Abstract
Monoclonal B-cell lymphocytosis (MBL) indicates the presence of less than 5 × 109/L circulating monoclonal B cells in otherwise healthy subjects. Recently, it has been reported that circulating chronic lymphocytic leukemia (CLL)–like B cells can be detected using 4- or 5-multicolor flow cytometry in 5% to 7% of adults with normal lymphocyte counts. We investigated the frequency of circulating monoclonal B cells in 608 healthy subjects older than 40 years with normal blood counts, using a highly sensitive 8-color flow cytometry approach and systematic screening for total PB leukocyte count higher than 5 × 106. We show that the frequency of PB monoclonal B cells is markedly higher than previously reported (12% for CLL-like B cells, found at frequencies of 0.17 ± 0.13 × 109 cells/L), the incidence progressively increasing with age. Most cases (62%) showed clonal B-cell levels below the maximum sensitivity of the techniques described by others (< 0.01%), supporting the notion that detection of MBL may largely depend on the sensitivity of the flow cytometry approach used.
Introduction
Monoclonal B-cell lymphocytosis (MBL) is a relatively recent diagnostic category, that indicates the presence of low numbers of circulating monoclonal B cells (< 5 × 109/L) in otherwise healthy subjects (absence of a history or signs of B-cell chronic lymphocytic leukemia [CLL] or any other lymphoproliferative or autoimmune/infectious disease).1 Although this entity has been recently included in the revised National Cancer Institute Working Group/International Workshop on CLL (NCI-WG/IWCLL) guidelines for the diagnosis and management of CLL,2 whether MBL represents an early stage of a chronic B-cell malignancy is still unknown,3-7 because no large series of subjects with MBL has yet been prospectively followed for a long period of time (> 10 years). In addition to MBL cases presenting with lymphocytosis, recently several groups have reported that low levels of peripheral blood (PB) monoclonal B cells can also be detected in healthy subjects with normal lymphocyte counts. Accordingly, Rawstron et al8 have found monoclonal B cells phenotypically identical to CLL cells at levels less than 3.5 × 109/L in the PB of approximately 5% of healthy subjects (78/1520) older than 60 years, and a similar percentage of cases showing circulating CLL-like B cells has been found by Dagklis et al9 in cases older than 40 years (88/1322; 6.6%). In the present study, we investigated the frequency of circulating monoclonal B cells in a total of 608 healthy subjects older than 40 years and with normal blood counts who belong to a population living in Salamanca (western Spain), using a highly sensitive multicolor flow cytometry approach. We show that the frequency of PB monoclonal B cells in these subjects is markedly higher than that previously reported (12% for CLL-like B cells).
Methods
Subjects of study
A total of 608 healthy subjects (284 men [47%] and 324 women [53%]) older than 40 years (62 ± 13 years, range: 40-97 years) randomly recruited from the Primary Health Care system region of Salamanca (Spain) were studied. The leukocyte and total lymphocyte counts were of 6.3 plus or minus 1.6 × 109/L and 2.2 plus or minus 0.7 × 109/L, respectively, and the absolute B-cell number was of 0.16 plus or minus 0.1 × 109/L. The research protocol was approved by the Ethics Committee of the Cancer Research Center of Salamanca and all participants gave their written informed consent in accordance with the Declaration of Helsinki.
