Abstract
In hematopoietic stem cell transplant (HSCT) recipients, the recognition of polymorphic antigens by the donor-derived immune system is an important mechanism underlying both graft-versus-host disease and graft-versus-leukemia (GVL) effect. Here we show that a subset of HSCT recipients (13.9%, n = 108) have antibodies directed to surface molecules of dendritic cells. We have used one such serum in conjunction with retroviral expression cloning to identify the highly polymorphic surface molecule immunoglobulin-like transcript 5 (ILT5) as one of the targets of dendritic cell-reactive antibodies. ILT5 reactive antibodies were found in 5.4% of HSCT patients but not in solid organ transplantation recipients, patients with collagen diseases, multiparous women, or polytransfused or healthy persons. We show that ILT5-specific antibodies can mediate killing of ILT5-bearing cells and furthermore demonstrate ILT5 expression in some leukemic cells, indicating that it might be a target for GVL effects. Thus, our results represent the first description of potent allogeneic antibody responses to a non–major histocompatibility complex cell surface molecule in hematopoietic stem cell transplanted patients and warrant further studies to elucidate the role of antibodies to polymorphic cell surface molecules in GVL and graft-versus-host responses.
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is a curative treatment for selected hematologic and oncologic diseases. Immune responses to residual malignant cells (graft-versus-leukemia [GVL]) can contribute to the eradication of the tumors in the transplantation recipients. However, graft-versus-host disease (GVHD) remains a frequent complication of HSCT, significantly contributing to nonrelapse morbidity and mortality in these patients. An important mechanism underlying both effects is the recognition of polymorphic antigens, such as minor histocompatibility antigens (mHAg), by donor-derived immune cells.1 The expression pattern of such antigens is assumed to significantly influence HSCT outcome in a way that recognition of polymorphic antigens mainly restricted to the hematopoietic system is beneficial whereas antigens expressed in nonhematopoietic tissues lead to GVH reactions in various target organs associated with increased mortality and impaired quality of life.
To date, most efforts to search for molecules recognized after allogeneic HSCT have focused on the identification of antigens targeted by T cells. This led to the identification of molecules, such as the HA antigens, and further work gave important insights into GVL effects elicited by them.2-8 However, the number of antigens identified remains limited, and this may in part be the result of the cumbersome screening for antigens using T cell–based methods. In addition, if the antigen does not persist, the specific T cells probably vanish from the peripheral blood. Miklos et al showed that H-Y minor histocompatibility antigens can elicit strong humoral immune responses that are associated with high incidence of chronic GVHD but also with maintenance of disease remission.9 In addition, a large number of molecules identified by serologic expression cloning (SEREX) has impressively demonstrated the usefulness of antibodies derived from sera of tumor patients to search for tumor antigens.10 Screening of leukemia-derived cDNA libraries with sera of HSCT patients given donor-lymphocyte infusions for treatment of relapsing leukemia led to identification of a panel of antigens overexpressed in leukemic cells. However, polymorphic antigens recognized because of sequence disparity between donors and recipients have not been reported in these studies.11,12
The SEREX methodology has predominantly led to the identification of intracellular antigens. One reason for this might be that the classic SEREX approach is based on the screening of prokaryotic libraries, which often might not afford the expression of properly folded surface antigens. However, antibody responses directed to membrane resident proteins are especially relevant because they might directly eliminate cells by antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cellular cytotoxicity (CDC). In this study, we have modified the SEREX methodology to identify cell surface antigens recognized by antibodies in sera of patients after allogeneic HSCT. We show that a subset of these patients has a strong antibody response to antigens expressed on allogeneic dendritic cells (DCs). DCs were chosen for our analysis because they can be regarded as model cell for the myeloid lineage and also because antibodies to these cells might have immunomodulatory functions. By screening a retroviral expression library with serum of such a patient, we have identified the polymorphic surface molecule immunoglobulin-like transcript 5 (ILT5) as inducer of extremely high titers of allo-specific IgG antibodies in several hematopoietic stem cell graft recipients. Furthermore, we show that ILT5-specific antibodies can kill ILT5-expressing cells and demonstrate ILT5 expression on leukemic cells, indicating that such antibodies have the potential to elicit GVL effects.
Methods
Patients
From October 2004 until April 2008, 737 serum samples were obtained from 240 adult HSCT recipients (1-9 samples/patient) during routine check-ups at the Outpatient Clinic at the Bone Marrow Transplant Unit of the Department of Internal Medicine I, Medical University Vienna and at the Department of Hematology, Leiden University Medical Center. Samples were taken 1 to 139 months after transplantation (median, 17 months [518 days]), and follow-up of patients' clinical data was until August 2008. High-resolution human leukocyte antigen (HLA) typing was performed by nucleotide sequencing of amplified alleles for the HLA-A, -B, -C, DRB1, DRB3/4/5, and DQB1 loci.
Sera derived from 108 of these patients were tested for DC reactivity using 10 different monocyte-derived DC (mdDC) preparations generated from monocytes derived from healthy volunteer donors as described.13 A total of 84% these 108 patients (30.4% sibling donors; 65.2% matched unrelated donors) had GVHD anamnesis.
In addition, sera of 105 renal allograft recipients, 40 patients with collagen diseases, 80 multiparous women, and 13 polytransfused persons were analyzed. Sera of renal graft recipients were sampled at a median of 26 days after transplantation (range, 1-1242 days; interquartile range, 15-121 days; 17 with antibody-mediated, 55 with acute cellular rejection). This collective has been described in detail.14 Informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and the study was approved by the local ethical committees of the participating institutions.
Cell culture
The murine thymoma cell line Bw5147 (referred to as Bw cells throughout this work) was maintained in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 10% fetal bovine serum (Invitrogen). The human embryonic kidney cell line 293T was maintained in Iscove modified Dulbecco medium containing 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by standard density centrifugation with Ficoll-Paque (Pharmacia Biotech). mdDCs were generated as described.13 For DC maturation, lipopolysaccharide was added to cultures of immature DCs on day 5 at 100 ng/mL and cells were analyzed 2 days later. For the preparation of lymphokine-activated killer cells, PBMCs of healthy donors were stimulated in presence of recombinant human interleukin-2 (1000 U/mL; R&D Systems) for 4 days.
