Abstract
AAV-1 is one of the most promising vectors for gene delivery to skeletal muscle. In this issue of Blood, Mingozzi and colleagues now demonstrate that AAV-1–mediated gene transfer into human skeletal muscle results in activation of capsid-specific T cells.1
Evidence continues to accumulate demonstrating that gene therapy may be therapeutically effective in patients suffering from different types of hereditary diseases.2,3 These recent successes in gene therapy reflect, at least in part, continued improvements in gene delivery technologies. In particular, gene delivery vectors derived from the nonpathogenic adeno-associated virus (AAV) have been used to cure monogenic diseases in preclinical animal models and thus far have yielded some promising results in clinical trials. Despite these advances, the immune response toward the therapeutic protein or the gene-engineered cells themselves represents a potential hurdle that may prevent long-term therapeutic effects. Unfortunately, the assessment of immune responses following gene therapy in preclinical animal models does not always accurately predict the outcome in humans.3 Hence, it is extremely important to fully study the immune consequences of gene transfer in human subjects enrolled in clinical trials.
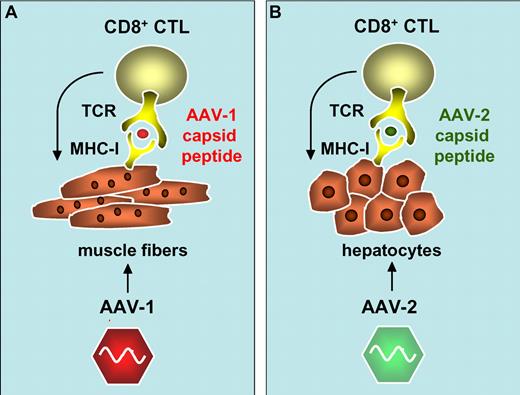
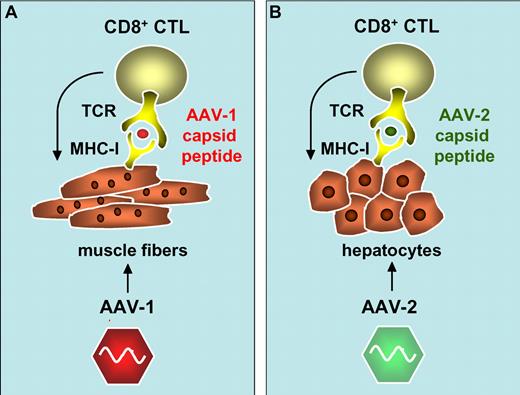
Activation of AAV-1 and AAV-2 capsid-specific CD8+ cytotoxic T cells (CTL) following cross-presentation of capsid-derived antigenic peptides onto major histocompatibility complex class I antigens (MHC-I) in transduced muscle fibers (A)1 and hepatocytes (B).3,5,6
In this issue, Mingozzi et al describe the immune consequences of AAV-mediated skeletal muscle gene delivery in humans. Their findings were based on an open-label dose escalation study in which an AAV-1 vector encoding a truncated lipoprotein lipase (LPLS447X) was administered into the limb muscles of subjects suffering from LPL-deficiency.4 These patients typically have abnormal lipid breakdown consisting of elevated triglyceride (TG) levels in the blood. A transient decrease in plasma TG levels was apparent in some of the subjects, suggesting a possible therapeutic effect. However, because dietary fluctuations may potentially interfere with TG levels and the number of patients was limited, conclusions regarding efficacy will need to be confirmed in subsequent larger trials.
The authors noted that one of the subjects who received the highest vector dose experienced a transient increase in muscle enzyme creatine phosphokinase (CPK). This elevation in CPK appeared to be associated with a therapeutic effect whereby TG levels transiently returned to baseline. Activation of AAV-1 capsid-specific CD4+ and CD8+ T-cell responses may have contributed to the clearance of the AAV-1–transduced muscle fibers. Interestingly, 50% of the subjects enrolled in this trial developed an AAV-1 capsid-specific T-cell response, whereas LPL-specific T-cell responses could not be detected. Increasing vector doses resulted in a more rapid onset of capsid-specific T-cell responses and an apparent shift toward mixed CD8+ and CD4+ AAV-1 capsid-specific T-cell responses. In contrast, the T-cell responses in the low-dose cohort was delayed and driven primarily by CD4+ instead of CD8+ T-cell capsid epitopes. Hence, AAV-1–transduced muscle fibers likely presented the capsid-derived antigenic peptides in association with major histocompatibility complex class I (MHC I) to CD8+ cytotoxic T cells, with subsequent elimination of transduced muscle cells, in a vector dose–dependent manner. Previous natural exposure to wild-type AAV in humans could possibly increase the likelihood of inducing these capsid-specific T-cell responses. Induction of AAV-1 capsid-specific MHC I–restricted CD8+ T-cell responses would de facto require “cross-presentation” of the exogenous AAV particles via the endogenous antigen presentation pathways, particularly because AAV-1 capsid antigens are not expressed de novo in transduced cells.
There are striking similarities between the results obtained in this trial and previous observations in a gene therapy trial for hemophilia B, which further strengthens the underlying hypothesis. In this trial, administration of AAV-2 vectors encoding clotting factor IX (FIX) resulted in a dose-dependent T-cell response that cleared the transduced hepatocytes expressing FIX. This is consistent with the observation of a decline in FIX expression levels coincident with transient elevation of liver enzymes in the plasma.3,5 This T-cell response was again directed at the AAV capsid-derived antigenic peptides presented in association with MHC I on the surface of the transduced hepatocytes, possibly via “cross-presentation.”6 Similarly, there was no evidence for T-cell responses against FIX, consistent with the lack of T-cell responses against LPL.
It has been proposed that the use of vectors derived from alternative AAV serotypes may reduce the likelihood of inducing capsid-specific T-cell responses, particularly if uptake of the vector particles by antigen-presenting cells is impaired. Because this uptake is likely mediated by heparan sulfate proteoglycan receptors (HSPGRs), AAV serotypes that exhibit reduced binding to HSPGRs may reduce activation of capsid-specific T cells.7 However, the present results challenge this notion by demonstrating that the use of AAV-1, which binds poorly to HSPGRs, does not prevent induction of a capsid-specific T-cell response.
This study has important implications for the design of future trials using AAV vectors, and suggests that switching AAV serotypes may not suffice to render the transduced cells impervious to immune attack, particularly because T-cell epitopes are relatively conserved among distinct AAV serotypes. Whether the T-cell responses to capsid curtail long-term transgene expression in some or all cases requires further investigation. The use of transient immune suppression and/or lower vector doses in conjunction with more robust transgene expression cassettes may potentially overcome some of these T-cell immune responses while maintaining stable transgene expression. The present study provides a conceptual framework to further test these hypotheses in future trials.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■