Immunophenotypic analyses
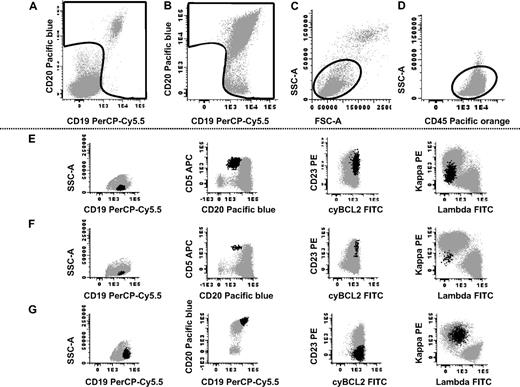
A total amount of approximately 4 mL EDTA-anticoagulated PB per case was immunophenotyped using a direct immunofluorescence stain-and-then-lyse technique,10 with the following antibody combinations: CD20-Pacific Blue (PB)/CD45-Pacific Orange (PO)/CD8-fluorescein isothiocyanate (FITC)+anti–sIgλ-FITC/CD56-phycoerythrin (PE)+anti–sIgκ-PE/CD4-peridin chlorophyll protein-cyanin 5.5 (PerCPCy5.5)/CD19-PE-cyanin 7 (PECy7)/CD3-allophycocyanin (APC)/CD38-Alexa Fluor 700 (AF700); CD20-PB/CD45-PO/cytBcl2-FITC/CD23-PE/CD19-PerCPCy5.5/CD10-PECy7/CD5-APC/CD38-AF700; and CD20-PB/anti–sIgλ-FITC/anti–sIgκ-PE/CD19-PerCPCy5.5/CD10-PECy7/CD5-APC. Reagents were purchased from Becton/Dickinson Biosciences (BDB) except for CD19-PECy7 (Beckman/Coulter), CD20-PB (e-Biosciences), CD38-AF700 (Exbio), CD45-PO (Caltag Laboratories), and Bcl2-FITC, anti–sIgλ-FITC, and anti–sIgκ-PE (Dako). Intracytoplasmic expression of bcl2 was evaluated after staining for cell surface antigens, using the Fix & Perm reagent kit (Invitrogen), according to the instructions of the manufacturer. Data acquisition was performed on a FACSCanto II flow cytometer (BDB) using the BDB FACSDiva software (V6.1), through a double-step procedure: first, information on 1 × 105 events corresponding to the whole sample cellularity was stored; in a second step, information was stored on CD19+ and/or CD20+ gated events, containing a minimum of 5 × 106 leucocytes/tube (Figure 1A-D). Instrument setup and calibration were performed according to well-established protocols11 ; to check for the consistent performance of the cytometer, and to ensure reliable and accurate results, a daily quality control program was strictly followed, based on the use of cytometer setup and tracking (CST) beads and CST module (BDB), according to the recommendations of the manufacturer. As isotype controls were not used, those cells that did not express a certain marker were considered as negative control for positive cells. In tubes 2 and 3, in which CD3 was not present in the panel of antibodies, the possibility that coincident T-cell events were included within the CD19/CD20 gate was excluded by the pattern of FSC/SSC, together with a phenotype incompatible with B/T-lymphocyte doublets (ie, CD5+/CD19+/CD20lo vs CD5+/CD19+/CD20hi; Figure 1E-F). The minimum number of cellular events considered to constitute a clonal B-cell cluster was 50 (Figure 1F).
Immunophenotypic identification of peripheral blood B-cell clones in otherwise healthy adults. Top panels (A-D) illustrate the sequential gating strategy used to identify B cells in one representative sample: in a first acquisition step, a gate was drawn on the cell fraction positive for CD19 and/or CD20 (A); information from only those events included in this gate was stored in a second step for more than 5 × 106 total leukocytes (B); finally only those events included in a wide gate drawn on a FSC/SSC and CD45 versus SSC dot plots, corresponding to small and eventually large CD45hi lymphocytes, were selected as B cells (C-D). Illustrative examples of 3 different samples displaying aberrant/clonal B cells are shown in panels E through G (aberrant/clonal B cells are shown as black dots, and residual polyclonal B cells are shown as gray dots): CLL-like cells present at low frequencies (0.01% of the whole cellularity of the sample and 0.7% of the whole B-cell population) are shown in panel E and CLL-like cells present at very low frequencies (0.0015% of the whole cellularity and 0.1% of the whole B-cell population) are displayed in panel F; non–CLL-like B cells proven to be clonal by polymerase chain reaction are shown in panel G.
Immunophenotypic identification of peripheral blood B-cell clones in otherwise healthy adults. Top panels (A-D) illustrate the sequential gating strategy used to identify B cells in one representative sample: in a first acquisition step, a gate was drawn on the cell fraction positive for CD19 and/or CD20 (A); information from only those events included in this gate was stored in a second step for more than 5 × 106 total leukocytes (B); finally only those events included in a wide gate drawn on a FSC/SSC and CD45 versus SSC dot plots, corresponding to small and eventually large CD45hi lymphocytes, were selected as B cells (C-D). Illustrative examples of 3 different samples displaying aberrant/clonal B cells are shown in panels E through G (aberrant/clonal B cells are shown as black dots, and residual polyclonal B cells are shown as gray dots): CLL-like cells present at low frequencies (0.01% of the whole cellularity of the sample and 0.7% of the whole B-cell population) are shown in panel E and CLL-like cells present at very low frequencies (0.0015% of the whole cellularity and 0.1% of the whole B-cell population) are displayed in panel F; non–CLL-like B cells proven to be clonal by polymerase chain reaction are shown in panel G.