Flow cytometry analyses
For fluorescence-activated cell sorter (FACS) analysis, cells were incubated with human sera for 20 minutes on ice, washed, and bound antibodies were detected by allophycocyanin- or phycoerythrin (PE)–labeled goat anti–human IgG (Fc-γ)–specific antibodies (Jackson ImmunoResearch Laboratories; no cross-reactivity with human IgM). Sera were used at a dilution of 1:200 unless stated otherwise. Titers of ILT5-specific antibodies were determined by analyzing serial dilutions of the sera with control Bw cells and Bw cell expressing ILT5 variant 1 or 2 (Bw-ILT5v1/Bw-ILT5v2) by FACS. Dilutions that yielded a 50% higher mean fluorescence intensity on the ILT5-expressing cells than on the control ones were scored positive. For elimination of xenoreactive (Bw-reactive) antibodies, 100 μL of serum samples was diluted 1:10 and incubated 4 times with 107 Bw cells at 4°C under constant rotation. To remove ILT5-specific antibodies, Bw cells transduced to express ILT5v1 were used for 4 rounds of incubation under the same conditions. Absorbed serum was further diluted for FACS sorting or staining assays to a final concentration of 1:200. Monoclonal antibodies (mAbs) were from BD Biosciences, except for CD85a/ILT5 mAb, which was obtained from R&D Systems.
For FACS analysis of whole blood and bone marrow, ADG lysis (An der Grub) was used. FACS analyses were done on a FACSCalibur using the CellQuest software (BD Bioscience).
Retroviral expression cloning
A previously described cDNA library generated from mdDCs was expressed in the Bw5147 cell line.15 The transfected cell pool was subjected to 2 rounds of flow cytometric sorting with serum G106/1 (1:200). For detection of bound antibodies, PE-labeled goat antihuman IgG antibodies were used. Subsequently, single-cell clones were established by limiting dilution. Genomic DNA was prepared from serum G106/1-reactive single-cell clones using Puregene (Gentra Systems) following the manufacturer's instructions. The integrated retroviral cDNA inserts were polymerase chain reaction (PCR)–amplified from genomic DNA as described.15 The PCR products were gel-purified and cloned into the retroviral expression plasmid pBMN. The resulting constructs were expressed in Bw cells as previously described.16 Plasmid DNA from selected clones was used for sequence analysis (MWG Biotech AG).
Cloning and expression of ILT5 variants and chimeric ILT5 molecules
cDNAs from mdDCs generated from patient G106 (after transplantation and thus donor-derived) were used to PCR amplify ILT5 encoding sequences. The PCR fragments were cloned into a retroviral vector and expressed on Bw cells as previously described.17 To generate chimeric ilt5 genes, where distinct parts of the cDNA encoding ILT5 variant 2 were replaced by the corresponding sequence of variant 1, fragments of interest were PCR-amplified with specific primers and combined with ilt5 variant 2 encoding sequences by 2 consecutive overlap extension PCR steps. The resulting genes encoding chimeric ILT5 variant 2 molecules harboring amino acids 3 to 278 (chimera 1 [C1]), 151 to 278 (C2), and 324 to 350 (C3) of ILT5v1 were cloned into a retroviral vector and expressed on Bw cells. To assess the amount of ILT5 reactivity directed to different areas of the extracellular domain of ILT5v1, serum G106 was depleted from antibodies reacting with amino acid 3 to 278, 151 to 278, or 324 to 350 of ILT5 variant 1. For this, serum G106 (diluted to 1:100) was repeatedly (15 times) incubated with Bw cells expressing the chimeric ILT5 molecules and for control purpose with Bw cells expressing ILT5v1 or control Bw cells. Complete depletion of antibodies directed to these molecules was confirmed by FACS analysis. Subsequently, these serum preparations were probed with Bw-ILT5v1 cells at a final dilution of 1:200 to assess the reduction of reactivity to ILT5v1 by FACS analysis. Percentage of reduction of antibody reactivity to ILT5v1 was calculated using the formula 100 × (MFIcontrol cell-treated serum − MFIBw-ILT5-chimera treated serum)/MFIcontrol cell-treated serum.
To express recipient-derived ilt5 sequences, genomic DNA (pretransplantation) from patient G106 was PCR amplified using primers specific for the codons 4 to 9 (forward) and 349 to 355 (backward). The resulting PCR products, which encode most of the extracellular part of the ILT5 molecule, were combined with ilt5 variant 2 encoding cDNA by 2 consecutive overlap extension PCR steps. The resulting products that represent chimeras of ilt5 variant 2 and ilt5 sequences derived from the genomic DNA of patient G106 were cloned into a retroviral expression vector. DNA sequence analysis was performed to confirm the integrity of the chimeric genes and also to identify constructs that correspond to the 2 allelic variants of the ilt5 gene of patient G106 and the donor of patient G106. The constructs were retrovirally expressed on Bw cells.
CDC and ADCC
CDC and ADCC were measured using a europium release assay kit (Delfia; PerkinElmer) following the manufacturer's protocol. Briefly, Bw cells expressing ILT5 variant 1 or 2 or mock-transduced Bw cells were labeled with europium and used at 5 × 104 cells per well. For determination of CDC, ILT5 antibody containing serum or control serum were added in the indicated concentrations. Guinea pig complement (0.5 μL /well) was added, and cell lysis was determined after 3 hours of incubation at 37°C. ADCC was assayed by incubation of ILT5 variant-expressing cells and control cells with interleukin-2–activated PBMCs of healthy donors (105/well; E/T ratio 20:1) for 4 hours at 37°C in presence or absence of serum containing ILT5-specific antibodies in different dilutions. For detection of cell lysis–associated europium release, 20 μL of supernatant was transferred to a 96 flat-bottom well plate, and 200 μL enhancement solution was added. Fluorescence was measured using a time-resolved fluorometer (Victor; PerkinElmer). The percentage of specific cytotoxicity was calculated by the formula: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100.
Statistical analysis
The Student t test (unpaired, 2-tailed) was used for statistical analyses. P values less than .05 were considered statistically significant.
