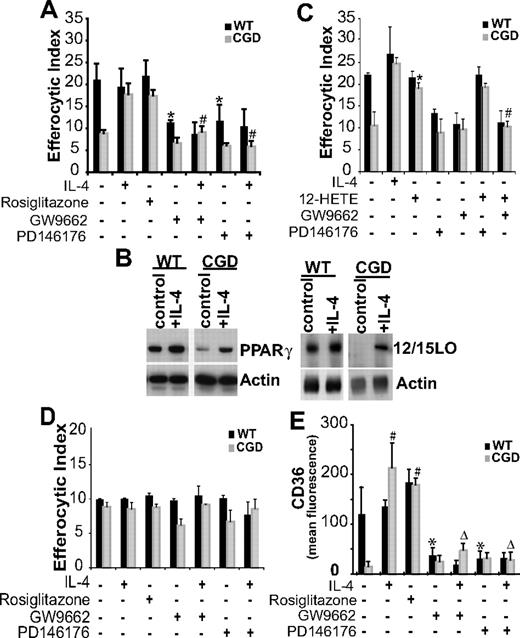
PPARγ, a nuclear transcriptional regulator, has been shown to be involved in uptake of apoptotic cells by human macrophages,33,34 and up-regulation of its expression and activation has been demonstrated in MØs after IL-4 treatment.35 To define the mechanism of IL-4–dependent efferocytosis, a role for PPARγ was examined. Significant enhancement of efferocytosis was observed when X-CGD RPMØs were treated ex vivo with a PPARγ agonist, rosiglitazone (Figure 5A). No effect was observed on WT RPMØs, suggesting the possibility of baseline signaling via PPARγ in these cells. This was supported by the observation that efferocytosis of WT MØs was inhibited by the PPARγ antagonist GW9662 to levels observed with X-CGD MØs (Figure 5A) while having no effect on X-CGD MØs (Figure 5A). In addition, reversal of the X-CGD MØ uptake defect by IL-4 was inhibited by the PPARγ antagonist (Figure 5A). These data are consistent with IL-4–dependent PPARγ activation as a requirement for efficient uptake of apoptotic cells. Lower levels of PPARγ protein were also observed in X-CGD MØ cell lysates compared with WT cells and were enhanced after IL-4 treatment (Figure 5B).
IL-4 enhances efferocytosis of X-CGD RPMØs via 12/15-LO–dependent PPARγ activation. (A) RPMØs were treated either with IL-4, rosiglitazone, GW9662, or PD146176 for 20 hours after which efferocytosis assays were performed as in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 6 experiments. * indicates P value less than or equal to .01 compared with untreated WT; #, P value less than or equal to .005 compared with IL-4–treated X-CGD. (B) RPMØs, either untreated or treated with 10 ng/mL IL-4 for 20 hours, were analyzed for protein expression by Western blot. (C) RPMØs were treated for 20 hours with either IL-4, 12-HETE, GW9662, or PD146176 or combinations as indicated before efferocytosis assays were performed. Data represent means plus or minus SEM; N = 5 experiments. *P ≤ .005 compared with untreated X-CGD; #P ≤ .005 compared with 12-HETE–treated X-CGD. (D) Assay was performed as in panel A, except that IgG-opsonized Jurkat T cells were used as targets. Data represent means plus or minus SEM; N = 3 experiments. (E) RPMØs were plated for 24 hours and then treated for 20 hours as indicated in panel A, lifted from the plate, fixed, stained, and analyzed by flow cytometry for CD36. Data represent means plus or minus SEM; N = 3 experiments. *P ≤ .01 compared with untreated WT; #P ≤ .005 compared with untreated X-CGD; and ΔP ≤ .005 compared with IL-4–treated X-CGD. Significant differences demonstrated in Figures 1–2 were maintained but not designated for clarity.
