Abstract
ADAMTS13 metalloprotease regulates the multimeric size of von Willebrand factor (VWF) by cleaving the Tyr1605-Met1606 bond in the VWF A2 domain. The mechanisms of VWF recognition by ADAMTS13 have yet to be fully resolved. Most studies have focused on the role of exosites within the VWF A2 domain, involved in interaction with the ADAMTS13 spacer domain. In the present study, we expressed different C-terminal domain VWF fragments and evaluated their binding to ADAMTS13 and its truncated mutants, MDTCS and del(TSP5-CUB). Using plate binding assay and surface plasmon resonance, we identified a novel ADAMTS13 binding site (KD ∼ 86 nM) in the region of VWF spanning residues 1874 to 2813, which includes the VWF D4 domain and that interacts with the C-terminal domains of ADAMTS13. We show that the interaction occurs even when VWF is in static conditions, assumed to be globular and where the VWF A2 domain is hidden. We demonstrate that C-terminal VWF fragments, as well as an antibody specifically directed toward the VWF D4 domain, inhibit VWF proteolysis by ADAMTS13 under shear conditions. We propose that this novel VWF C-terminal binding site may participate as the initial step of a multistep interaction ultimately leading to proteolysis of VWF by ADAMTS13.
Introduction
Von Willebrand factor (VWF) is a large multimeric glycoprotein that promotes the tethering of platelets at sites of vascular injury.1-3 VWF is synthesized by endothelial cells and megakaryocytes and secreted into plasma as ultralarge multimers.4,5 It is known that the largest VWF multimers have enhanced thrombogenic potential, due to the presence of multiple sites of interaction for platelets and vessel wall components.6
The size of plasma VWF multimers is regulated by the metalloprotease ADAMTS13, which cleaves VWF at a single peptide bond, Tyr1605-Met1606, in the VWF A2 domain.6,7 Proteolysis can occur only once VWF has been unraveled from its globular conformation, either by high fluid shear stress in vivo or in the presence of denaturants in vitro, conditions that are able to promote the exposure of the VWF scissile bond.8 The importance of VWF cleavage by ADAMTS13 is highlighted by genetic or acquired deficiency of the protease, which can lead to microangiopathy and thrombotic thrombocytopenic purpura, characterized by the formation of platelet-rich thrombi in the microcirculation.9-11
The precise mechanisms of ADAMTS13 recognition of VWF have yet to be fully defined. However, accumulating results suggest that different domains of the 2 proteins participate in complex interactions. Several studies have characterized a high-affinity binding site in the VWF A2 domain.12-15 A series of ADAMTS13 truncated mutants have been evaluated for binding to full-length VWF or to a short fragment derived from VWF A2 domain (VWF73).16,17 These studies indicated a crucial role for the ADAMTS13 spacer domain in substrate recognition. This is consistent with the observation that the spacer domain is functionally essential for VWF cleavage under static conditions.18,19 It has been also demonstrated that the ADAMTS13 spacer domain binds to an exosite located in the C-terminal region of VWF A2 domain.14 However, ADAMTS13 deletion mutants lacking the spacer domain are still able to cleave VWF, albeit at a appreciably slower rate.17 A recent study has identified an important role for the disintegrin-like domain in ADAMTS13 function.20
VWF A2 domain becomes available for binding interaction only once the VWF has unfolded from its globular conformation. A recognition mechanism that enables proximity of the globular form of VWF and ADAMTS13 may be necessary to position the 2 molecules favorably for flow-induced unfolding and interaction of the VWFA2 domain. Certain studies have suggested a possible role for the distal C-terminal domains of ADAMTS13 in VWF recognition and proteolysis. Truncation mutants of ADAMTS13 after the spacer domain retained most of their activity in cleaving VWF in static conditions.18,19,21 In contrast, ADAMTS13 murine strains, lacking the C-terminal TSPs and the CUB domains, showed appreciable reductions in VWF proteolysis in vitro.22 Furthermore, a peptide from the first CUB domain has been found to inhibit VWF proteolysis under flow, but not under static, conditions.23 The importance of ADAMTS13 C-terminal domains under flow has also been highlighted when the ADAMTS13 truncation mutant MDTCS was shown to have a reduced activity against VWF under the shear stress generated by a mini-vortex,24 but the same activity as wild-type ADAMTS13 in a static assay.18,24
In the present study, we have re-examined ADAMTS13 binding to VWF using domain fragments of both molecules. We demonstrate that the ADAMTS13 C-terminal distal domains (TSP5-CUB) bind to a novel binding site in the C-terminal region of VWF (spanning residues 1874-2813 and including the VWF D4 domain), which, critically, is constitutively exposed on the surface of VWF in solution without flow.