Results
Sera from HSCT recipients react with mdDCs
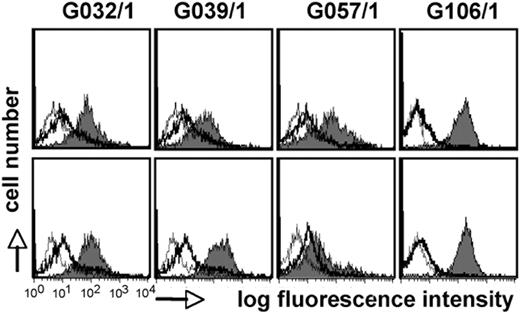
To assess whether HSCT recipients develop a humoral immune response against surface antigens of leukocytes, we generated mdDCs from healthy volunteer donors and tested 108 HSCT patients by FACS for the presence of DC-reactive IgG antibodies. We found such antibodies in 15 patients (13.9%, n = 108) but not in healthy persons (n = 30). Interaction of DC-reactive sera with immature and LPS-matured DCs is shown in Figure 1. Because most of the sera are derived from HSCT patients who received HLA class I– and class II–matched stem cell grafts, it is doubtful that a significant portion of these sera recognize major histocompatibility complex (MHC) antigens. In addition, DC-reactive sera showed consistent binding to cells derived from different donors (data not shown). Furthermore, although DCs strongly up-regulate MHC class I and class II molecules on maturation, some of the sera reacted with immature and mature DCs to the same degree, also arguing against HLA molecules as target antigens for these antibodies.
Sera of stem cell–transplanted patients contain DC-reactive antibodies. Allogeneic immature (top panels) and mature (bottom panels) mdDCs were probed with sera (final concentration 1:200) of stem cell–transplanted persons by FACS (gray histograms). Sera of healthy persons (thick line) and phosphate-buffered saline (thin line) were used as controls. Bound antibodies were detected with PE-labeled goat anti–human IgG antibodies.
Sera of stem cell–transplanted patients contain DC-reactive antibodies. Allogeneic immature (top panels) and mature (bottom panels) mdDCs were probed with sera (final concentration 1:200) of stem cell–transplanted persons by FACS (gray histograms). Sera of healthy persons (thick line) and phosphate-buffered saline (thin line) were used as controls. Bound antibodies were detected with PE-labeled goat anti–human IgG antibodies.
Identification of ILT5 as target antigen
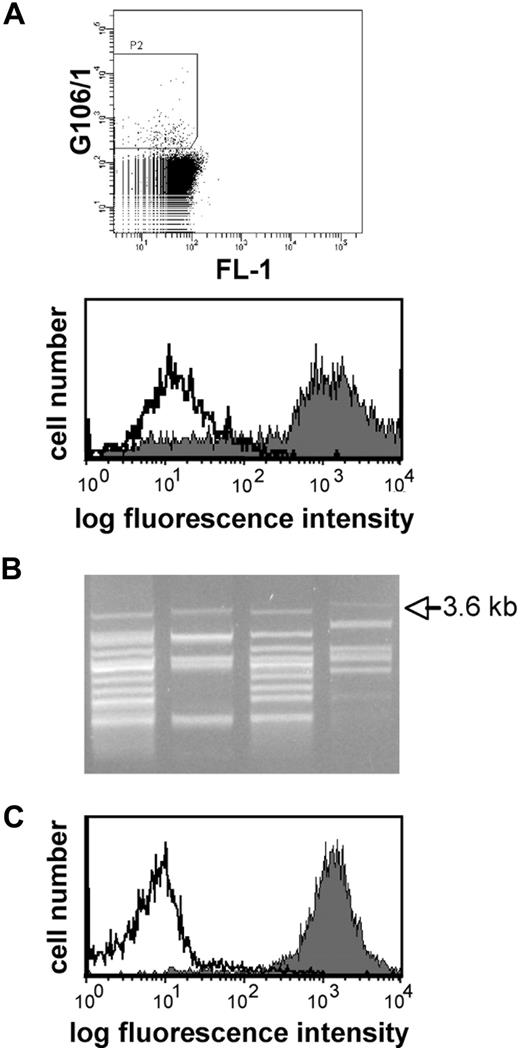
To identify antigens recognized by DC-reactive antibodies, we applied retroviral expression cloning in conjunction with flow cytometric sorting. Serum G106/1 derived from patient G106, which showed strong reactivity with DCs (Figure 1), was selected for this purpose. A previously described retroviral cDNA library derived from mdDCs was expressed in the murine thymoma cell line Bw5147.15,17 To eliminate xenoreactive antibodies, serum G106/1 was subjected to 4 rounds of adsorption with native Bw cells, which reduced xenoreactivity to almost background levels (> 90% reduction). Importantly, the DC reactivity of serum G106/1 was not affected by this procedure (data not shown). The transduced cell pool expressing the cDNA library was incubated with the adsorbed serum, and bound human IgG antibodies were detected by PE-labeled anti–human IgG antibodies. Reactive cells were isolated by 2 rounds of flow cytometric sorting followed by single-cell cloning and PCR-based rescue of the retrovirally introduced cDNA (Figure 2). A PCR band of 3.6 kb that was present in all cell clones that reacted with serum G106 was recloned into the retroviral vector pBMN. Retroviral reexpression of this cDNA insert in the Bw cell line conferred reactivity with serum G106/1, demonstrating that it encodes an antigen recognized by this serum (Figure 2C). DNA sequence analysis identified it as ILT5, also named LILRB3, LIR3, or CD85a. Depletion of ILT5 reactive antibodies from serum G106/1 demonstrated that ILT5 accounts for all DC reactivity in this serum (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Identification of ILT5 as target antigen by eukaryotic expression cloning. (A) Screening of a DC library expressed in Bw cells with serum G106/1. (Top panel) Bw cells expressing the cDNA library derived from DCs were incubated with serum G106/1 (final concentration 1:200) and bound antibodies were detected by PE-labeled antihuman IgG antibodies. PE-positive cells were gated for sorting. (Bottom panel) Serum G106/1 was incubated with the cell pool obtained after 2 rounds of sorting (gray histogram) or with control Bw cells (open histogram). (B) PCR recovery of the retroviral cDNA inserts from single-cell clones reacting with serum G106/1. Retroviral cDNAs were recovered from the genomic DNA of reactive clones by PCR. A 3.6-kb band was present in the PCR products of all clones. (C) The 3.6-kb cDNA encodes an antigen recognized by G106/1. Serum G106/1 reacts with Bw cells transduced to express a 3.6-kb cDNA (gray histogram) but not control-transduced Bw cells (open histogram).