IL-4 enhances efferocytosis of X-CGD RPMØs via 12/15-LO–dependent PPARγ activation. (A) RPMØs were treated either with IL-4, rosiglitazone, GW9662, or PD146176 for 20 hours after which efferocytosis assays were performed as in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 6 experiments. * indicates P value less than or equal to .01 compared with untreated WT; #, P value less than or equal to .005 compared with IL-4–treated X-CGD. (B) RPMØs, either untreated or treated with 10 ng/mL IL-4 for 20 hours, were analyzed for protein expression by Western blot. (C) RPMØs were treated for 20 hours with either IL-4, 12-HETE, GW9662, or PD146176 or combinations as indicated before efferocytosis assays were performed. Data represent means plus or minus SEM; N = 5 experiments. *P ≤ .005 compared with untreated X-CGD; #P ≤ .005 compared with 12-HETE–treated X-CGD. (D) Assay was performed as in panel A, except that IgG-opsonized Jurkat T cells were used as targets. Data represent means plus or minus SEM; N = 3 experiments. (E) RPMØs were plated for 24 hours and then treated for 20 hours as indicated in panel A, lifted from the plate, fixed, stained, and analyzed by flow cytometry for CD36. Data represent means plus or minus SEM; N = 3 experiments. *P ≤ .01 compared with untreated WT; #P ≤ .005 compared with untreated X-CGD; and ΔP ≤ .005 compared with IL-4–treated X-CGD. Significant differences demonstrated in Figures 1–2 were maintained but not designated for clarity.
Although there are several ligands known to activate PPARγ, products of 12/15-LO have been shown to perform this function and there is precedence for coordinate up-regulation of 12/15-LO and PPARγ by IL-4.35,36 Accordingly, the involvement of 12/15-LO was assessed: WT MØs treated with a 12/15-LO inhibitor (PD146176) demonstrated reduced efferocytic capacity as did IL-4–treated X-CGD MØs (Figure 5A). Cell lysate analysis confirmed that 12/15-LO protein levels in untreated X-CGD RPMØs were lower than in WT RPMØs, and after IL-4 treatment, increased 12/15-LO expression was observed (Figure 5B). Furthermore, 12S-hydroxyl-5,8,10,14 eicosatetraenoic acid (12-HETE), a product of 12/15-LO, was found to enhance efferocytosis of X-CGD MØs but not WT MØs (Figure 5C). This enhancement was not influenced by the 12/15-LO inhibitor and inhibition of PPARγ signaling prevented uptake regardless of the presence or absence of 12-HETE (Figure 5C). These observations suggest that 12-HETE functions as a ligand for PPARγ activation and that signaling via PPARγ is responsible for the enhanced efferocytosis by X-CGD MØs. Further, these data establish that although both 12/15-LO and PPARγ expression are coordinately up-regulated by IL-4, 12/15-LO acts upstream of PPARγ. Treatment with IL-4 in the presence or absence of either the 12/15-LO inhibitor or PPARγ antagonist had no effect on the phagocytosis of IgG opsonized cells that are ingested through Fc receptors, demonstrating that these observations are specific to efferocytosis (Figure 5D).
CD36, has been reported to play a role in apoptotic cell uptake.31,37 In addition, it is associated with alternatively activated MØs and has been shown to be up-regulated after IL-4 treatment in a 12/15-LO– and PPARγ-dependent manner.34,35 Accordingly, CD36 expression was examined. Whereas IL-4 and rosiglitazone enhanced CD36 expression on X-CGD and WT MØs, the PPARγ antagonist GW9662, in the presence or absence of IL-4, inhibited CD36 expression (Figure 5E). Similar inhibition was also observed when MØs were pretreated with the 12/15-LO inhibitor PD146176 in the presence or absence of IL-4 (Figure 5E).
