Abstract
The microenvironment of the lung in asthma is acidic, yet the effect of acidity on inflammatory cells has not been well established. We now demonstrate that acidity inhibits eosinophil apoptosis and increases cellular viability in a dose-dependent manner between pH 7.5 and 6.0. Notably, acidity induced eosinophil cyclic adenosine 5′-monophosphate (cAMP) production and enhanced cellular viability in an adenylate cyclase–dependent manner. Furthermore, we identify G protein-coupled receptor 65 (GPR65) as the chief acid-sensing receptor expressed by eosinophils, as GPR65-deficient eosinophils were resistant to acid-induced eosinophil cAMP production and enhanced viability. Notably, GPR65−/− mice had attenuated airway eosinophilia and increased apoptosis in 2 distinct models of allergic airway disease. We conclude that eosinophil viability is increased in acidic microenvironments in a cAMP- and GPR65-dependent manner.
Introduction
Asthma is a chronic lung disease characterized by episodes of inflammation and narrowing of the airways in response to diverse stimuli.1 Over the past 2 decades, the incidence of asthma has increased worldwide and is now one of the chief diagnoses responsible for pediatric hospitalization. Extracellular acidosis is commonly observed in inflammatory diseases such as asthma. Several studies have shown that the pH of exhaled breath condensate (EBC) in patients with acute asthma is significantly lower than that of normal subjects. The pH of EBC is mildly alkaline in control persons (7.65 ± 0.20), reaching 5.23 plus or minus 0.21 during asthma exacerbations.2 Furthermore, the EBC pH of pediatric and adult patients with chronic asthma is also significantly decreased compared with healthy people and correlates with asthma severity.3,4 Whereas EBC is not a direct measurement of airway lining fluid, airway acidification in asthma is supported by alternative approaches, including direct measurements of bronchial secretions.2 Although EBC pH is not an asthma-specific biomarker, early clinical studies have suggested that acidification contributes to the disease process as inhalation of buffers that increase airway pH alleviates asthma symptoms.5
G protein-coupled receptor 65 (GPR65), also known as T-cell death-associated gene 8, belongs to a group of acid-sensing receptors in the G2A G protein–coupled receptor (GPCR) family.6-8 This group includes G2A, GPR65, ovarian cancer GPCR1 (OGR1), and GPR4. Some members of the G2A family, including GPR65, have been shown to be proton sensors in lymphocytes. Acid-sensing capacity is mediated by proton transfer to histidines in the first extracellular loop of GPR65, presumably causing a conformational change in GPR65 that activates Gαs. This acid-sensing activity has been demonstrated by measuring intracellular cyclic adenosine 5′-monophosphate (cAMP) in GPR65-deficient lymphocytes incubated at several proton concentrations.7
To better understand the functional implications of acidic microenvironments on eosinophils, hallmark cells of asthmatic inflammation,9 we assessed the function of eosinophils exposed to acid. Recent studies in patients with asthma and experimental models of asthma suggest that eosinophils have a causative role in asthma under certain conditions.10-13 Eosinophils may affect the pathophysiologic processes of asthma through an array of mechanisms, including immunomodulation, inducing tissue damage and dysfunction by releasing toxic granule proteins, and effects on nerves leading to bronchoconstriction.9
Importantly, we found that both human and murine eosinophils respond to protons with increasing cellular viability and decreasing early apoptosis. We demonstrate that eosinophils respond to increased acidity by increasing intracellular cAMP production; furthermore, the activity of adenylate cyclase is required for acidity-enhanced eosinophil viability. We identified an acid-sensing receptor GPR65 that has increased expression in asthmatic inflammation and is expressed on eosinophils. Importantly, we demonstrate that the acidic pH-induced increase in eosinophil cAMP and viability is dependent upon GPR65. Finally, we show that GPR65−/− mice have attenuated airway eosinophilia in 2 distinct models of allergic asthma, and this is associated with increased apoptosis in GPR65−/− compared with wild-type allergen-elicited airway eosinophils.
Methods
Mice
Male and female 8- to 12-week-old, CD2–interleukin 5 (IL-5) transgenic (Tg) mice (BALB/c)14 were housed under specific pathogen-free conditions and used as a source of eosinophils, as previously reported.15 CD2–IL-5 transgenic mice were crossed with GPR65−/− BALB/c mice16 as a source of GPR65-deficient eosinophils. BALB/c and GPR65−/− mice were used for allergen challenge experiments. All animal studies were reviewed and approved by the Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee.
Human eosinophil purification
Eosinophils were purified from healthy or mildly atopic volunteers by negative immunomagnetic selection, as reported,17 and approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board, with informed consent obtained in accordance with the Declaration of Helsinki. Briefly, granulocytes were isolated from heparin-anticoagulated whole blood by dextran sedimentation, Percoll centrifugation, and hypotonic lysis of red blood cells. Cells were resuspended in Hanks buffered salt solution (Life Technologies) with 2% fetal calf serum and incubated with 0.75 μL/106 cells of anti-CD16–conjugated microbeads (magnetic activated cell sorting [MACS]; Miltenyi Biotec) for 30 minutes at 4°C. The cell suspension was then applied onto a CS MACS column, and negative populations were collected through a magnetic field. The isolates routinely contained more than 95% eosinophils with viability of more than 95% as assessed by trypan blue exclusion.
Murine eosinophils
Eosinophils were obtained from spleen cells derived from CD2–IL-5 transgenic mice, in which approximately 30% to 50% of the cells are eosinophils. Both the ammonium chloride and hypotonic methods of red blood cell lysis were used with similar results. Eosinophils were purified (80%-85% purity) from the spleen by immunomagnetic negative selection using anti-Thy1.2 and anti-CD19 using the MACS system (Miltenyi Biotec), as previously described.15 The results from 2A were also confirmed on murine eosinophils purified to more than 95% using polarized light to sort the eosinophils by flow cytometry, as described previously.18 Purified eosinophils were used for measurement of cAMP, whereas unpurified spleen cells were used for viability assays.
Assessment of viability
For acidic exposure, RPMI with 10% fetal bovine serum was prepared by using 30 mM biologic buffer (either HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2-[N-morpholino]ethanesulfonic acid (MES), acetate, or citrate for pH ranges 8.5-7.0, 6.5-5.5, 5.0-4.5, and 4.0, respectively). After the media was adjusted to the appropriate pH, NaCl was added to maintain osmolarity at 330 mOsM. For mouse studies, IL-5 Tg spleen cells (5-10 × 105 cells) were incubated in 1 mL buffered media for 3 to 7 hours at 37°C with 5% CO2. After 5 hours of incubation, the pH increased by 0.25 plus or minus 0.08 at pH 7.5 and 0.2 plus or minus 0.1 at pH 6. In some experiments, cells were first stained with anti–C-C chemokine receptor (CCR)3 or anti–Siglec-F to identify eosinophils. For human studies, purified eosinophils were incubated for 18 to 24 hours. Apoptosis and viability were determined using annexin V–allophycocyanin and 7-aminoactinomycin D (7AAD), according to the manufacturer's instructions (BD Biosciences). Viability was defined as annexin V–negative, 7AAD-negative. Early apoptosis was defined as annexin V–positive, 7AAD-negative. Briefly, cells were washed and resuspended in annexin V–binding buffer and stained with annexin V–allophycocyanin and 7AAD. Samples were then incubated at room temperature for 15 minutes. Cells were analyzed immediately on the FACSCalibur.