Identification of ILT5 as target antigen by eukaryotic expression cloning. (A) Screening of a DC library expressed in Bw cells with serum G106/1. (Top panel) Bw cells expressing the cDNA library derived from DCs were incubated with serum G106/1 (final concentration 1:200) and bound antibodies were detected by PE-labeled antihuman IgG antibodies. PE-positive cells were gated for sorting. (Bottom panel) Serum G106/1 was incubated with the cell pool obtained after 2 rounds of sorting (gray histogram) or with control Bw cells (open histogram). (B) PCR recovery of the retroviral cDNA inserts from single-cell clones reacting with serum G106/1. Retroviral cDNAs were recovered from the genomic DNA of reactive clones by PCR. A 3.6-kb band was present in the PCR products of all clones. (C) The 3.6-kb cDNA encodes an antigen recognized by G106/1. Serum G106/1 reacts with Bw cells transduced to express a 3.6-kb cDNA (gray histogram) but not control-transduced Bw cells (open histogram).
ILT5-specific antibodies arise from disparities in the ILT5 protein between donor and recipient
ILT5 is a member of the immunoglobulin-like transcript family, which has at least 10 members.18 Currently, no functional data on ILT5 are available, but it is known to be a highly polymorphic protein. Colonna et al identified 15 different ilt5 sequences in a cDNA sample derived from 50 donors.19 The polymorphic amino acids are not randomly dispersed but clustered at certain positions. The ILT5 form isolated from our DC expression library differed from these sequences and was designated ILT5 variant 1 (ILT5v1). The nucleotide sequences of ILT5 variant 1 and variant 2 have been submitted to the GenBank (accession nos. EU814627 and EU814628, respectively).20
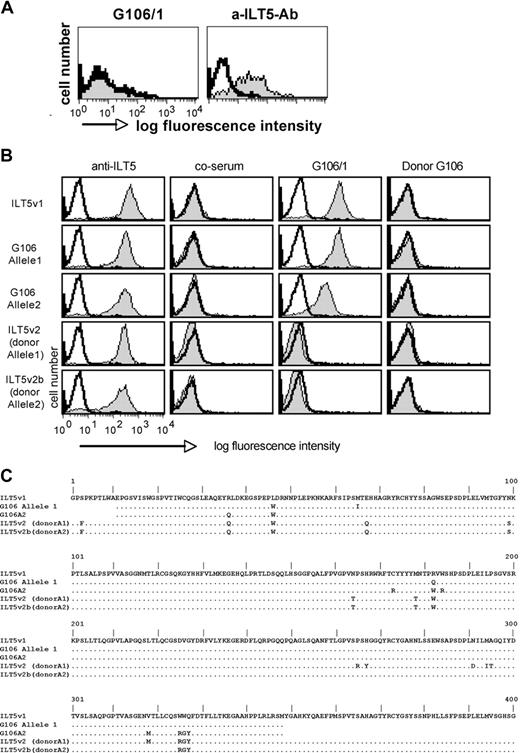
Patient G106 achieved full donor chimerism; thus, all hematopoietic cells, including antibody-producing B cells and the ILT5 expressed on the DCs, are of donor origin. To analyze whether allogeneic disparity in the ILT5 protein was responsible for the antibody production in this HSCT patient, we probed serum G106/1 with her mdDCs, which are genetically donor derived. As shown in Figure 3A, despite high ILT5 expression on these cells, serum G106/1 did not react with these DCs. We then cloned the ilt5 cDNA from DCs of patient G106 and subjected it to sequence analysis. The deduced amino acid sequence of this cDNA was found to significantly differ from the ILT5v1 that was isolated from our DC library and was termed ILT5 variant 2 (ILT5v2). Analysis of ilt5 sequences encoded by the DC-cDNA of patient G106 revealed that the second ILT5 variant (variant 2b) expressed in these cells was highly similar to ILT5 variant 2. DNA sequence analysis of genomic DNA derived from the HSC donor of patient G106 confirmed that ILT5 variant 2 and variant 2b were of donor origin. Because no ILT5-expressing pretransplantation recipient cells were available, we PCR-retrieved ilt5 sequences encoding the extracellular part from (pretransplantation) genomic DNA of patient G106. The PCR products were combined with ilt5 variant 2 sequences to generate 2 chimeric ilt5 genes, where the extracellular part is encoded by the 2 allelic ilt5 sequences derived from patient G106. The resulting ILT5 cDNAs were cloned into retroviral vectors and expressed on Bw cells. Serum G106 reacted with these ILT5 chimeras. Whereas the reactivity of this serum to cells expressing the extracellular part of allele 1 was comparable with the reactivity observed with ILT5v1, a weaker reactivity was seen with cells expressing allele 2. In contrast, serum G106 did not react with the 2 ILT5 variants (v2 and v2b) cloned from cells derived from the donor of patient G106 (Figure 3B). Furthermore, serum derived from the HSC donor of patient G106 did not bind to ILT5, indicating that antibodies to the ILT5 molecules of the recipient have developed in the patient after HSCT (Figure 3B). In support for this, we found a marked difference in the amino acid sequences of ILT5 molecules of the donor and the recipient. Importantly, in line with the reactivity of serum G106/1 to the cells expressing ilt5 sequences of the recipient, we found that allele 1 had a higher similarity to ILT5v1 and differed in more amino acid positions from ILT5v2 and 2b than allele 2 (Figure 3C).
ILT5-specific antibodies arise from disparities in the ILT5 protein. (A) G106 does not react with autologous DCs. mdDCs (donor derived) generated from patient G106 were probed with serum G106/1 (left panel, gray histogram) and a murine anti-ILT5 antibody (right panel, gray histogram). Reactivity of a control serum (left panel) or the isotype control antibody (right panel) is depicted as open histograms. (B) G106/1 reacts with recipient-derived ILT5 but not donor-derived ILT5. The ilt5 products of the recipient G106 (pretransplantation; alleles 1 and 2) and posttransplantation (donor-derived; ilt5v2 and v2b) were cloned and expressed on Bw cells. The transfectants were probed with an ILT5-PE mAb (left panels), a healthy control serum (middle left panels), serum G106/1 (middle right panels), and donor serum (right panels) shown as gray histograms. Reactivity of anti–ILT5-PE mAb and sera to untransduced Bw cells is depicted as open histograms. Bound human sera were detected with PE-labeled goat anti–human IgG (Fcμ-specific) antibodies. (C) Alignment of the deduced amino acid sequence of ilt5v1 and ilt5 variants derived from patient G106 pretransplantation (ilt5 recipient alleles 1 and 2) and posttransplantation ILT5 donor alleles 1 (= ilt5v2) and 2 (= ilt5v2b).
