The plasma protein CFH is a critical complement regulator with relevance to human diseases. The function of several smaller, CFH-related proteins (CFHRs) has been a mystery. In this issue of Blood, Heinen and colleagues identify CFHR-1 as a novel inhibitor of C5 convertase and the terminal complement pathway.1
The complement system is a branch of innate immunity crucial to the recognition and clearance of pathogens and cellular debris. Whereas it is essential to the health of the host, complement also has the potential to harm the host if not tightly regulated.
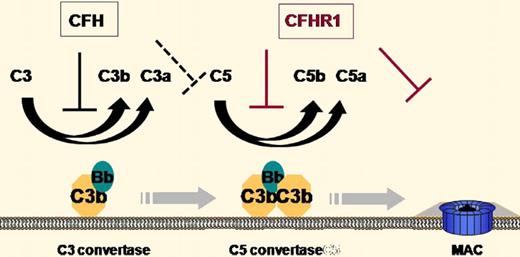
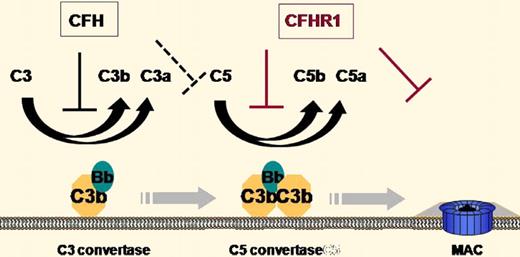
The plasma protein complement factor H (CFH) has long been known as a crucial regulator of complement activation both in the fluid phase and on the cell surface.2 CFH inhibits complement activation by accelerating the decay of C3 and C5 convertases of the alternative complement pathway and by acting as a cofactor for factor I–mediated cleavage of surface deposited C3b (see figure). It is composed of 20 short consensus repeat (SCR) domains, with SCRs 1-4 being necessary and sufficient for complement inhibition and SCRs 19-20 being critical for C3b/C3d binding and host cell interaction. Mutations in CFH have been linked to rare renal diseases such as membranoproliferative glomerulonephritis (MPGN) and atypical hemolytic uremic syndrome (aHUS), as well as a common ocular disease, age-related macular degeneration (AMD).2
Illustration of the sites of action of CFH and CFHR-1 in the regulation of complement activation.
Illustration of the sites of action of CFH and CFHR-1 in the regulation of complement activation.
It has been recognized for some time that several shorter, CFH-related proteins (CFHRs), encoded by separate genes next to the CFH locus, also exist in human plasma but their functions have remained a mystery.3 Among them, CFHR-1 is composed of 5 SCRs with SCRs 3-5 being identical or highly homologous to SCRs 18-20 of CFH (100%, 100%, and 98% identical), and SCRs 1-2 showing moderate homology to SCRs 6-7 of CFH (36% and 45% identical).3 Notably, most aHUS-related mutations in CFH are located within SCRs 19-20 and the AMD-related CFH polymorphism (Y402H mutation) occurs within SCR 7. Interest in CFH-related proteins intensified with the finding that deletion of CFHR-1/CFHR-3 increases the risk of aHUS,4 but paradoxically the same deletion seems to confer protection for AMD.5 The mechanism underlying this association is unknown.
In a highly significant paper appearing in this issue of Blood, Heinen et al present evidence that CFHR1 acts as an inhibitor of the C5 convertase and the lytic pathway of complement.1 They demonstrate that like CFH, CFHR-1 can bind to heparin, C3b/C3d, and host cells. They propose that CFH and CFHR-1 compete for the same binding sites, implying that an increase in CFHR-1 activity on the host cell surface is at the expense of CFH activity. Because CFHR-1 does not contain domains homologous to the N-terminus of CFH (SCRs 1-4), it should not affect C3 activation. Indeed, Heinen et al confirmed that CFHR-1 does not regulate C3 convertase activity. Surprisingly, they found that CFHR-1 can regulate the activity of the C5 convertase C3bBbC3b, lowering C5a production. Furthermore, they demonstrate that CFHR-1 can bind to C5 and C5b6 and thus is also capable of inhibiting the formation of the membrane attack complex independent of its activity on the C5 convertase. These results identify CFHR-1 as a unique complement regulator that regulates C5 activation and terminal complex formation without affecting the C3 activation step (see figure).
The revelation of CFHR-1 activity may help us understand the genetic data linking CFHR-1/CFHR-3 deletion to AMD and aHUS. Given the functional difference between CFH and CFHR-1 (see figure) and the presumed competition between the 2 proteins in host cell binding, one interpretation of the genetic data is that control of C5 activation and the terminal complement pathway is important for reducing the risk of aHUS, whereas regulation of C3 activation is perhaps more critical for preventing AMD. Such a view is probably too simplistic as the relative roles of CFH and CFHR-1 in vivo are likely to be determined also by their plasma concentrations, relative affinity for C3b/C3d and host cell surface, and respective potency as a direct or indirect C5 convertase inhibitor. Additionally, CFHR-1/CFHR-3 deficiency is often associated with CFH autoantibodies,6 which may significantly impact aHUS and AMD risks as well.
Whether or not the findings of Heinen et al can readily explain the observed correlation between CFHR-1/CFHR-3 deletion and altered aHUS and AMD risks, their work raises the possibility that other CFH-like (CFHL1, derived from alternative splicing of the CFH gene) and CFH-related (CFHR-2 to CFHR-5, encoded by separate genes) plasma proteins2,3 may also possess complement-regulating activities. It also raises the question as to whether CFHR-1 might be involved in pathogen evasion of host immunity. Apart from protecting host cells from complement attack, CFH is frequently recruited and used by pathogens to thwart the host complement system.2,3 It has been shown previously that some pathogens can recruit and bind CFHR-1.7 Thus, it will be interesting to determine whether these organisms can use this and other CFHRs for immune evasion as well. It is hoped that the current study will serve as a catalyst to stimulate additional investigations into these questions.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■