Analysis of IgH gene rearrangements
Assessment of clonality was performed on FACSorted B cells (purity: 98% ± 0.8%) in 8 cases. Genomic DNA preparation, polymerase chain reaction amplification, heteroduplex analysis, and sequencing and analysis of IgHV genes were performed following well-established protocols, previously described in detail.12
Interphase fluorescence in situ hybridization studies
The most common genetic abnormalities associated with both CLL and non–CLL-B-chronic lymphoproliferative disorder were screened by multicolor interphase fluorescence in situ hybridization, performed on slides containing fixed, FACSorted (purity: 98% ± 0.8%) aberrant B cells (n = 37), as previously described.13
Statistical methods
Descriptive and comparative statistics (either the Pearson χ2 test or the Student t test or the Mann-Whitney U test) were performed using the SPSS software program (SPSS 15.0; SPSS, Chicago, IL). P values less than .05 were considered to be associated with statistical significance.
Results and discussion
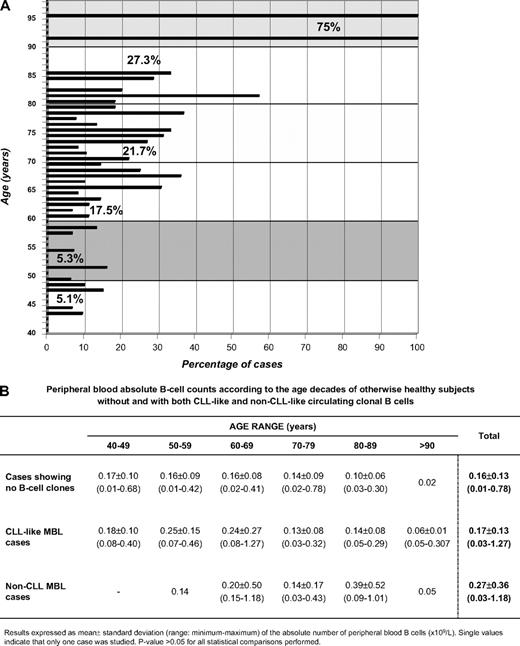
By applying a highly sensitive multicolor flow cytometry approach, we have found that the frequency of circulating monoclonal B cells with a CLL phenotype in healthy subjects older than 40 years showing normal lymphocyte counts was at least double than previously reported8,9 (73/608 subjects from a population-based cohort; 12%); of note, the proportion of cases displaying circulating CLL-like monoclonal B cells remained stable throughout the recruitment period (12 months): 10%, 10.5%, 14%, and 12.2% in the first 150, 300, 400, and 500 cases, respectively. Forty-eight (66%) of those 73 cases expressed surface kappa light chain immunoglobulins (sIgκ+) and 11 (15%) expressed sIgλ; such an imbalance in favor of sIgκ+ cases was significantly different (P < .05) from the expected frequency for normal and CLL B cells. In the remaining 14 cases (19%), 2 clonal B-cell subsets were detected (one being sIgκ+ and the other sIgλ+ in 13 cases and 2 distinct sIgκ+ B-cell subpopulations in 1 case). Interestingly, no statistically significant different PB B-cell counts were found between subjects with and without circulating clonal B cells (Figure 2B); actually, clonal B cells represented a minor proportion (median: 0.38%; interquartile range: 0.14%-4.2%) of the whole B-cell population. In line with other recent reports,4,9 the frequency at which PB clonal B-cell populations were detected progressively increased with age (Figure 1), whereas no association was found between age and the size of the clone, or between the presence of circulating clonal B cells and sex (11% of women vs 13.8% of men; P > .05). The higher frequency at which we detected PB clonal B cells in otherwise healthy subjects is most likely due to the higher sensitivity of the flow cytometry approach applied versus that used by others, as we have screened at least 10 times more PB nucleated cells/sample (5 × 106/case vs 2 × 105 and 5 × 105/case8,9 ) and used 8-color staining panels versus 4-color8 or 5-color9 protocols. This is supported by the fact that in more than half of the cases (62%) the percentage of aberrant/clonal B cells was below the maximum sensitivity of the latter approaches (< 0.01%). However, clonality was confirmed only by additional methods in 18 cases, all except one with more than 0.01% aberrant/clonal B cells (median percentage of CLL-like B cells: 0.10%; interquartile range: 0.025%-0.68%). Clonality was confirmed by the presence of clonal IGH gene rearrangements (8/8 cases tested) and/or the presence of concordant genetic abnormalities: del(13q14.3) in 10 of 37 cases tested (including 1 case showing 0.007% CLL-like cells) and/or trisomy 12 in 2 of 37 cases tested. IGHV sequencing was performed in 7 cases that used the following IGHV genes (% mutation from germ line): VH1-01 (4%); VH1-2 (0%); VH1-3 (1.5%); VH1-08 (1.8%); VH3-21 (0%); VH3-23 (0%); and VH5-51*01 (6%). There are insufficient data to make definitive conclusions about whether they show different VH repertoire versus CLL, as previously suggested.9 Of note, all biclonal cases analyzed displayed 2 different molecular markers. As in other