ILT5-specific antibodies arise from disparities in the ILT5 protein. (A) G106 does not react with autologous DCs. mdDCs (donor derived) generated from patient G106 were probed with serum G106/1 (left panel, gray histogram) and a murine anti-ILT5 antibody (right panel, gray histogram). Reactivity of a control serum (left panel) or the isotype control antibody (right panel) is depicted as open histograms. (B) G106/1 reacts with recipient-derived ILT5 but not donor-derived ILT5. The ilt5 products of the recipient G106 (pretransplantation; alleles 1 and 2) and posttransplantation (donor-derived; ilt5v2 and v2b) were cloned and expressed on Bw cells. The transfectants were probed with an ILT5-PE mAb (left panels), a healthy control serum (middle left panels), serum G106/1 (middle right panels), and donor serum (right panels) shown as gray histograms. Reactivity of anti–ILT5-PE mAb and sera to untransduced Bw cells is depicted as open histograms. Bound human sera were detected with PE-labeled goat anti–human IgG (Fcμ-specific) antibodies. (C) Alignment of the deduced amino acid sequence of ilt5v1 and ilt5 variants derived from patient G106 pretransplantation (ilt5 recipient alleles 1 and 2) and posttransplantation ILT5 donor alleles 1 (= ilt5v2) and 2 (= ilt5v2b).
The epitopes of ILT5-specific antibodies are dispersed over the entire extracellular region of the molecule
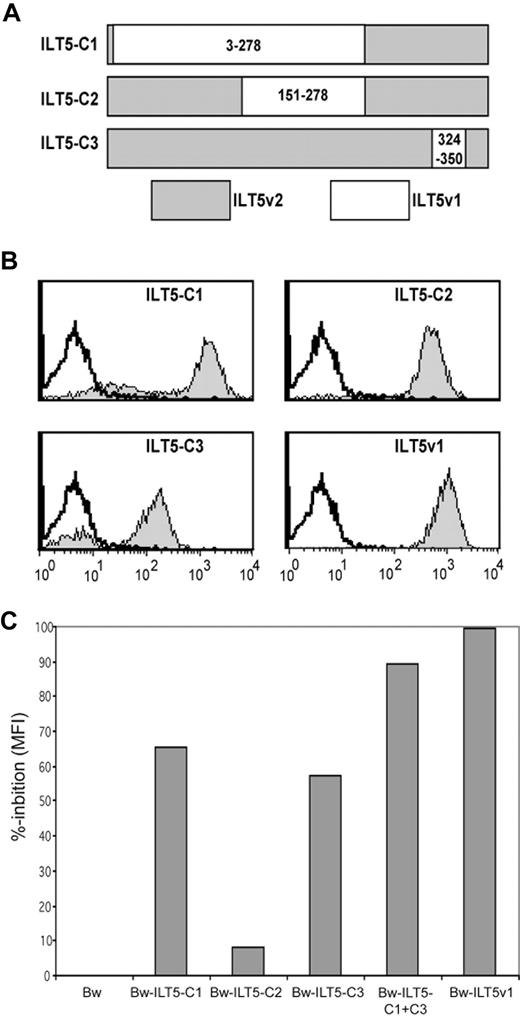
Because the sequence disparities between the ILT5 molecules of the donor and the recipient are dispersed over the extracellular domain of the ILT5 molecule, we wanted to assess which part of the molecule harbors the epitopes of the ILT5-specific antibodies. We thus generated and expressed 3 chimeric ILT5 variant 2 molecules (C1-C3) that express sequences derived from the ILT5 variant 1 (Figure 4A). These molecules were retrovirally expressed, and the resultant cells were probed with an ILT5 mAb (supplemental Figure 2) These cells were on one hand probed with serum from patient G106, and on the other hand we used these cells to deplete this serum from ILT5-reactive antibodies directed to distinct regions of the ILT5 molecule by repeatedly incubating the serum with these Bw cells and removing cell-bound antibodies by centrifugation. This treatment yielded sera that contained antibodies only to ILT5v1 epitopes that were not present on the chimeric molecules. We found that serum of patient G106 strongly reacted with all chimeric molecules that were generated (Figure 4B). The majority of ILT5-reactive antibodies in this serum were directed to amino acids 3 to 278 of the ILT5v1 molecule, which is represented by chimera C1. Adsorption of ILT5 reactive antibodies with Bw-C1 cells resulted in a reduction of the antibody reactivity to ILT5v1 by almost 70% (Figure 4C). Adsorption of antibodies with Bw-C2 that harbors amino acids 151 to 278 of ILT5 variant 1 resulted in a modest reduction of reactivity to cells expressing the whole ILT5v1 molecule, indicating that the majority of antibodies that react with the chimera C1 bind to the N-terminal part (amino acids 3-150) of the molecule. The reactivity of serum G106 to Bw-C3 cells, which express amino acids 324 to 350 of variant 1, was somewhat weaker, probably the result of a lower expression of the chimeric ILT5 (Figure 4B, supplemental Figure 2). However, a substantial portion of the ILT5 reactivity is directed to this part of the molecule because adsorption with Bw-C3 cells also led to a strong reduction of the reactivity to ILT5v1 (Figure 4C). Thus, these data show that the binding sites of ILT5 antibodies are dispersed over the entire extracellular region of the ILT5 molecule.
Identification of binding sites of ILT5-specific antibodies. (A) Scheme of chimeric ILT5v1/v2 molecules representing amino acids 3 to 278 (ILT5-C1), 150 to 278 (ILT5-C2), and 324 to 350 (ILT5-C3) of ILTv1. (B) Plasmids encoding ILT5 chimeras C1 to C3 and ILT5v1 were retrovirally expressed on Bw cells and tested with serum G106/1 (gray histograms). Reactivity of serum G106/1 with untransduced Bw cells is depicted as open histograms. (C) Bw cells expressing ILT5v1 were stained with serum G106/1 preincubated with Bw cells or Bw cells expressing ILT5 chimera (C1-C3) or ILT5v1 as indicated. Percentage reduction of reactivity compared with control Bw treated serum is shown.