Experimental asthma induction
Allergic lung disease was established by 2 mouse models, ovalbumin (OVA) and Aspergillus fumigatus, as described.19,20 Briefly, the OVA model involved 2 intraperitoneal sensitizations of OVA with alum, followed by 2 intranasal challenges with either OVA or saline. The Aspergillus model involved 9 intranasal challenges with Aspergillus extract. After the mice were killed, bronchoalveolar lavage (BAL) differential cell counts were performed, as previously described.20
Preparation of RNA and microarray hybridization
Northern blot
Human RNA samples
Nasal brushing samples were obtained from children with stable asthma, children undergoing an asthma exacerbation, and nonatopic children with asthma, as described previously.21
RT-PCR
Gene-specific primers (designed by using Beacon Designer software [Applied Dynamics International]) were chosen to span at least 1 intron in the genomic sequence to enable the mRNA-derived product to be distinguished from any possible contaminating genomic product. The sequences of primers for GPR65 are forward, CGGAAGAAACATGGAAGGAA and reverse, TTGGCTAAAGGGGTCAACTG. Before cDNA synthesis (using SuperScript II, RNase H−; Invitrogen), 1 to 2 μg of each RNA sample was pretreated with DNase I (Invitrogen). Reverse transcription–polymerase chain reaction (RT-PCR) analysis was conducted with the iCycler (Bio-Rad) by using the iQ SYBR Green Supermix Taq polymerase mix (Bio-Rad). The amount of double-stranded DNA product, indicated by SYBR Green fluorescence, was measured at the end of each extension cycle. The relative mRNA levels of each target gene were normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (forward, TGGAAATCCCATCACCATCT; reverse, GTCTTCTGGGTGGCAGTGAT).
Measurement of cAMP in eosinophils
Human eosinophils isolated from peripheral blood and murine eosinophils purified from the spleen by immunomagnetic negative selection were used to assess cAMP levels. Cells were incubated in buffered media and 0.5 mM 3-isobutyl-1-methylxanthine (IBMX). After a 30-minute incubation, cells were lysed for cAMP quantification using enzyme-linked immunosorbent assay (ELISA; Amersham Biosciences). For data in Figure 2E, human eosinophils were incubated without treatment, or with IBMX or adenylate cyclase inhibitor for 10 minutes.
Bone marrow eosinophil CFU analysis and eosinophil blood levels
ELISA measurements
Protein levels were measured in eosinophil lysates, BAL fluid (BALF), and lung homogenates using ELISA kits specific for IL-5, IL-13, and eotaxin-2 (R&D Systems). The sensitivity of the ELISAs were as follows: 31.25 pg/mL, 250 pg/mL, and 15.6 pg/mL for IL-5, IL-13, and eotaxin-2, respectively. OVA-specific immunoglobulin G1 was measured, as previously described.24
Chemotaxis
Chemotactic responses were determined by chemotaxis through a Transwell plate, as previously described.25 Eosinophils (1.5 × 106) in buffered saline (HEPES or 2-[N-morpholino]ethanesulfonic acid for pH 7.5 and 6.0, respectively; osmolarity was adjusted to equal value in all conditions) with 0.5% bovine serum albumin (low endotoxin; Sigma-Aldrich) were placed in the upper chamber, and the chemoattractant (eotaxin-2 at various doses in buffered saline with 0.5% bovine serum albumin) was placed in the lower chamber. Chemotaxis was allowed to proceed for 1 hour (before changes in viability occur) at pH 7.5 and 6.0; both top and bottom chambers were adjusted to the same pH.
Actin polymerization
Agonist-induced actin polymerization was assessed using nitrobenzoxadiazole-phallacidin (Molecular Probes). For experiments measuring pH-induced actin polymerization, eosinophils were resuspended in pH-buffered saline and sampled at 0 to 120 seconds. For eotaxin-2–elicited actin polymerization, eosinophils were resuspended at 2.5 × 106 cells/mL in pH-buffered saline and stimulated with eotaxin-2 (10 nM) for indicated amounts of time. After stimulation, cells were fixed in 3.7% formaldehyde for 60 minutes. Lysophosphatidylcholine (100 mg/mL; Sigma-Aldrich) and 3.3 × 10−7 M nitrobenzoxadiazole-phallacidin were added to the cells and incubated for 1 hour in the dark. Cells were analyzed on a FACSCalibur with a linear fluorescence channel (FL1) in which the fluorescence is proportional to F-actin content. Relative F-actin content is expressed as the ratio of the mean channel fluorescence between chemokine- and media alone–stimulated cells.
Statistical analysis
Within experiments, analysis of variance with appropriate posttest (for experiments with more than 2 groups) and Student t test (for experiments with 2 groups) were used to assess statistical significance. Paired t tests were used to assess significance over multiple experiments.