Phosphatidylserine enhances the efferocytosis of X-CGD MØs in an IL-4– and PPARγ-dependent manner
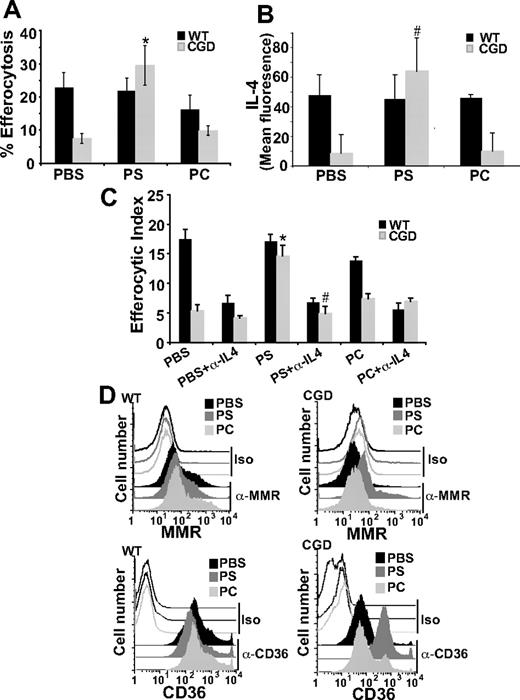
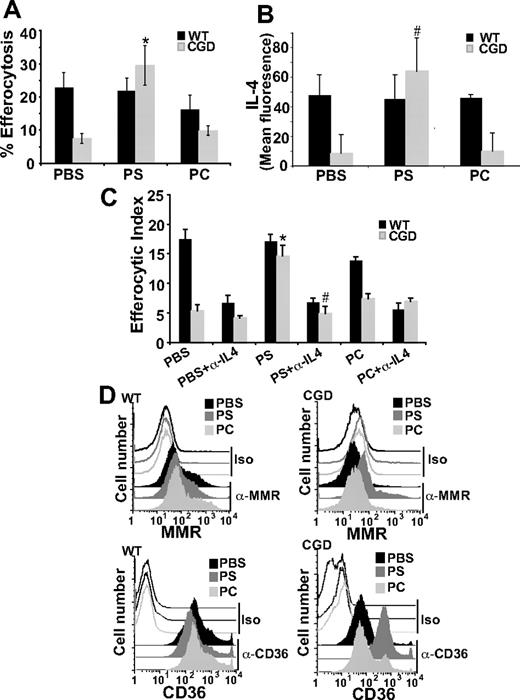
PS externalized to the outer leaflet of the plasma membrane of cells undergoing apoptosis is an important signaling molecule required for apoptotic cell recognition and removal and also suppression of proinflammatory mediator production.5,37,38 A host of PS receptors and PS-recognizing bridge molecules/receptor pairs used by various phagocytes have been described for its recognition (see “Discussion”). As there is evidence in the literature that PS exposure is lacking on apoptotic neutrophils in CGD,17 it was hypothesized that a lack of PS signaling in X-CGD might result in deficient IL-4 production and subsequent signaling via the PPARγ pathway. To test this, PS liposomes provided in vivo were assessed for their ability to enhance efferocytosis of X-CGD MØs. Liposomes, containing 100% POPC (carrier lipid) or 30% POPS (containing 70% POPC), or PBS was injected intraperitoneally into WT and X-CGD mice 24 hours before the injection of fluorescently labeled apoptotic thymocytes. A significant increase in the efferocytosis of X-CGD MØs was observed after treatment with PS liposomes, whereas WT MØs were unaffected (Figure 7A). PC carrier liposomes or PBS had no appreciable effect on efferocytosis (Figure 6A).
PS-containing liposomes enhance efferocytosis of X-CGD MØs in an IL-4–dependent manner. (A) PBS, PS, or PC liposomes were injected intraperitoneally for 24 hours and in vivo efferocytosis assay was performed as in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 6 experiments. *P ≤ .005 compared with PBS-treated X-CGD. (B) After identical treatment, freshly isolated F4/80-positive MØs were analyzed for intracellular IL-4 by flow cytometry. Data represent means plus or minus SEM; N = 3 experiments. #P ≤ .005 compared with PBS-treated X-CGD. (C) After in vivo treatment as in panel A, RPMØs were plated in the presence or absence of neutralizing antibody to IL-4 (500 ng/mL) ex vivo for 24 hours and efferocytosis assays performed. Data represent means plus or minus SEM; N = 5 experiments. *P ≤ .005 compared with PBS-treated X-CGD; #P ≤ .005 compared with PS-treated X-CGD. (D) After in vivo treatment as in panel A, RPMØs were analyzed for surface receptors by flow cytometry; histograms are representative of N = 3 experiments.