Identification of binding sites of ILT5-specific antibodies. (A) Scheme of chimeric ILT5v1/v2 molecules representing amino acids 3 to 278 (ILT5-C1), 150 to 278 (ILT5-C2), and 324 to 350 (ILT5-C3) of ILTv1. (B) Plasmids encoding ILT5 chimeras C1 to C3 and ILT5v1 were retrovirally expressed on Bw cells and tested with serum G106/1 (gray histograms). Reactivity of serum G106/1 with untransduced Bw cells is depicted as open histograms. (C) Bw cells expressing ILT5v1 were stained with serum G106/1 preincubated with Bw cells or Bw cells expressing ILT5 chimera (C1-C3) or ILT5v1 as indicated. Percentage reduction of reactivity compared with control Bw treated serum is shown.
Identification of additional HSCT patients with strong antibody responses to ILT5
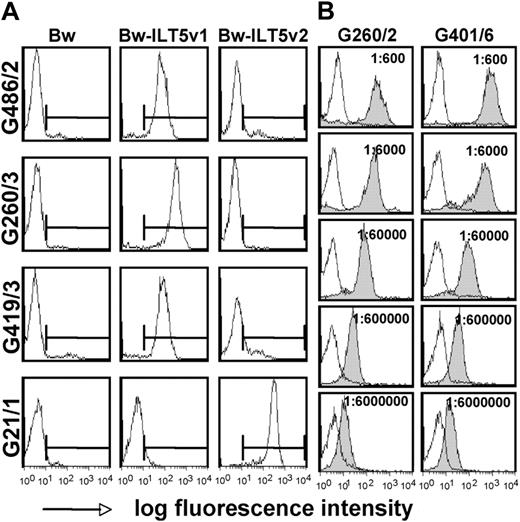
To assess whether ILT5-reactive antibodies are only found in patient G106 or represent a broader phenomenon in patients after HSCT, we tested the sera of 240 HSCT recipients for reactivity to cells expressing ILT5v1 or ILT5v2. In these experiments, we could identify 10 additional patients with high titers of ILT5v1-specific antibodies; and interestingly, we also identified 2 patients with ILT5v2-specific antibodies (ILT5v1: n = 11, 4.6%; ILT5v2: n = 2, 0.8%; n = 240). The clinical data of the patients with ILT5-reactive antibodies are summarized in Table 1. In all cases, the sera reacted with one variant of this molecule (Figure 5A). We tested serial dilutions of 2 sera that showed especially strong reactivity to ILT5v1 and found that ILT5-specific antibodies were still detectable by FACS at a titer of 6 × 106 (Figure 5B). Sera derived from several ILT5-reactive patients were probed with Bw cells expressing chimeric molecules representing different regions of the extracellular domain of ILT5v1. Similar to results obtained with serum G106, we found these sera to contain antibodies directed to different areas of the ILT5 molecule (supplemental Figure 2).
Identification of additional stem cell-transplanted patients with high titers of antibodies specific for ILT5 variants 1 and 2. (A) Control cells (Bw) and cells expressing ILT5 variant 1 (Bw-ILT5v1) or 2 (Bw-ILT5v2) and control cells (Bw) were probed with sera reacting with ILT5 variant 1 (G486/2, G260/3, and G419/3) or ILT5 variant 2 (G21/1). (B) Serial dilutions of indicated ILT5 variant 1-reactive sera were probed with control Bw cells (open histograms) or Bw cells expressing ILT5 variant 1 (gray histograms).
Identification of additional stem cell-transplanted patients with high titers of antibodies specific for ILT5 variants 1 and 2. (A) Control cells (Bw) and cells expressing ILT5 variant 1 (Bw-ILT5v1) or 2 (Bw-ILT5v2) and control cells (Bw) were probed with sera reacting with ILT5 variant 1 (G486/2, G260/3, and G419/3) or ILT5 variant 2 (G21/1). (B) Serial dilutions of indicated ILT5 variant 1-reactive sera were probed with control Bw cells (open histograms) or Bw cells expressing ILT5 variant 1 (gray histograms).
ILT5-reactive antibodies could be detected as early as 97 days after HSCT, but in some patients ILT5-reactive antibodies were detected at much later time points (data not shown). ILT5-specific antibodies appear to persist for a long time in the patient, and analysis of follow-up sera did not point to a significant loss of reactivity to this molecule. No ILT5 reactivity was detected in any serum sample that was obtained less than 3 months after transplantation (data not shown). Most importantly, none of the sera that were collected before HSCT contained ILT5-reactive antibodies (supplemental Figure 3A). Pretransplantation sera were only available for one donor/recipient pair of an ILT5-reactive person (patient H47). Neither serum reacted with ILT5, which again demonstrates that ILT5 reactivity developed in the patient after HSCT (supplemental Figure 3B). To assess whether the incidence of ILT5-specific antibodies is restricted to HSCT patients, we screened sera of renal allograft recipients (n = 105) for reactivity to ILT5v1 or ILT5v2. However, ILT5-specific antibodies were not detectable in these patients. In addition, we did not detect antibodies in sera derived from polytransfused patients (n = 13), patients with collagenous diseases (n = 40), multiparous women (n = 80), or any healthy control (n = 30; Table 2).
ILT5 is expressed on leukemic cells
As depicted in Table 1, the patients showing ILT5 reactivity are highly heterogeneous regarding primary disease, conditioning for HSCT, and stem cell source received. Furthermore, analysis of their HLA genotypes did not reveal common HLA molecules that might be associated with the development of ILT5-specific antibodies (data not shown). Comparing the conditioning protocols (myeloablative vs reduced), stem cell source, degree of HLA matching, or use of sibling/matched unrelated donors as well as the incidence of GVHD, relapse, and death in HSCT recipients with ILT5-reactive antibodies and those without ILT5 reactivity no statistically significant differences or associations could be detected (Table 3).