Results
Exposure to acidity increases eosinophil viability and decreases eosinophil apoptosis
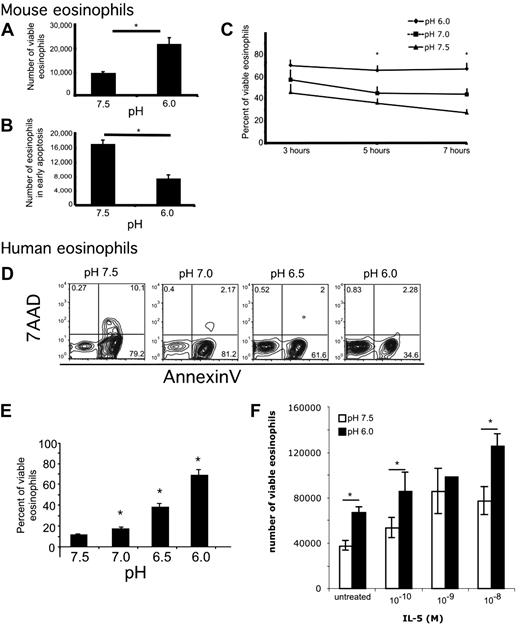
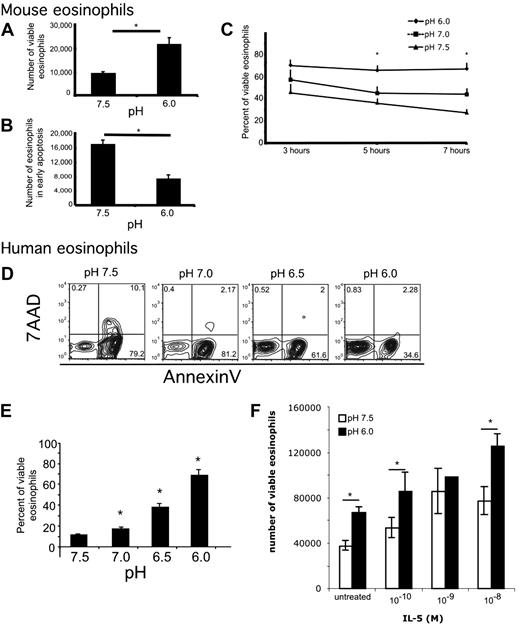
To examine the effects of acidity on eosinophils, we incubated murine eosinophils in media buffered to various pH. The viability dyes annexin V and 7AAD were used to assess eosinophil viability and apoptosis by measuring the percentage of cells in each quadrant and the number of cells at the end of the incubation. Viable cells were defined as annexin V–negative, 7AAD-negative. Early apoptotic cells were defined as annexin V–positive, 7AAD-negative. Mouse eosinophils incubated in acidic pH were found to have increased viability and decreased apoptosis (Figure 1A-B). Specifically, at pH 6, there were 2.0- plus or minus 0.4-fold more viable eosinophils and 1.75- plus or minus 0.5-fold fewer eosinophils in early apoptosis compared with eosinophils incubated at pH 7.5 (average ± SD; n = 13 experiments, paired t test, P = .008 and P = .009, respectively). Furthermore, increased viability and decreased early apoptosis with decreased pH occurred in a time- and dose-dependent manner (Figure 1C). When examined over a 7-hour time course, there were no significant differences in viability at 3 hours, whereas at 5 hours eosinophils were more viable at pH 6.0 compared with pH 7.5; at 7 hours, the pH-induced effect on eosinophil viability was significantly increased compared with 5 hours (Figure 1C). This pH-induced effect on eosinophil viability was also dose-dependent in human peripheral blood eosinophils incubated for 24 hours (Figure 1D-E). Mild acidity has an additive effect on IL-5–induced increased viability of human eosinophils (Figure 1F). In summary, both human and murine eosinophils respond to increased acidity (decreased pH) with increased viability and decreased apoptosis.
Eosinophils have decreased early apoptosis and increased viability in acidic pH. Murine (A-C) and human eosinophils (D-F) were incubated in media buffered to various pH. Viability analysis shows that incubation of murine eosinophils in acidic media for 4 hours affects number of viable eosinophils (A) and number of early apoptotic murine eosinophils (B). Viability analysis of murine eosinophils over a 7-hour time course shows that incubation of murine eosinophils in acidic media affects percentage of viable eosinophils (C). Representative flow cytometric analysis of human eosinophils incubated at various pH for 24 hours (D) with quantification showing average percentage of viable eosinophils with SD (E). Human eosinophils were incubated in media buffered to pH 7.5 and 6.0 in the presence of indicated doses of IL-5 for 18 hours (F). Results show the average ± SD of triplicate incubations. *P < .05. Representative experiment (of > 11 and 3) is shown for murine and human eosinophils, respectively.
Eosinophils have decreased early apoptosis and increased viability in acidic pH. Murine (A-C) and human eosinophils (D-F) were incubated in media buffered to various pH. Viability analysis shows that incubation of murine eosinophils in acidic media for 4 hours affects number of viable eosinophils (A) and number of early apoptotic murine eosinophils (B). Viability analysis of murine eosinophils over a 7-hour time course shows that incubation of murine eosinophils in acidic media affects percentage of viable eosinophils (C). Representative flow cytometric analysis of human eosinophils incubated at various pH for 24 hours (D) with quantification showing average percentage of viable eosinophils with SD (E). Human eosinophils were incubated in media buffered to pH 7.5 and 6.0 in the presence of indicated doses of IL-5 for 18 hours (F). Results show the average ± SD of triplicate incubations. *P < .05. Representative experiment (of > 11 and 3) is shown for murine and human eosinophils, respectively.
To show that the effects of acid on eosinophils are specific to viability, we assessed other pH-induced effects, including actin polymerization and chemotaxis toward the eosinophil chemoattractant eotaxin-2. There were no significant differences in actin polymerization in response to acidic exposure, eotaxin-2–induced actin polymerization in media of different pH, or chemoattraction toward eotaxin-2 in media of different pH (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article; and data not shown).
Acidic pH increases intracellular cAMP production, which is a critical signaling molecule for eosinophil viability
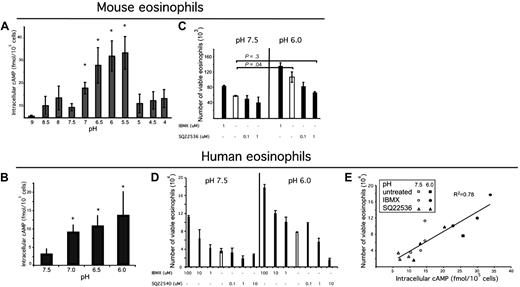
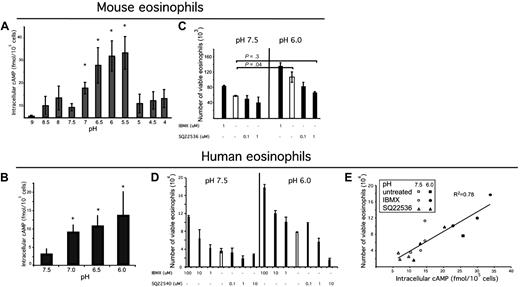
We hypothesized that the effects of exposure of eosinophils to acidic microenvironments would involve intracellular signaling events. Indeed, we found that purified murine eosinophils responded to increasing exposure to acid with a significant up-regulation of cAMP production in a pH-dependent manner to pH 5.5 (Figure 2A). Similar to murine eosinophils, human eosinophils responded to increased acidity with a dose-dependent increase in cAMP production (Figure 2B).
Acidic pH increases intracellular cAMP, which is a critical signaling molecule in eosinophil viability. Eosinophils were isolated from mouse spleen or human peripheral blood; eosinophils were incubated in media of over a pH range (A-B) for 30 minutes in the presence of IBMX, and accumulated intracellular cAMP was measured via an ELISA from eosinophil lysates. n = 3 mice per group. Results shown as the mean intracellular cAMP ± SD. Representative experiments (of 3) are shown. Murine eosinophils were incubated in IBMX (a phosphodiesterase inhibitor) or an adenylate cyclase inhibitor (C) for 4 hours. Human eosinophils were exposed to various concentrations of adenylate cyclase inhibitor SQ22536 and IBMX at pH 7.5 and pH 6.0 for 24 hours. Human eosinophil viability was assessed (D), and intracellular cAMP was measured from the lysates of a sample of each well (taken at 10-minute time point); (E) demonstrates human eosinophil viability as a function of intracellular cAMP. Viability analysis was performed using annexin V and 7AAD. Results show the average ± SD (of triplicate incubations). *P < .05.