PS-containing liposomes enhance efferocytosis of X-CGD MØs in an IL-4–dependent manner. (A) PBS, PS, or PC liposomes were injected intraperitoneally for 24 hours and in vivo efferocytosis assay was performed as in “Efferocytosis assay.” Data represent means plus or minus SEM; N = 6 experiments. *P ≤ .005 compared with PBS-treated X-CGD. (B) After identical treatment, freshly isolated F4/80-positive MØs were analyzed for intracellular IL-4 by flow cytometry. Data represent means plus or minus SEM; N = 3 experiments. #P ≤ .005 compared with PBS-treated X-CGD. (C) After in vivo treatment as in panel A, RPMØs were plated in the presence or absence of neutralizing antibody to IL-4 (500 ng/mL) ex vivo for 24 hours and efferocytosis assays performed. Data represent means plus or minus SEM; N = 5 experiments. *P ≤ .005 compared with PBS-treated X-CGD; #P ≤ .005 compared with PS-treated X-CGD. (D) After in vivo treatment as in panel A, RPMØs were analyzed for surface receptors by flow cytometry; histograms are representative of N = 3 experiments.
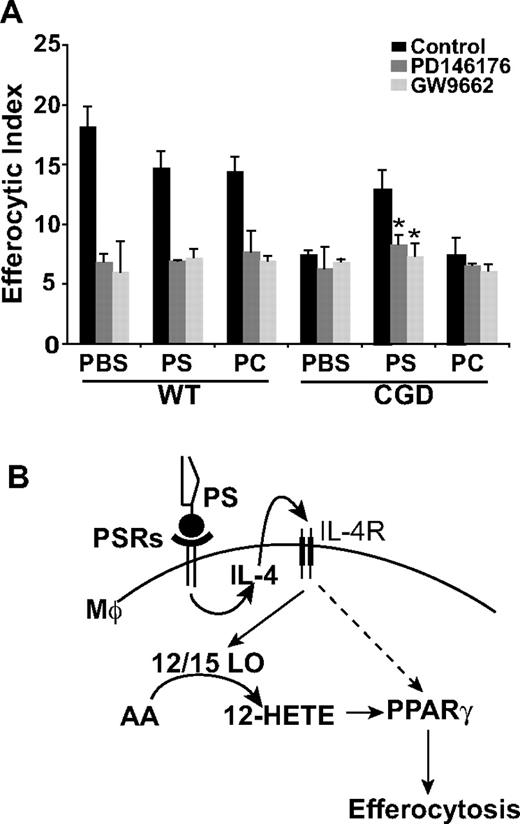
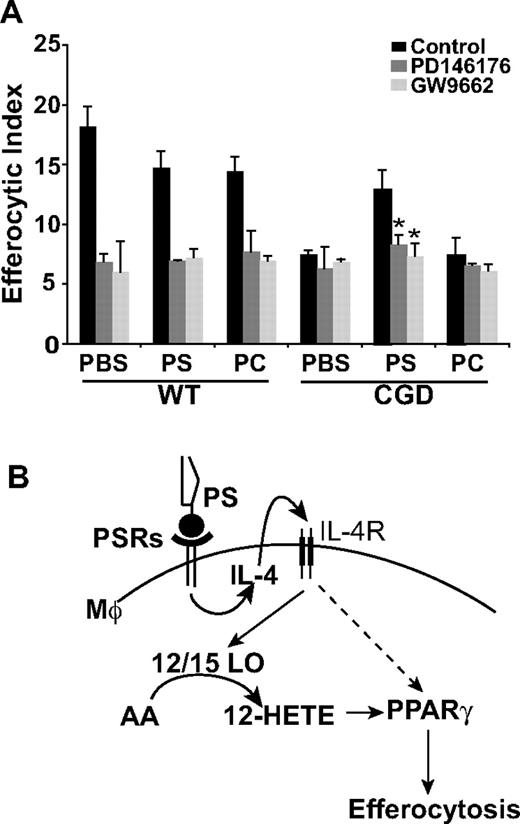
12/15-LO and PPARγ are downstream effectors of the PS-dependent enhancement of X-CGD MØ efferocytosis. (A) PBS, PS, or PC liposomes were injected intraperitoneally for 24 hours and RPMØs plated ex vivo for 24 hours in the presence or absence of either GW9662 or PD146176 before efferocytosis assays were performed. Data represent means plus or minus SEM; N = 3 experiments.*P ≤ .005 compared with PS-treated X-CGD. Significant differences demonstrated in Figure 6 were maintained but not designated for clarity. (B) Model: PS-dependent enhancement of efferocytosis is mediated by IL-4–dependent PPARγ activation and production of PPARγ activation ligands by 12/15-LO.