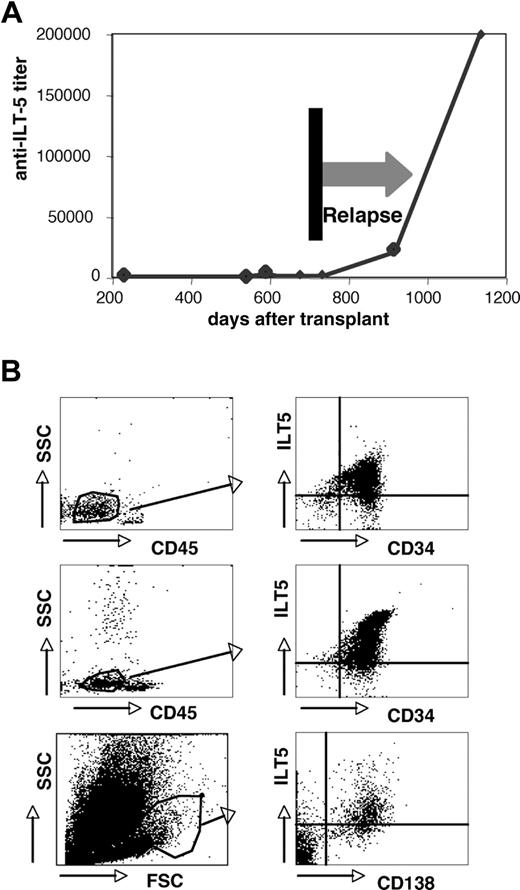
Three HSCT patients with ILT5-specific antibodies experienced leukemic relapse (Table 1). For one of these persons (patient G189), several sera obtained at different time points were analyzed for ILT5-specific antibodies. Importantly, in this patient, we observed a very strong increase in the ILT5 antibody titer after leukemic relapse (Figure 6A), indicating that the target antigen is expressed on the leukemic cells of this patient. An increase in the ILT5-specific antibody titers after relapse was also observed in a second patient where sera collected before a leukemic relapse were available (data not shown). When analyzing healthy donors, we found ILT5 to be surface-expressed on monocytes and granulocytes, but not on lymphocytes (supplemental Figure 4). FACS analyses of bone marrow samples derived from leukemic patients demonstrate that, in addition, ILT5 can be expressed on leukemic cells of myeloid origin, on myeloma cells, and other lymphoid neoplasias (Figure 6B). None of the patients with very strong ILT5 reactivity had relapsed, which might indicate that ILT5-specific antibodies have antileukemic activity. We therefore assessed whether these antibodies are able to mediate killing of ILT5-bearing cells. In these experiments, we found sera containing ILT5-specific antibodies to be potent mediators of both CDC and ADCC (Figure 7).
Expression of ILT5 on leukemia cells. (A) The titers of ILT5-specific antibodies were determined in serum samples derived from a patient who relapsed from AML. (B) B cell (top panels) and myeloid (middle panels) leukemia samples were analyzed for ILT5 expression. Blasts were identified by side scatter analysis and low CD45 expression (left panels). CD34/ILT5 expression on the gated cells is shown (right panels). (Bottom panels) Myeloma cells were identified by forward scatter/side scatter analysis (left panel) and CD138 expression (right panel).
Expression of ILT5 on leukemia cells. (A) The titers of ILT5-specific antibodies were determined in serum samples derived from a patient who relapsed from AML. (B) B cell (top panels) and myeloid (middle panels) leukemia samples were analyzed for ILT5 expression. Blasts were identified by side scatter analysis and low CD45 expression (left panels). CD34/ILT5 expression on the gated cells is shown (right panels). (Bottom panels) Myeloma cells were identified by forward scatter/side scatter analysis (left panel) and CD138 expression (right panel).
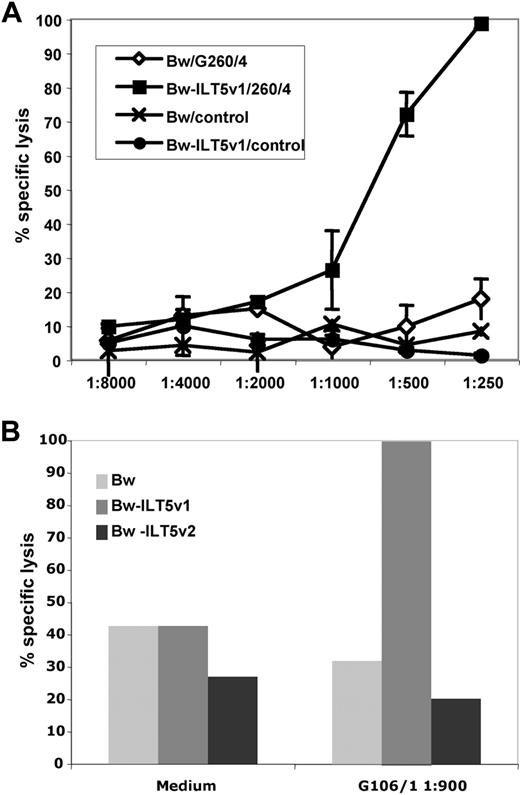
ILT5-specific antibodies can induce cytotoxicity. (A) Complement-mediated cytotoxicity of ILT5-reactive serum (G260/4) and control serum (control) using Bw cells (Bw) or Bw cells expressing ILT5 variant 1 (Bw-ILT5v1). (B) Control Bw cells and Bw cells expressing either ILT5 variant 1 (Bw-ILT5v1) or variant 2 (Bw-ILT5v2) were incubated with interleukin-2–activated lymphocytes in presence of serum G106/1 to test the ability of ILT5-specific antibodies to induce ADCC.
ILT5-specific antibodies can induce cytotoxicity. (A) Complement-mediated cytotoxicity of ILT5-reactive serum (G260/4) and control serum (control) using Bw cells (Bw) or Bw cells expressing ILT5 variant 1 (Bw-ILT5v1). (B) Control Bw cells and Bw cells expressing either ILT5 variant 1 (Bw-ILT5v1) or variant 2 (Bw-ILT5v2) were incubated with interleukin-2–activated lymphocytes in presence of serum G106/1 to test the ability of ILT5-specific antibodies to induce ADCC.