series,3,9,14 we also detected additional cases showing non–CLL-like B-cell clones, at a frequency similar to that found in other studies9 (14/608; 2.3%), and not as high as we expected from our results on CLL-like MBL; a possible explanation is that the 8-color panel used here might not be optimal to detect aberrancies other than CLL-like, in addition to the fact that for the unequivocal identification of certain B-cell chronic lymphoproliferative disorder groups (ie, those derived from marginal zone), neither specific nor highly suggestive phenotypic marker profiles exist. Because it has been suggested that there is no cutoff point that could be used for a 100% efficient discrimination of those subjects at risk of progression and that the fundamental biologic issue is the presence of a clonal population,15 the precise identification of cases having circulating clonal B cells using highly sensitive flow cytometry becomes a key question. Nevertheless, given the high frequency of such cell populations (> 100 times more frequent than CLL), and the fact that this frequency strongly depends on the sensitivity of the approach used, prospective, long-term follow-up studies16 are needed to define the natural history of MBL.
PB B-cell counts and frequency of cases with monoclonal B cells in healthy subjects older than 40 years with normal lymphocyte counts, grouped according to age. (A) Frequency of PB monoclonal B cells in healthy subjects older than 40 years with normal lymphocyte counts, grouped according to age. Bars represent the percentage of cases within each age subgroup (from 40 to 97 years) displaying circulating B-cell clones. The numbers inside the picture represent the proportion of cases displaying circulating B-cell clones per decade: 40 to 49, 50 to 59, 60 to 69, 70 to 79, 80 to 89, and older than 90 years. (B) Peripheral blood absolute B-cell counts according to age of otherwise healthy adults without and with both CLL-like and non–CLL-like circulating clonal B cells.
PB B-cell counts and frequency of cases with monoclonal B cells in healthy subjects older than 40 years with normal lymphocyte counts, grouped according to age. (A) Frequency of PB monoclonal B cells in healthy subjects older than 40 years with normal lymphocyte counts, grouped according to age. Bars represent the percentage of cases within each age subgroup (from 40 to 97 years) displaying circulating B-cell clones. The numbers inside the picture represent the proportion of cases displaying circulating B-cell clones per decade: 40 to 49, 50 to 59, 60 to 69, 70 to 79, 80 to 89, and older than 90 years. (B) Peripheral blood absolute B-cell counts according to age of otherwise healthy adults without and with both CLL-like and non–CLL-like circulating clonal B cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was financially supported by the following grants: FIS 06-0824 from the Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo, Madrid, Spain; GRS206/A/08 from the Gerencia Regional de Salud de Castilla y León, Valladolid, Spain; and RTICC RD06/0020/0035 from the Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Madrid, Spain.
Authorship
Contribution: W.G.N. performed research and analyzed data; J.A. designed research, analyzed data, and wrote the paper; A. Romero selected the cohort of subjects and performed the coordination with the Primary Health Care Group; C.T. and M.G. performed research and data analysis; A.L. analyzed data; A.F.H., M.L.S., M.J.-A., and A. Rasillo performed the techniques; P.F.-N. collected samples and data from healthy subjects; T.V. supervised the epidemiologic selection of subjects; and A.O. designed research, analyzed data, and wrote the paper. Members of the Primary Health Care Group of Salamanca for the Study of MBL directly collected samples and data from the cohort of the healthy adults recruited.
For a complete list of Primary Health Care Group of Salamanca for the Study of MBL participants, see the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Orfao, Laboratorio 11, Centro de Investigación del Cáncer, Av Universidad de Coimbra s/n, Campus Miguel de Unamuno, 37007 Salamanca, Spain; e-mail: orfao@usal.es.