12/15-LO and PPARγ are downstream effectors of the PS-dependent enhancement of X-CGD MØ efferocytosis. (A) PBS, PS, or PC liposomes were injected intraperitoneally for 24 hours and RPMØs plated ex vivo for 24 hours in the presence or absence of either GW9662 or PD146176 before efferocytosis assays were performed. Data represent means plus or minus SEM; N = 3 experiments.*P ≤ .005 compared with PS-treated X-CGD. Significant differences demonstrated in Figure 6 were maintained but not designated for clarity. (B) Model: PS-dependent enhancement of efferocytosis is mediated by IL-4–dependent PPARγ activation and production of PPARγ activation ligands by 12/15-LO.
Given the prominent role of IL-4 in efferocytosis, it was hypothesized that intraperitoneal injection of PS containing liposomes might drive IL-4 production. Interestingly, pretreatment with PS has been shown to induce production of IL-4 in dendritic cells T-cell cocultures.39 After intraperitoneal treatment with either PS or PC liposomes or PBS for 24 hours, RPMØs were collected and stained for IL-4. Significantly increased levels of intracellular IL-4 were observed in X-CGD MØs after treatment with PS containing liposomes compared with the PBS control, whereas PC liposomes had no appreciable effect (Figure 6B). In addition, treatment of mice with PS liposomes reduced constitutive proinflammatory cytokine production in X-CGD MØs plated ex vivo (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). No effects were seen on WT MØs. To establish whether the observed up-regulation of IL-4 was required for enhanced efferocytosis, RPMØs were harvested after intraperitoneal treatment with PS liposomes (or control PC liposomes or PBS) for 24 hours, and plated ex vivo in the presence or absence of an IL-4–neutralizing antibody. As predicted, whereas in vivo PS liposome treatment (but not control treatments) enhanced ex vivo efferocytosis by X-CGD MØs similar to the previous in vivo experiment (Figure 6A,C), ex vivo enhancement was lost after overnight IL-4 neutralization (Figure 6C). No effect of isotype control antibody was observed in any of the treatment groups (data not shown). These data demonstrate that IL-4 is key in the PS-dependent enhancement of X-CGD MØ efferocytosis. Further, in concert with enhanced efferocytosis, in vivo treatment with PS liposomes (but not control treatments) up-regulated levels of both the MMR and CD36 (Figure 6D). Since this PS-dependent enhancement of efferocytosis after in vivo treatment of X-CGD MØs was demonstrated to be IL-4 dependent, it was hypothesized that downstream signaling from PS would be via 12/15-LO and PPARγ. After in vivo treatment with either PS liposomes or controls, peritoneal MØs were plated ex vivo in the presence or absence of either the 12/15-LO or PPARγ inhibitor. As predicted, the enhanced efferocytosis of X-CGD MØs after PS liposome treatment was abrogated by both 12/15-LO inhibitor and PPARγ antagonist (Figure 7A).