Discussion
It is generally accepted that the recognition of (non-HLA) sequence polymorphisms by the donor-derived immune system has an important impact on the outcome of HSCT. Consequently, many investigators have studied the role of mHAg in GVH reactions or recurrence of leukemias and reported a significant impact for such polymorphisms in this context.5,7,21-26 Most of the known mHAgs are intracellular proteins; thus, these antigens can only be targeted by cytotoxic T cells, although it is well documented that the recognition of such intracellular antigens can be accompanied by strong humoral immune responses.9,27 In contrast, cells bearing immunogenic polymorphic surface molecules could be efficiently eradicated by antibodies directed to them. We show here that a subset of HSCT patients has antibodies directed to DC surface antigens. Using the serum of one such patient in conjunction with retroviral expression cloning, we have identified the ILT5 as one target of DC-reactive antibodies in recipients after allogeneic HSCT. ILT5 belongs to the ILT family also known as leukocyte immunoglobulin-like receptors. Like the members of the killer cell immunoglobulin-like receptor, which are distantly related to the ILTs, they are encoded on human chromosome 19.28 Whereas various functions, including binding of classic and nonclassic MHC molecules and tolerization of dendritic cells, have been ascribed to members of the ILT family,29-31 no functions or binding partner has been reported for ILT5. However, it has been known for a long time that ILT5 is a highly polymorphic protein, and our results clearly point to sequence disparities between donors and recipients as the reason for this antibody response. By screening a collective of sera derived from 240 HSCT patients, we identified 13 patients (5.4%) who had ILT5-specific antibodies. Because we have only evaluated the antibody response to 2 variants of this molecule, it is possible that the incidence of ILT5-specific antibodies in HSCT recipients might be considerably higher as antibody responses to other variants might have gone undetected. One of the striking findings of this study is the extremely high titers of antibodies to ILT5 that were seen in some persons. This points to a very strong immune response to the ILT5 variant expressed by the recipients' cells. The presence of ILT5 or any other member of the ILT family on nonhematopoietic cells has not been described,18 and analysis of mRNA expression in different human tissues did only detect significant levels of ILT5 mRNA in natural killer cells, monocytes, and CD33+ myeloid cells.32 Despite high titers of ILT5 antibodies, 4 of these patients never had any sign of GVHD and the incidence of GVHD was not higher in the ILT5-reactive group compared with patients without ILT5-specifc antibodies (Table 3). Furthermore, we did not observe ILT5 reactivity in sera of renal allograft recipients. Thus, it is probable that malignant cells persisting or recurring in the patients have elicited this strong antibody response to ILT5. Interestingly, in 2 patients, we observed a strong increase in the ILT5 antibody titer after extramedullary leukemic relapse. Importantly, both patients achieved complete remission after short course radiotherapy with a follow-up of 12 and 30 months. We show that ILT5 can be expressed on leukemic cells of myeloid origin and demonstrate expression of this molecule on myeloma cells and lymphoid neoplasias. Furthermore, ILT5-specific antibodies can efficiently kill ILT5-expressing cells by ADCC and CDC. Thus, it is possible that ILT5 disparities between donors and recipients can elicit antibody-mediated GVL effects. Comparison of HCST patients who developed ILT5-specific antibodies with patients not reacting with this molecule showed that, although the incidence of relapse was higher in the ILT5-reactive group, none of these patients died. Both parameters did not, however, reach statistical significance, possibly because of the small number of patients with ILT5-specific antibodies available for analysis. Thus, additional studies to determine whether patients with ILT5-positive leukemias can benefit from an allogeneic HSCT that is mismatched in the ILT5 locus are highly warranted.
It can be expected that the generation of the very strong IgG response to ILT5 is accompanied by a parallel T-cell response to polymorphic ILT5 peptides. We have generated an immunoglobulin fusion protein representing ILT5v1 to analyze cellular responses of PBMCs derived from patients with antibodies to this ILT5 variant. However, we were unable to detect ILT5-specific T-cell responses (data not shown). It is possible that ILT5-specific T cells might have vanished from the periphery perhaps resulting from the lack of antigen because the samples we analyzed were obtained 400 days after transplantation or later.
To our knowledge, this study is the first report of an antibody response to allogeneic non-HLA leukocyte surface antigens in HSCT patients. Amino acid sequence polymorphisms in cell surface molecules is not an uncommon phenomenon: Maruya et al found common amino acid variations in 7 of 14 human membrane resident proteins.33 Thus, it is probable that allogeneic disparities in additional surface molecules also induce humoral immune responses in HSCT patients. This hypothesis is further supported by the fact that additional DC-reactive sera we have identified did not react with ILT5 but other not yet identified DC antigens.
The efficacy of biologicals, such as rituximab (anti-CD20 mAb), underlines the potency of antibodies to leukocyte antigens for therapy of neoplasias and autoimmune diseases. We propose that also antibodies to polymorphic surface antigens could have a currently unrecognized role in GVL reactions. Recently, Bellucci et al showed that after donor-lymphocyte infusion patients with myeloma developed humoral immune responses to the surface molecule B-cell maturation antigen, a nonpolymorphic surface antigen that is overexpressed on myeloma cells.34 Thus, antibodies directed to membrane resident leukemic antigens might, in addition to humoral immune responses to polymorphic surface antigens, combat leukemias after HSCT. Serologic identification of additional polymorphic surface antigens using the method described in this study or related approaches will help to assess the contribution of such antibodies to the eradication of leukemias but also to HSCT-associated complications. Furthermore, the identification of common immunogenic surface antigens could lead to new strategies for the treatment of relapsing leukemia, eg, the development of therapeutic antibodies selectively binding to polymorphic leukocyte surface antigens on recipients' cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Martin Aringer for providing serum samples; Ulrike Körmöczi, Imke Lohmann, Arno Rottal, Petra Cejka, Claus Wenhardt, Kuzmina Zoya, and Karin Feldmann for excellent technical assistance; Gerhard Fritsch and Dieter Printz (Childrens' Cancer Research Institute) for cell sorting; and Dr Garry Nolan et al for providing the retroviral vector pBMN-Z.
This work was supported by the Children's Cancer Research Institute (Vienna, Austria; grant 7003) and the Austrian Federal Bank (grants AP12185 and AP12731).
This work is dedicated, in grateful memory, to Walter Knapp, whose ideas initiated this work.
Authorship
Contribution: K.P. and P.S. designed research and wrote the manuscript; K.P., P.S., A.L., C.K., and J.L. performed experiments; K.P., P.S., A.L., H.T.G., R.W., M.H.M.H., W.F.P., O.M., G.A.B., and G.F.F. analyzed and interpreted data; and A.L., H.T.G., R.W., M.H.M.H., W.F.P., O.M., G.A.B., J.L., C.K., and G.F.F. critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Steinberger, Institute of Immunology, Medical University of Vienna, Borschkegasse 8A, 1090 Vienna, Austria; e-mail: peter.steinberger@meduniwien.ac.at.