Acidic pH increases intracellular cAMP, which is a critical signaling molecule in eosinophil viability. Eosinophils were isolated from mouse spleen or human peripheral blood; eosinophils were incubated in media of over a pH range (A-B) for 30 minutes in the presence of IBMX, and accumulated intracellular cAMP was measured via an ELISA from eosinophil lysates. n = 3 mice per group. Results shown as the mean intracellular cAMP ± SD. Representative experiments (of 3) are shown. Murine eosinophils were incubated in IBMX (a phosphodiesterase inhibitor) or an adenylate cyclase inhibitor (C) for 4 hours. Human eosinophils were exposed to various concentrations of adenylate cyclase inhibitor SQ22536 and IBMX at pH 7.5 and pH 6.0 for 24 hours. Human eosinophil viability was assessed (D), and intracellular cAMP was measured from the lysates of a sample of each well (taken at 10-minute time point); (E) demonstrates human eosinophil viability as a function of intracellular cAMP. Viability analysis was performed using annexin V and 7AAD. Results show the average ± SD (of triplicate incubations). *P < .05.
Previous studies have shown a role for cAMP in increasing human eosinophil viability.26-30 We hypothesized that murine eosinophils would also respond to agents that increased cAMP with increased viability. To test this hypothesis, we exposed spleen-derived eosinophils to IBMX, a phosphodiesterase inhibitor; dibutyryl cAMP, a cell-permeable cAMP analog; and forskolin, an activator of adenylate cyclase. Dibutyryl cAMP and forskolin increased eosinophil viability (data not shown; consistent with Machida et al31 ). A dose-dependent increase in viability with increased IBMX exposure was found at both pH 7.5 and 6.0; however, a corresponding dose-dependent decrease in viability with an increasing dose of adenylate cyclase inhibitor SQ2254032 was found only at pH 6.0 (Figure 2C). Treatment with adenylate cyclase inhibitor at pH 6.0 decreased the eosinophil viability to the level observed at pH 7.5. To further understand the mechanism of increased viability in acidic pH, human eosinophils were exposed to various doses of phosphodiesterase inhibitor, IBMX, and adenylate cyclase inhibitor, SQ22540,32 at pH 7.5 and 6.0. IBMX increased viability in a dose-dependent manner at both pH 7.5 and 6.0. The adenylate cyclase inhibitor decreased eosinophil viability in a dose-dependent manner at pH 6.0, but not pH 7.5 (Figure 2D), thus demonstrating that acid-induced increased viability is dependent on cAMP. When eosinophil viability was assessed as a function of intracellular cAMP, we found a strong association (R2 of 0.78, P < .001; Figure 2E). In summary, acidic pH leads to cAMP accumulation in eosinophils, and this is required for acidic pH-enhanced eosinophil viability.
GPR65 is a proton-sensing GPCR that has increased expression in models of asthmatic inflammation
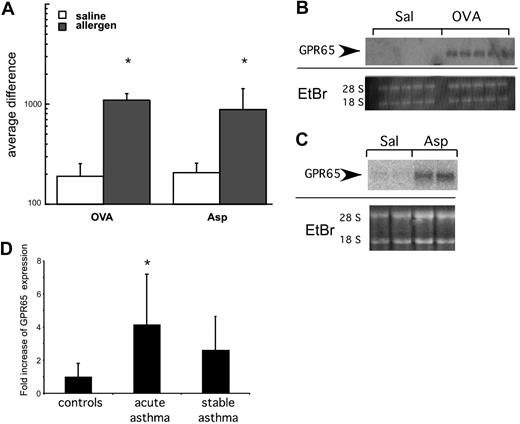
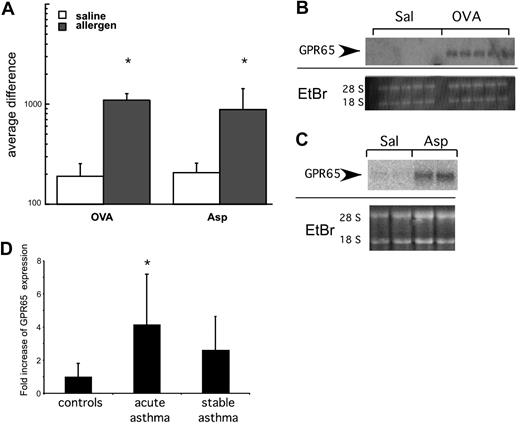
The mechanism of pH recognition by inflammatory cells remains largely unclear. We have previously defined a group of “asthma signature genes” based on transcript expression profile analysis of lung tissue derived from 2 independent murine models of allergen-induced experimental asthma that both have a major eosinophilic component.19,20 This analysis revealed that an acid-sensing receptor gpr65 is among the genes whose expression is up-regulated in the lung by the induction of experimental allergic asthma (Figure 3). This was specific for GPR65 as other related receptors (G2A and endothelial differentiation gene [EDG]-1, -3, -5, -6, and -8) did not exhibit increased expression in mouse models of asthma (data not shown). The expression results for GPR65 were confirmed by Northern blot analysis. In contrast to control saline-challenged mice that had no detectable levels of GPR65 mRNA, GPR65 mRNA expression was significantly increased by allergen challenge (Figure 3B-C). To assess relevance to human disease, we measured expression of GPR65 in nasal brushings of patients with stable and acute asthma and control patients.21 GPR65 mRNA expression was increased by 4.1- plus or minus 3-fold in patients with acute asthma compared with controls (P = .02). In samples from patients with stable asthma, there was a trend toward increased GPR65 mRNA expression (2.6- ± 2-fold, P = .08; Figure 3D). In summary, GPR65 expression is increased in multiple murine models of allergic asthma and in human populations during acute asthma exacerbations.
GPR65 has increased expression in allergic airway inflammation. (A-C) Lung RNA from allergen-treated mice was analyzed by microarray analysis (A) and Northern blot (B-C) for expression of GPR65. Ethidium bromide (E + Br) is shown as a loading control. (D) Quantitative RT-PCR analysis of GPR65 expression from pediatric patients. *P < .05 compared with saline in panel A and control subjects in panel D.
GPR65 has increased expression in allergic airway inflammation. (A-C) Lung RNA from allergen-treated mice was analyzed by microarray analysis (A) and Northern blot (B-C) for expression of GPR65. Ethidium bromide (E + Br) is shown as a loading control. (D) Quantitative RT-PCR analysis of GPR65 expression from pediatric patients. *P < .05 compared with saline in panel A and control subjects in panel D.