Discussion
Diverse examples of exaggerated inflammation in CGD suggest that broad mechanisms necessary to resolve inflammation are deficient. Specifically, it was hypothesized that in addition to delayed neutrophil apoptosis and inadequate PS signaling, impaired engulfment of apoptotic cells might explain the exaggerated inflammation that contributes to the considerable morbidity of this disease. Data presented here using the murine model of X-CGD demonstrate that efferocytosis by several MØ populations is defective both ex vivo and in vivo (Figure 1). Our observations that RPMØ activation state is associated with differential abilities to engulf apoptotic cells corroborate published in vitro data. Classical activation (LPS + IFNγ) of MØs reduces efferocytosis.25,,,,–30 Alternative activation of MØs with IL-4, IL-13, IL-10, or M-CSF enhances efferocytosis.25,,,,–30 In this investigation, X-CGD RPMØs demonstrated markers of classical activation, whereas WT RPMØs were alternatively activated, and these phenotypes were associated with significant differences in efferocytosis (Figures 2,4,5). Further, endogenous expression levels of IL-4 between WT and X-CGD RPMØ were different (Figure 3A), suggesting that this cytokine was involved in the modulation of MØ activation and efferocytic capacity in vivo. In support of this, production of IL-4 by unstimulated WT RPMØs was found to be required for their efficient efferocytosis (Figure 3B), and notably, impaired efferocytosis by X-CGD RPMØs was corrected after pretreatment with IL-4 ex vivo (Figure 2). Although decreased efferocytosis was demonstrated for all X-CGD MØ populations tested, BMDMØs were the least impaired (approximately 25% impairment compared with 50%-75% for the other macrophage populations; Figure 1). Unlike the other macrophage populations, which were investigated either in vivo or ex vivo immediately after harvest, BMDMØs were derived after prolonged culture in M-CSF, a cytokine also associated with alternative activation,27 which likely diminished or reversed CGD MØ phenotypic differences. Similarly, in the only other investigation of efferocytosis in CGD to date, human blood monocytes from CGD patients cultured for 6 days ex vivo in M-CSF for derivation of MØs (HMDMØs), did not show impaired efferocytosis relative to control HMDMØs.10
Importantly, the lack of a functioning NADPH oxidase in X-CGD MØs does not contribute to their efferocytic impairment. Rather, it is the cytokine milieu that programs MØs and thereby inhibits or supports efferocytosis. Although other alternatively activating cytokines can substitute for IL-4 in vitro (eg, M-CSF in BMDMØs27 and IL-13 in RPMØs; Figure 3B), the ex vivo and in vivo data point to a central role for endogenously produced IL-4 in efferocytosis (Figures 3A,7C). Further downstream mechanisms are also elucidated (Figure 7B). Other investigations have shown that IL-4 coordinately up-regulates both 12/15-LO, a source of PPARγ ligands, and PPARγ expression and/or activity.35,40 PPARγ has recently been shown to be required for efferocytosis of apoptotic cells by MØs.33,34 In accordance with this observation, rosiglitazone, a PPARγ activator, normalized efferocytic capacity of X-CGD MØs, and a PPARγ inhibitor prevented the IL-4–induced enhancement, suggesting a PPARγ-dependent pathway (Figure 5). Although WT MØ efferocytosis appeared maximal at baseline with little stimulation by the PPARγ agonist, the necessity for PPARγ signaling was apparent: a PPARγ antagonist reduced efferocytosis of WT MØs to levels seen in X-CGD MØs. The mechanism underlying IL-4–dependent efferocytic function was further investigated and a role for up-regulation of 12/15-LO activity upstream of PPARγ, not demonstrated previously, was shown to be responsible (Figure 6). In addition, IL-4–dependent up-regulation of PPARγ activity resulted in heightened expression of receptors implicated in efferocytosis (eg, CD36 and CD1431,32 ). These data linking IL-4 with enhanced expression of CD36 and PPARγ concur with previous observations in other in vitro and in vivo systems.34,35
Studies suggest that ROS produced by the activated NADPH oxidase play significant, although at times seemingly paradoxical, anti-inflammatory roles that are lost in CGD.15 Importantly, the nature of these anti-inflammatory signals has not been characterized. Impaired degradation of leukotriene B4 (LTB4), LTC4, and formyl-methionyl-leucyl-phenylalanine (fMLP) in the absence of a functioning NADPH oxidase has been demonstrated,13,41 but seems inadequate to explain the global enhancement of inflammation seen in CGD. Alternatively, the deficiency or delay in PS exposure by activated and then apoptosing neutrophils, as demonstrated in our own and other investigations,16,–18 may significantly contribute to the overexuberant inflammation in CGD. PS is a major ligand on apoptotic cells and is recognized by several bridge molecule-receptor combinations (eg, MFG-E8 and αvβ3 and αvβ5 integrins or Gas6 and Mer receptor tyrosine kinase42 ) and several newly described PS receptors (eg, Tim4 [T-cell immunoglobulin and mucin domain-containing molecule 4],43 BAI1 [brain-specific angiogenesis inhibitor 1],44 and stabilin-245 ). The specific PS receptors or bridge molecule-receptor combinations involved in recognition and clearance of apoptotic cells in specific settings ex vivo and in vivo are still being defined.46 Of note, Tim4 expression that has been described on resident peritoneal macrophages was not different on X-CGD compared with WT RPMØs (data not shown).
