To the editor:
JAK2V617F is a gain-of-function somatic mutation of JAK2 tyrosine kinase present in some but not all BCR/ABL-negative myeloproliferative neoplasms (MPN). High JAK2V617F allelic burdens correspond with homozygosity generated by uniparental disomy1 present in some progenitors and are seen in most patients with polycythemia vera and primary myelofibrosis. A JAK2V617F mutational burden in myeloproliferative disorder (MPD) granulocytes correlates with morbidity in polycythemia vera,2,3 justifying the clinical use of quantitative JAK2V617F assays. The use of plasma for detection of the JAK2V617F mutation and zygosity state has been reported.4,5
We questioned the utility of detection of cellular DNA and messenger RNA (mRNA) in plasma and tested its validity by comparing JAK2V617F mutational burden in paired granulocyte and plasma samples over time using a highly sensitive quantitative assay.6 Twelve milliliters of peripheral blood were collected from 17 consecutive JAK2V617F-positive MPD patients, each divided into 6 (2-mL) aliquots. Approval was obtained from the University of Utah Institutional Review Board. Informed consent was provided according to the Declaration of Helsinki. Plasma and granulocytes were isolated on days 0, 2, 4, 7, 9, and 11 from samples kept at room temperature by Histopaque (Sigma-Aldrich; 1.077 g/mL density gradient).6 Genomic DNA was isolated from GNC at 3 time points (days 0, 4, and 11) and plasma at 6 time points (days 0, 2, 4, 7, 9, and 11). RNA was isolated from 2 patients whose 12 plasma aliquots were collected at 6 time points, each aliquot contained RNase inhibitor. RNA was reverse transcribed and cDNA analyzed for JAK2V617F allelic burden using specific primers (see supplementary Table 1). Data of 165 aliquots from 17 MPD patients were analyzed using SAS software, Version 9.1 (SAS Institute).
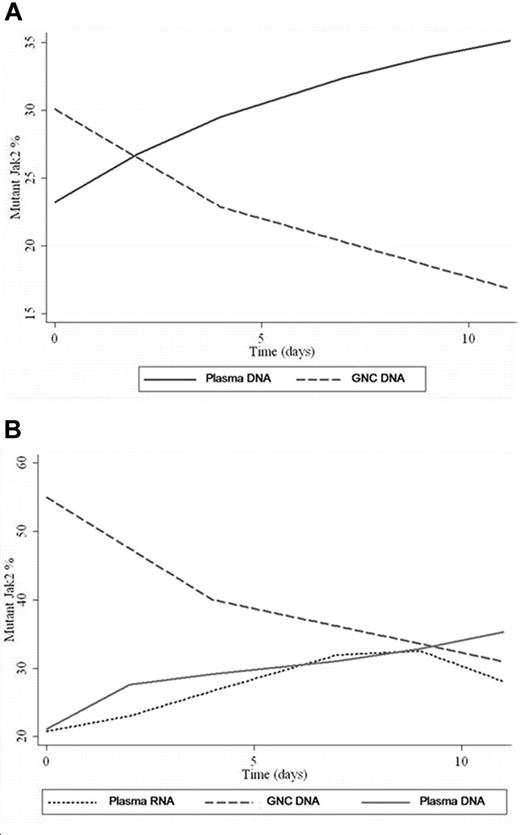
The JAK2V617F mutant allele frequency in genomic DNA from granulocytes was (0.2%-90%) and (0%-88%) in genomic DNA from plasma with no concordance of corresponding values in any patient at any given time point. There was a progressive increase of JAK2V617F frequency in plasma and a reciprocal decrease of JAK2V617F in paired granulocytes samples in all cases with time (Figure 1A), indicating preferential lysis of the granulocytes bearing the JAK2V617F mutation. Value ranges, mean, and standard deviation at each time point can be found in supplemental Table 2 (available on the Blood website; see the Supplemental Materials link at the top of the online article). A repeated-measure ANOVA was calculated to determine whether there was interaction between granulocytes versus plasma and time. The rate of change in JAK2V617F values did indicate an interaction and was significantly different (P < .001) between granulocytes and plasma. One patient had 0.7% JAK2V617F in granulocytes but not in plasma sample on day 0. However, JAK2V617F was detectable on day 11 in both granulocytes and plasma at a level of 0.2 and 2, respectively.
