Abstract
BMP2 and BMP4 play crucial inductive roles during development. In this issue of Blood, Shao et al demonstrate that an intricate network of paracrine BMP2/4 signals also regulates angiogenesis—and will very likely interact with endocrine BMP cues during wound repair.1
The bone morphogenetic proteins (BMPs) were initially identified as a chaotrope-extractable activity purified from bone that induced ectopic bone formation in soft tissues. Marshal Urist described this activity in landmark publications in 1965.2,3 Subsequent to protein sequencing and cloning by Wozney, Rosen et al revealed that the original BMP activity was, in fact, a heterogeneous admixture of multiple BMPs, members of the TGFβ superfamily of polypeptide ligands that both stimulated (BMP2) and limited (BMP3) bone formation.4,5 Murine and human molecular genetics rapidly established important roles of BMPs in physiology beyond that of bone. Recent studies of BMP6 and the activin-like kinase (ALK) coreceptor HJV/HFE2 have established a BMP-ALK-hepdicin endocrine axis that controls iron homeostasis.6 Heterozygous loss of function alleles in the receptor ALK1 give rise to hereditary hemorrhagic telangectasia type II, and this genetic linkage first hinted that BMP signaling has an important role in human vascular physiology.7 ALK1 is expressed selectively in endothelial cells (ECs), and TGFβ is the prototypic agonist for ALK1 activation. However, ten Dijke, Bailly, and colleagues identified that BMP9 is also an important agonist for ALK1, that it inhibits angiogenesis and vascular endothelial growth factor (VEGF) expression via ALK1, and circulates at 5 ng/mL to 10 ng/mL—well above the minimum concentrations necessary for significant ALK1 activity in vitro.8-10 Thus, a new BMP9 endocrine axis has emerged, with BMP9 serving as a hepatocyte-derived humoral regulator that prevents excessive angiogenic responses. Nevertheless, the intricacies of ALK1 activation by different ligands and net impact upon EC physiology remain to be determined.
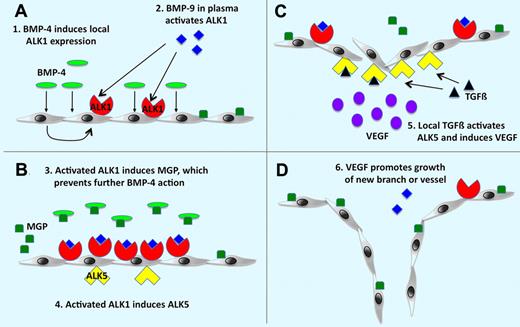
Working model for the roles of ALK1 and ALK5 in angiogenesis. (A) BMP-4 as an angiogenic stimulus causes local induction of ALK1, which is activated by BMP-9 normally circulating in the plasma. (B) ALK1 signaling induces expression of MGP, which prevents excessive angiogenic stimulation by binding to BMP-4, and expression of ALK5. (C-D) ALK5 is activated by local TGFß, which results in VEGF induction and stimulation of endothelial cell growth and angiogenesis. See the complete figure in the article beginning on page 2197.
Working model for the roles of ALK1 and ALK5 in angiogenesis. (A) BMP-4 as an angiogenic stimulus causes local induction of ALK1, which is activated by BMP-9 normally circulating in the plasma. (B) ALK1 signaling induces expression of MGP, which prevents excessive angiogenic stimulation by binding to BMP-4, and expression of ALK5. (C-D) ALK5 is activated by local TGFß, which results in VEGF induction and stimulation of endothelial cell growth and angiogenesis. See the complete figure in the article beginning on page 2197.
In this issue of Blood, Shao and colleagues have significantly extended our understanding of BMP-ALK signaling in EC biology. Her investigative team identified that, via a complex relay, ALK1 signaling may also elicit a later stage of angiogenesis via paracrine mechanisms. Activation of ALK1 by BMP4 up-regulates both matrix GLA protein (MGP) and ALK5, the former an antagonist of further paracrine activation by BMP4. Up-regulation of ALK5 via ALK1 signaling is permissive for a later phase of EC proliferation and angiogenesis via TGFβ and induction of VEGF. Thus, unlike the endocrine regulation of the axis by BMP9—the ALK1 agonist NOT inhibited by MGP—the paracrine signaling via BMP4-ALK1 potentially provides 2 phases of EC regulation. They are transient quiescence followed by TGFβ-ALK5–dependent angiogenesis.
Why such an elaborate network of regulation? This endocrine-paracrine interplay may serve an important role in the biology of wound repair. In the event of injury and in the presence of poor blood flow and relative tissue hypoxia, BMP9 delivery will be impaired. Inflammation-dependent up-regulation of BMP2 in ECs and BMP4 and TGFβ production by smooth muscle will synergize with hypoxia to increase VEGF production. With angiogenesis and the restoration of tissue perfusion, humoral delivery of oxygen and the MGP-insensitive ALK1 ligand, BMP9, will reduce VEGF expression and actively suppress angiogenesis, respectively. This will facilitate the resolution of the repair process. Beneath this emerging model (see figure), angiogenesis and wound repair should be significantly impaired in MGP-null mice. Because of the profound impact of MGP deficiency on arterial mineralization and vascular integrity11 (also likely arising from dysregulated BMP2/4 signaling12 ), most studies have emphasized vascular calcification. The multiplicity of negative regulators for paracrine BMP2/4 signaling suggests that MGP deficiency may impact wound repair in specific venues. Given the circulatory relationships between liver and lung, pulmonary repair processes may be particularly susceptible to MGP deficiency.13 Finally, because skeletal BMP depots, tissue hypoxemia, and TGFβ signaling are prominent components of the tumor microenvironment within bone metastasis,14 the newly appreciated BMP-ALK1/ALK5 biology from the Shao et al study may provide insights useful for devising novel strategies for controlling bone tumor growth.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health