It is hypothesized that under normal conditions, neutrophils continuously migrate into tissues, undergo apoptosis, and expose PS providing a tonic stimulus for alternative activation of MØs capable of apoptotic cell removal. Recent data demonstrate that neutrophil migration into tissues and their subsequent apoptosis and recognition results in programming of MØs for the synthesis of cytokines controlling neutrophil production.47 Maintenance of the circulating neutrophil pool thus requires a balanced equilibrium of continuous production and turnover. In CGD, delays in neutrophil apoptosis and deficient PS in tissues are hypothesized (based on our studies) to lead to classical activation of MØs with low IL-4 production. Downstream consequences are deficient expression and activity of 12/15-LO and PPARγ, leading to delays in engulfment of apoptotic cells. Delayed apoptotic cell clearance is associated with cell cytolysis and the spilling of phlogistic intracellular contents (eg, proteases) and exposure of neoepitopes spurring autoimmunity.4,5 A critical role for PS in this model is the programming of MØs toward alternative activation. PS exposure has been linked with the production of IL-4 by dendritic cells T-cells cocultures in vitro, and here, it was demonstrated that pretreatment of classically activated MØs with PS liposomes enhanced IL-4 production, alternative activation, and efferocytic capacity in vivo (Figures 6,7). Alternatively activated MØs have a beneficial role in the resolution of inflammation.48,49 Recent studies have shown that injection of alternatively activated MØs limits kidney injury and reduces apoptotic cell accumulation in severe combined immunodeficiency (SCID) mice.50 So potent an anti-inflammatory stimulus is PS,22 that the infusion of apoptosing leukocytes after extracorporeal photophoresis is being investigated in the induction of tolerance in transplantation and control of inflammation in a variety of inflammatory diseases.51,52 Implications from this work, specifically the deliberate skewing of MØs toward alternative activation in CGD (after the risk of infection is limited with antibiotics), might diminish the exaggerated inflammation that contributes significantly to the morbidity in this disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jenai Kailey for performance of Luminex assays, Karla Kenyon for help with efferocytosis assays, Linda Remigo for help with BMDMØ preparation, Dianne Ashby for help with mice injections, and Brenda Sebern for aiding in paper preparation.
This work was funded by the National Institutes of Health (NIH, Bethesda, MD; nos. AI058228, HL34303, HL81151, GM61031, GM008947, HL68628, and HL55549) and the Immunodeficiency Foundation (IDF, Towson, MD).
National Institutes of Health
Authorship
Contribution: R.F.F.-B. designed and performed research, analyzed data, and wrote the paper; S.C.F. designed research and analyzed data; K.M., R.W.V., and B.L.H. performed research; D.W.H.R. contributed reagents and helped with the paper; P.M.H. designed research and helped with the paper; and D.L.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donna L. Bratton, Department of Pediatrics, National Jewish Medical and Research Center, 1400 Jackson Street, Denver, CO 80206; e-mail: brattond@njc.org.