GPR65 is expressed by eosinophils
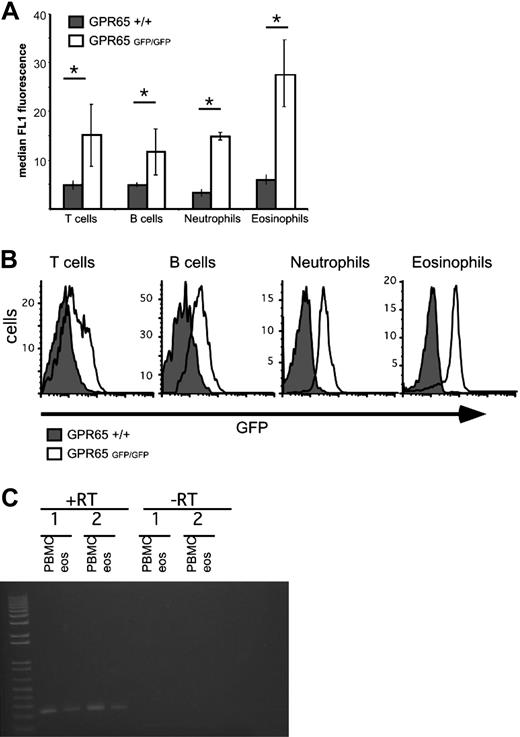
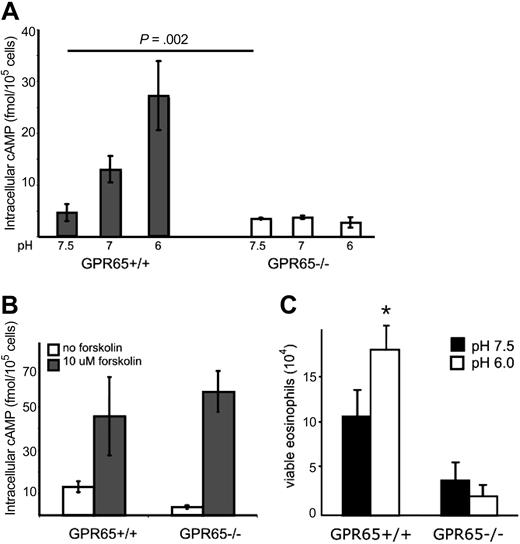
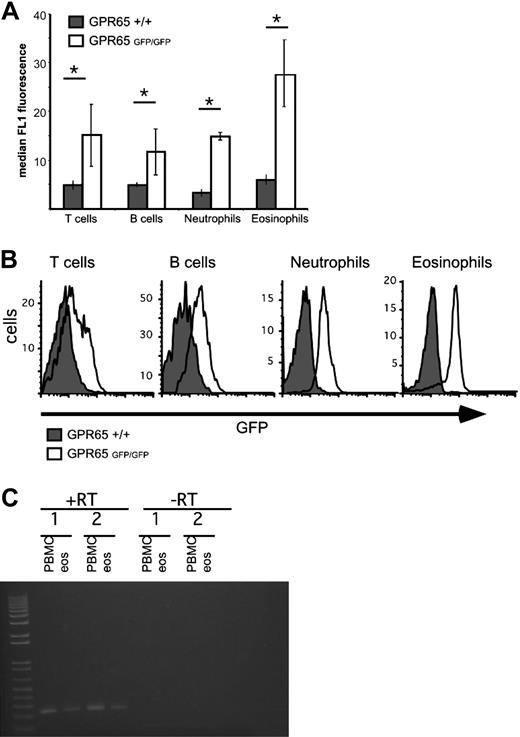
We hypothesized that GPR65 was expressed by eosinophils. To address this, we examined GPR65 gene-targeted mice that contain the GPR65 exon 2 replaced with a construct encoding promoterless IRES–enhanced green fluorescent protein (EGFP) sequences, thus allowing for GPR65 promoter activity to be assessed by EGFP expression.7 Notably, our data demonstrate strong GPR65 promoter activity in murine eosinophils (Figure 4). As a control, peripheral blood T cells, B cells, and neutrophils also expressed GPR65, as measured by the difference in median FL1 intensity between GPR65+/+ and GPR65−/− cells (Figure 4). In mouse spleen and blood cells, the EGFP expression was 2.4 plus or minus 0.8 times higher in eosinophils than in T cells (average of 5 experiments). In mouse bone marrow, we detected EGFP expression in CD3+, CD19+, Gr1hi, and Siglec-F+ cells, but not Ter119+ cells (supplemental Figure 2). Similarly, in human eosinophils (96% pure), RT-PCR analysis demonstrated expression of GPR65 mRNA (Figure 4C); this is consistent with microarray data in the public domain33 (Gene Expression Omnibus [GEO] accession number GDS1775). In summary, these data demonstrate substantial expression of GPR65 by eosinophils.
Eosinophils express significant GPR65 promoter activity. GPR65+/+ and GPR65−/− (GFP knockin) peripheral blood was assessed for median GFP expression in the FL1 channel using flow cytometry. Cell types were defined as follows: T cells (CD3+), B cells (CD19+), neutrophils (CCR3−Gr-1hi), and eosinophils (CCR3+Gr-1lo). Quantification of a representative experiment is shown in panel A (n = 3 mice per genotype). (B) Histograms demonstrating GFP expression in different cell populations are shown. Results show the median FL1 ± SD. *P < .05. Representative experiment (of 4) is shown. (C) PCR analysis of GPR65 expression in cDNA made from human eosinophils and PBMC from 2 individual donors. −RT indicates negative control without reverse transcriptase.
Eosinophils express significant GPR65 promoter activity. GPR65+/+ and GPR65−/− (GFP knockin) peripheral blood was assessed for median GFP expression in the FL1 channel using flow cytometry. Cell types were defined as follows: T cells (CD3+), B cells (CD19+), neutrophils (CCR3−Gr-1hi), and eosinophils (CCR3+Gr-1lo). Quantification of a representative experiment is shown in panel A (n = 3 mice per genotype). (B) Histograms demonstrating GFP expression in different cell populations are shown. Results show the median FL1 ± SD. *P < .05. Representative experiment (of 4) is shown. (C) PCR analysis of GPR65 expression in cDNA made from human eosinophils and PBMC from 2 individual donors. −RT indicates negative control without reverse transcriptase.