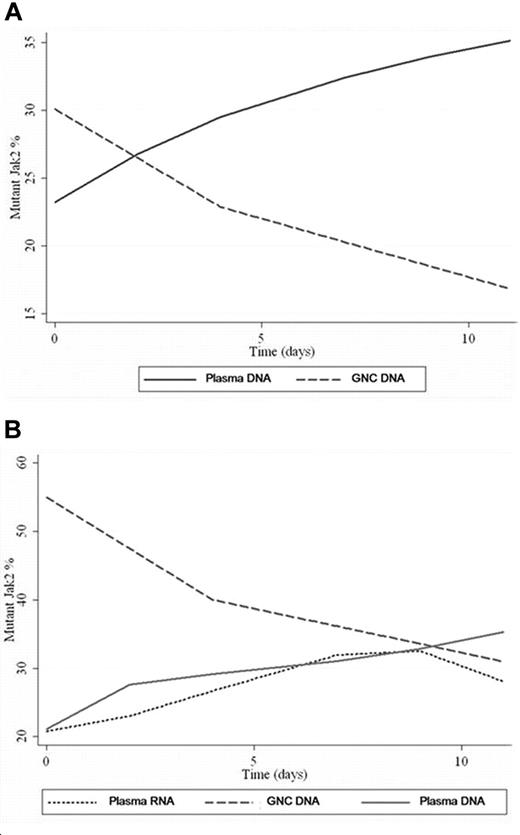
GNC/plasma JAK2 values overtime. (A) This plot is derived from polynomial regression from all patient samples. The regression produced best-fit curves showing the trend of mutant JAK2 values changes over time for GNC DNA (decreasing) and plasma DNA (increasing). (B) Variation of mutant JAK2 values in GNC-DNA, plasma-DNA versus plasma-RNA samples from the same patient over time. Plasma JAK2 DNA and plasma JAK2 RNA show comparable values with progressive increase in values over time. A drop in plasma RNA level was noted at day 9 while plasma DNA level continued to rise.
GNC/plasma JAK2 values overtime. (A) This plot is derived from polynomial regression from all patient samples. The regression produced best-fit curves showing the trend of mutant JAK2 values changes over time for GNC DNA (decreasing) and plasma DNA (increasing). (B) Variation of mutant JAK2 values in GNC-DNA, plasma-DNA versus plasma-RNA samples from the same patient over time. Plasma JAK2 DNA and plasma JAK2 RNA show comparable values with progressive increase in values over time. A drop in plasma RNA level was noted at day 9 while plasma DNA level continued to rise.
In concurrent samples evaluated for granulocyte DNA, plasma DNA and plasma RNA, JAK2V617F levels were higher in granulocyte DNA than in plasma DNA or RNA on day 0 (Figure 1B). Plasma JAK2V617F DNA and mRNA levels progressively increased with time. A decrease in plasma JAK2V617F mRNA level was noted after day 9, likely because of expected RNA degradation in plasma, while the plasma DNA level continued to rise throughout the study.
We conclude that detection of JAK2V617F DNA and mRNA in plasma is due to progressive lysis of granulocytes during storage compounded by decreased viability of the JAK2V617F bearing cells and is a storage artifact; as such it should not be used in clinical practice to quantify JAK2V617F or address zygosity. It might be reasonable to consider plasma for qualitative detection if blood has been left unprocessed for an extended period of time; only as a screen but not as a measure of JAK2V617F allelic burden. However, it should be kept in mind that the analysis of freshly prepared plasma sample from JAK2V617F-positive blood can be interpreted falsely as “negative.” In addition, the interval between drawing of blood and purification of granulocytes must be kept below 48 hours to avoid significant decrease in the apparent JAK2V617F allele burden.
Authorship
Acknowledgments: This work was supported by grant no. 1P01CA108671-O1A2 (National Cancer Institute, Bethesda, MD) awarded to the Myeloproliferative Disorders Consortium (project #1, PI J.T.P.).
Conflict-of-interest disclosure: M.E.S., A.W., and J.T.P. are affiliated with ARUP laboratory, which offers testing of JAK2 mutations. The other authors declare no competing financial interests.
Correspondence: Mohamed E. Salama, MD, or Josef T. Prchal, MD, Department of Pathology, University of Utah School of Medicine, 500 Chipeta Way, Salt Lake City, UT 84108; e-mail: mohamed.salama@path.utah.edu or Josef.Prchal@hsc.utah.edu.
References
National Institutes of Health