GPR65 is an eosinophil pH sensor
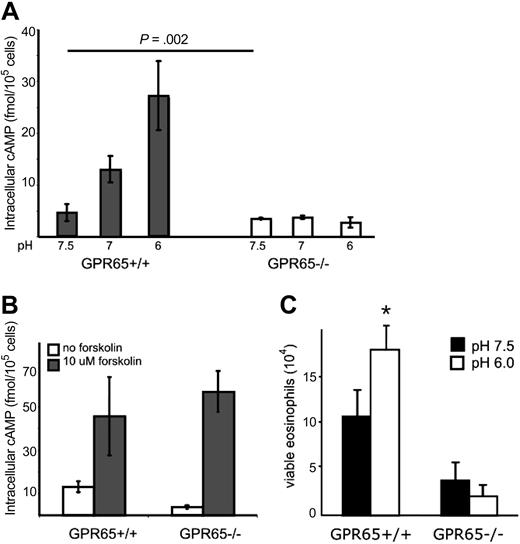
We tested the hypothesis that GPR65 was an eosinophil pH sensor. We first compared the response of GPR65+/+ and GPR65−/− murine eosinophils to increasing acid concentrations: importantly, the up-regulation of cAMP production in eosinophils in response to increased acid was ablated in GPR65−/− eosinophils (Figure 5A). GPR65+/+ and GPR65−/− eosinophils have equivalent viability at a 30-minute time point across the pH range (data not shown). As a control, GPR65+/+ and GPR65−/− eosinophils up-regulated cAMP production in response to forskolin, an activator of adenylate cyclase at comparable levels (Figure 5B), showing that GPR65−/− eosinophils do not have a global defect in cAMP production. Thus, GPR65 is a nonredundant acid-sensing receptor on eosinophils that controls cAMP production.
GPR65 regulates eosinophil cAMP production and viability. Eosinophils were isolated from the spleens of GPR65+/+ or GPR65−/− mice; eosinophils were incubated in media of various acidity (A) or forskolin at pH 7.0 (B) for 30 minutes in the presence of IBMX, and accumulated intracellular cAMP was measured via an ELISA from eosinophil lysates. n = 3 mice per group. Results shown as the mean intracellular cAMP ± SD. Representative experiments (of 3) are shown. Viability analysis of GPR65+/+ and GPR65−/− eosinophils after a 5-hour ex vivo incubation at pH 7.5 and 6.0 (C). Results are representative of 3 time-course and 8 5-hour incubation experiments. Results are shown as the mean ± SD of triplicate incubations.
GPR65 regulates eosinophil cAMP production and viability. Eosinophils were isolated from the spleens of GPR65+/+ or GPR65−/− mice; eosinophils were incubated in media of various acidity (A) or forskolin at pH 7.0 (B) for 30 minutes in the presence of IBMX, and accumulated intracellular cAMP was measured via an ELISA from eosinophil lysates. n = 3 mice per group. Results shown as the mean intracellular cAMP ± SD. Representative experiments (of 3) are shown. Viability analysis of GPR65+/+ and GPR65−/− eosinophils after a 5-hour ex vivo incubation at pH 7.5 and 6.0 (C). Results are representative of 3 time-course and 8 5-hour incubation experiments. Results are shown as the mean ± SD of triplicate incubations.
GPR65 regulates eosinophil survival in vitro
To test the role of GPR65 in acidic pH-induced eosinophil viability, we assessed the viability of eosinophils from GPR65 wild-type and GPR65-deficient mice at pH 7.5 and 6.0. Whereas wild-type eosinophils had 1.8- plus or minus 0.4-fold increased numbers of viable eosinophils at pH 6.0 compared with eosinophils at pH 7.5 (n = 8 experiments; average ± SD), GPR65−/− eosinophils had 35% plus or minus 30% decrease in the numbers of viable eosinophils incubated at pH 6.0 compared with pH 7.5 (n = 8 experiments; average ± SD; Figure 5C). Thus, the acid-induced increase in eosinophil viability was not observed in GPR65-deficient cells.
GPR65 nonredundantly regulates eosinophil survival in vivo
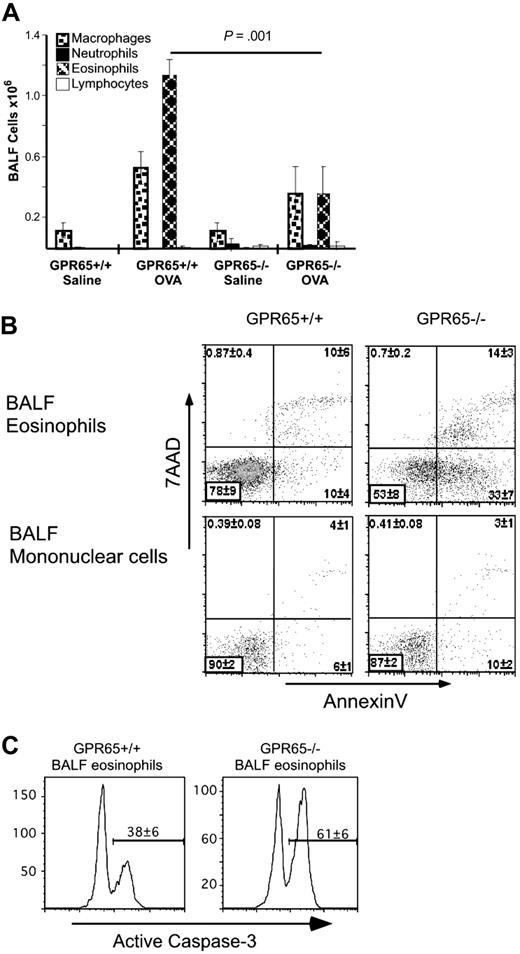
We hypothesized that GPR65 would affect eosinophil viability in murine models of asthma. Previously sensitized mice were euthanized 24 hours after the second allergen challenge, and differential cell counts were determined by staining cytocentrifuge preparations of the BALF (representative experiment in Figure 6A). GPR65-deficient mice had 76% plus or minus 12% attenuation of airway eosinophilia compared with normal controls (n = 8 experiments; P = .01-.001). Based on CCR3 and Siglec-F costaining, there was 82% plus or minus 8% attenuation of eosinophilia in GPR65-deficient mice compared with wild-type mice (data not shown; n = 3 experiments). In comparison, other cell types in the BALF of allergen-challenged mice were not reproducibly affected by GPR65 expression (Figure 6A). Furthermore, we assessed the airway eosinophilia after 9 intranasal challenges with the allergen A fumigatus and found a statistically significant decrease in airway eosinophilia in the absence of GPR65. Specifically, in the A fumigatus model, we found attenuated airway eosinophilia (representative experiment, 8.8 × 104 ± 1.8 × 104 and 3.0 × 104 ± 1.2 × 104 BAL eosinophils in GPR65+/+ and GPR65−/−, respectively; average of 3 experiments, 2.5 ± 0.5-fold decrease in BAL eosinophilia P < .05-.01). Taken together, these data demonstrate an in vivo effect of GPR65 in regulating allergen-induced eosinophilia.
GPR65 is a critical regulator of BAL eosinophilia and viability in murine asthma model. Sensitized GPR65+/+ and GPR65−/− were challenged with intranasal OVA or saline. The differential cell counts were performed by staining cytocentrifuge preparations of the BALF (A). n = 4-6 mice per group. Flow cytometry–based viability analysis of eosinophils and mononuclear cells in representative in vivo experiment using annexin V/7AAD (B) and anti-active caspase-3 (C). n = 4-6 mice per group. Results are shown as the mean ± SD. Representative experiments (of 3-5) are shown.
GPR65 is a critical regulator of BAL eosinophilia and viability in murine asthma model. Sensitized GPR65+/+ and GPR65−/− were challenged with intranasal OVA or saline. The differential cell counts were performed by staining cytocentrifuge preparations of the BALF (A). n = 4-6 mice per group. Flow cytometry–based viability analysis of eosinophils and mononuclear cells in representative in vivo experiment using annexin V/7AAD (B) and anti-active caspase-3 (C). n = 4-6 mice per group. Results are shown as the mean ± SD. Representative experiments (of 3-5) are shown.
As a control, GPR65-deficient mice have normal levels of CFU-eosinophil (GPR65+/+, 7 ± 5 and GPR65−/−, 5 ± 4 CFU-eosinophil colonies/105 low-density bone marrow cells). We found comparable levels of peripheral blood eosinophils in GPR65 wild-type and deficient animals (GPR65+/+, 2.1 ± 0.4 × 105 eosinophils/mL, and GPR65−/−, 2.2 ± 0.1 × 105 eosinophils/mL). Furthermore, GPR65−/− and GPR65+/+ IL-5 Tg mice had comparable levels of peripheral blood eosinophilia (GPR65+/+, 42 ± 6 × 105 eosinophils/mL, and GPR65−/−, 48 ± 1 × 105 eosinophils/mL). Together, these data suggest that eosinophils develop and egress into the circulation normally in GPR65−/− mice.
The attenuated BALF eosinophilia in the allergen-challenged GPR65−/− mice could have been due to insufficient recruitment in the airway. In vitro analysis demonstrated normal chemotactic responses of GPR65−/− eosinophils (supplemental Figure 1). Furthermore, levels of Th2 cytokines (IL-5 and IL-13) in lung tissue, and the eosinophil chemokine eotaxin-2 in BALF were not altered in allergen-challenged GPR65-deficient mice (supplemental Figure 3). In addition, GPR65−/− mice were appropriately and systemically sensitized to the allergen, as determined by the levels of OVA-specific immunoglobulin G1 in plasma (data not shown). The lack of defects in sensitization or cytokine production suggests that the concerted action of other cell types, for example, lymphocytes, was normal and that the defect was intrinsic to eosinophils.
Based on decreased numbers of airway eosinophils with normal levels of eosinophilic chemotactic agents, we hypothesized that GPR65 functions to maintain eosinophil viability. Eosinophils derived from the BALF of GPR65-deficient mice were significantly less viable, as assessed by 7AAD and annexin V staining (GPR65+/+, 78% ± 9% viable; GPR65−/−, 53% ± 8% viable; P < .001; Figure 6B). Specifically, the majority of GPR65+/+ eosinophils were annexin V−/7AAD−, whereas 3 plus or minus 0.1 times more GPR65−/− than GPR65+/+ eosinophils were annexin V+/7AAD− (average of 3 experiments). As a control, mononuclear cells in the BALF of GPR65+/+ and GPR65−/− did not differ in viability, further suggesting that in allergic inflammation GPR65-deficient mice have a specific defect in eosinophils (90% ± 2% and 87% ± 2% mononuclear cell viability in GPR65+/+ and GPR65−/−, respectively). We also found a defect in BAL eosinophil viability following the Aspergillus model in GPR65−/− compared with GPR65+/+ mice (representative experiment: 67% ± 8% and 55% ± 9% of BALF eosinophils that were viable in GPR65+/+ and GPR65−/− mice, respectively; average ± SD of 3 experiments, 12% ± 4% decrease in viability in GPR65−/− compared with GPR65+/+ BAL eosinophils). To identify changes in caspase-3 activity in the BALF eosinophils, we used flow cytometry to measure the percentage of eosinophils with caspase-3 activation in the BALF of OVA-challenged GPR65+/+ and GPR65−/− mice. There was a significant increase in active caspase-3 in the GPR65−/− BALF eosinophils compared with the GPR65+/+ BALF eosinophils: 38% plus or minus 6% and 61% plus or minus 6% of BAL eosinophils had activated caspase-3 in GPR65+/+ and GPR65−/−, respectively (Figure 6C). In summary, GPR65 regulates viability of allergen-elicited BALF eosinophils, most likely through a caspase-dependent mechanism.
Discussion
Acidity of the airways in asthma has been demonstrated in multiple studies and is in trials as a noninvasive biomarker with clinical utility to diagnose and/or guide treatment of asthma (reviewed in Hunt34 ). However, the effect of airway acidity on inflammatory cells that accumulate in asthma is an understudied area of research. Prior studies have mainly concentrated on neutrophils, demonstrating that their functional responses (eg, chemokinesis and survival) are enhanced in acidic pH.35-37 Similarly, lymphocyte motility is activated by acidic pH.38 Dendritic cell activation as well as uptake of antigen are also increased with mild acidosis.39 In macrophages, a recently published study highlights the role of GPR65, called T-cell death–associated gene 8 in their study, in mediating acid-induced inhibition of cytokine production, for example, tumor necrosis factor-α, in response to endotoxin.40 In this study, we establish that acidic pH enhances the viability of eosinophils in a manner dependent upon adenylate cyclase activity and cAMP production.
Recently, a novel hypothesis has been proposed that protons may act as intercellular signal transmitters. For instance, a recent publication demonstrated that protons are released from cells in a regulated way and that adjacent cells have proton receptors; together, this leads to a physiologic response, namely muscle contraction in Caenorhabditis elegans.41 Extracellular acidosis may affect cells by multiple mechanisms, including the following: (1) affecting cell surface receptors (such as the acid-sensing family of GPCRs), leading to intracellular signal transduction; (2) activating acid-sensing ion channels (such as the neuronal subgroup of epithelial sodium [Na] channels), leading to acid-evoked currents42 ; or (3) indirectly by affecting the action of other ligands (eg, chemokines,43 growth factors whose activity may be pH-dependent) or by affecting intracellular pH. For instance, in osteoclasts, ovarian cancer GPCR1 (OGR1) is a crucial proton sensor that regulates survival.44 In this study, we provide evidence that the effect of extracellular acidosis on eosinophils involves GPR65, a novel cell surface proton-sensing GPCR, and the intracellular second messenger cAMP, at least in part. We demonstrate the importance of this pathway in vivo on eosinophil viability and accumulation in models of allergic airway inflammation.
cAMP accumulation has been shown to induce eosinophil survival. Treatment of eosinophils with the adenylate cyclase activator forskolin, β2 adrenergic agonists, or other stimulators of Gαs-coupled GPCRs, or phosphodiesterase inhibitors, leads to increased cAMP and enhanced eosinophil survival.26-31 As a side note, whereas the commonly used phosphodiesterase inhibitor, theophylline, leads to eosinophil cell death, this effect is independent of cAMP.29 However, cAMP has been associated with inducing cell death in other cell types,45,46 thus demonstrating eosinophil specificity of this effect. Furthermore, the effect of pH-induced regulation of cAMP on eosinophil viability was previously unknown.
We further demonstrate the importance of a novel acid-sensing receptor, GPR65, in regulating eosinophil viability in response to acidic pH in vitro and allergen challenge in vivo. We first identified GPR65 as an allergen-induced gene19,20 (Figure 3). GPR65 was previously shown to control acidic pH-induced cAMP accumulation in lymphocytes.7 Second, we show that GPR65 is expressed on eosinophils at a higher level than other hematopoietic cells. This is consistent with microarray data in the public domain showing the highest level of GPR65 on human eosinophils, followed by neutrophils and basophils, and lymphocytes33 (GEO accession number GDS1775). Unfortunately, we were unable to obtain GPR65-specific staining with commercially available antibodies (clones sc-9702 and sc9705 from Santa Cruz Biotechnology). Furthermore, we show that GPR65 regulates acidic pH-induced cAMP accumulation in eosinophils, as well as acid pH-enhanced eosinophil viability. Consistent with the in vitro observations, our in vivo studies demonstrate that GPR65 critically regulates airway eosinophilia in a murine model of allergic airway inflammation. We provide evidence that the mechanism of GPR65's regulation is mediated by regulation of eosinophil viability. Eosinophil apoptosis has been hypothesized to regulate the maintenance and clearance of eosinophils during allergic airway inflammation. Factors that affect the balance of antiapoptotic and proapoptotic proteins in eosinophils in the microenvironment of allergic airway inflammation are not well understood. The clearance of dead eosinophils from the airways of allergen-challenged mice is rapid and most likely meditated by macrophage and epithelial cell phagocytosis. The difference in viability seen in our study may explain the attenuation of eosinophilia, as once eosinophils begin the death pathway, surrounding cells quickly phagocytose them. This is supported by our finding that GPR65−/− mice have a 3-fold increase in early apoptotic cells (annexin V+/7AAD−), but no change in late apoptotic/necrotic cells (annexin V+/7AAD+; see Figure 6B), suggesting that late apoptotic cells are phagocytosed and removed. There have been descriptions of apoptotic eosinophils in the airways going through secondary necrosis.47 Although we demonstrate that GPR65-deficient eosinophils have decreased survival in the BALF of allergen-challenged mice, future studies will directly address whether phagocytosis or secondary necrosis is responsible for the observed decreased BALF eosinophilia. It also remains possible that GPR65-deficient eosinophils undergo activation-induced death in the lung parenchyma and can thus not be transported into the alveolar space, which has been suggested as a clearance mechanism for apoptotic eosinophils.47 Finally, our in vivo studies in allergen-challenged mice do not directly test the role of acidic pH in eosinophil viability and accumulation. Our preliminary analysis demonstrates that the airway pH is indeed acidic in the OVA model of allergic airway inflammation.48 Future studies will focus on the in vivo effect of acidic pH on eosinophil viability, effect of acidic pH on other eosinophil functions (respiratory burst, degranulation), as well as any consequences on asthma outcomes.
Previous studies have shown expression of GPR65 in multiple hematopoietic cells. Similarly, we compare the expression of GPR65 (inferred from GFP expression in GPR65−/− mice) in eosinophils, neutrophils, and T and B lymphocytes. Whereas the level was highest in eosinophils, other cells also express GPR65. However, our in vivo experiments demonstrate eosinophil-selective effects in that eosinophils are the only cell type consistently decreased in the BALF of allergen-challenged GPR65−/− mice. There are several potential explanations for this finding. First, as we demonstrate that the GPR65 level is higher in eosinophils compared with other hematopoietic cells, eosinophils may be more sensitive to GPR65 than other cell types. Similarly, GPR65 function may be nonredundant in eosinophils, whereas other related receptors may compensate in other cell types. Alternatively, cAMP may have different functions in different cell types that may stem from cross-talk with other pathways or differential levels of cAMP at baseline in individual cell types.45,46 For instance, the level of cAMP in regulatory T cells is greater than 10-fold higher than in CD4 effector T cells.45 Future studies will specifically address the cell selectivity of the GPR65 effect. However, our study clearly demonstrates that, despite its original name (T-cell death-associated gene 8) and early studies in T cells,49,50 GPR65 is expressed in eosinophils and it functions in a survival-promoting fashion.
In summary, our results have established the following: (1) acidic pH regulates eosinophil survival; (2) intracellular cAMP is a crucial regulator of acid-enhanced eosinophil survival; (3) GPR65 is expressed and functions as an acid-sensing receptor on eosinophils; and (4) GPR65 regulates allergen-induced airway eosinophilia.
Some of the data in this study were presented in abstract form at the 2007 annual meeting of the American Academy of Asthma, Allergy, and Immunology, San Diego, CA, February 26, 2007, the 2009 annual meeting of the American Academy of Asthma, Allergy, and Immunology, Washington, DC, March 17, 2009, and the 2007 International Eosinophil meeting, Snowbird, UT, July 20, 2007
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs David Hildeman, Kimberly Risma, and Umasundari Sivaprasad for advice and critical input regarding this study; Drs Christopher Karp, Fred Finkelman, and Simon Hogan for critical review of this manuscript; and Mark Ericksen and Drs Yoshiyuki Yamada and Jesus Guajardo for technical help.
This work was supported in part by a Cincinnati Children's Hospital Medical Center Trustee grant to N.Z. and National Institutes of Health grant R21A1079251 to N.Z. We acknowledge an American Academy of Allergy, Asthma, and Immunology Strategic Training in Allergy Research (ST*AR) award (to L.C.K.). L.C.K. is a Philanthropic Educational Organization (PEO) scholar. O.N.W. is an Investigator of the Howard Hughes Medical Institute.
National Institutes of Health
Authorship
Contribution: L.C.K., A.R.C., K.H.C., K.A.N., M.H., and G.K.H. performed research; L.C.K., A.R.C., G.K.H., M.E.R., and N.Z. analyzed and interpreted data; C.G.R. and O.N.W. contributed vital new reagents (GPR65-deficient mice); and L.C.K., M.E.R., and N.Z. wrote the manuscript.
Conflict-of-interest disclosure: M.E.R. and N.Z. received research funding from Merck related to GPR65. The remaining authors declare no competing financial interests.
Correspondence: Nives Zimmermann, Division of Allergy and Immunology, Cincinnati Children's Hospital, 3333 Burnet Ave, ML 7028, Cincinnati, OH 45229-3039; e-mail: nives.zimmermann@cchmc.org.