Methods
Expression and purification of recombinant full-length ADAMTS13 and truncated variants
Recombinant wild-type ADAMTS13 with a C-terminal myc/His tag was expressed and purified.25 For cleavage experiments, wild-type ADAMTS13 in conditioned media was concentrated using Labscale TFF System (Millipore) and dialyzed in 20 mM Tris-HCl (pH 7.8). The concentrations of wild-type ADAMTS13 were determined using enzyme-linked immunosorbent assay.26
The ADAMTS13 variants truncated after the spacer domain, MDTCS (amino acids 34-685), and after the fourth TSP-1 repeat, del(TSP5-CUB) (amino acids 34-894), were generated by polymerase chain reaction (PCR) using wild-type ADAMTS13 pcDNA 3.1/myc-His vector as a template and then cloned into the EcoRI and XbaI sites of pcDNA 3.1/myc-His vector. MDTCS and del(TSP5-CUB) constructs were transiently transfected in HEK293T cells using linear polyethylenimine (Polysciences Inc).27 MDTCS and del(TSP5-CUB) were purified by Ni2+-Hi-Trap–chelating columns (GE Healthcare), similarly to wild-type ADAMTS13.25
The purity of each recombinant protein was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a NuPAGE 4% to 12% Bis-Tris gel (Invitrogen), stained by Coomassie blue Safe Stain (Invitrogen).
Expression and purification of VWF fragments
VWFD′D3 (amino acids 764-1247), VWFA1A2A3 (amino acids 1260-1874), and VWFA1CK (amino acids 1260-2813) were expressed in BHK cells overexpressing furin and purified as previously described.28 VWFD4 (amino acids 1947-2299) construct was kindly donated by Dr Tom Vink (University Medical Center Utrecht).29 It was also expressed in BHK cells and purified by anion exchange chromatography.
VWFA2 (amino acids 1472-1668) pcDNA 3.1/myc-His vector was expressed in HEK293T cells and purified as previously described.30 VWFA3CK (amino acids 1671-2813), VWFD4CK (amino acids 1874-2813), and VWFB1CK (amino acids 2296-2813) pcDNA 3.1/His vectors were generated by inverse polymerase chain reaction, using wild-type VWF pcDNA3.1(A+)/His30 as a template. VWFA2, VWFD4CK, VWFA3CK, and VWFB1CK were transiently expressed in HEK293T cells, purified by Ni2+-Hi-Trap–chelating columns, as for wild-type ADAMTS13.
Purity of proteins was assessed by SDS-PAGE. Quantitation was performed using a BCA protein assay kit (Perbio).
Purification of plasma-derived VWF
Plate binding assays
VWF fragments, 30 nM of each, in 50 mM sodium carbonate buffer (pH 9.6) were immobilized onto the wells of a 96-well microtiter plate (Nunc). The plate was washed with phosphate-buffered saline (PBS)–0.1% Tween and the wells were blocked with 200 μL PBS–2.5% bovine serum albumin (BSA) for 1 hour. After repeated washing, increasing concentrations of purified wild-type ADAMTS13 (0-400 nM) in PBS–1% BSA, in the presence of 10 mM EDTA, were applied for 2 hours at 37°C. Bound ADAMTS13 was detected by first adding 0.1 μg/mL of biotinylated anti–TSP2-4 polyclonal antibody26 in PBS–1% BSA for 1 hour. Streptavidin conjugated with horseradish peroxidase (HRP) (GE Healthcare) in PBS–1% BSA, 100 μL, was applied, before adding Sigma color fast OPD substrate. Alternatively, a mouse HRP-conjugated anti-myc antibody (Invitrogen) was used for the detection of bound ADAMTS13, MDTCS, and del(TSP5-CUB). Binding curves were fitted to the one-binding site model using GraphPad Prism 4 software, to determine KD(app).
A modified version of the assay was used to assess the binding of immobilized ADAMTS13, MDTCS, and del(TSP5-CUB) to soluble full-length VWF. ADAMTS13, MDTCS, and del(TSP5-CUB), all at 30 nM, were immobilized on a plate. After blocking with PBS–2.5% BSA, soluble VWF (0-400 nM) in PBS–1% BSA, 10 mM EDTA was added for 2 hours. Bound VWF was detected using an HRP-conjugated anti-VWF IgG (Dako). VWF concentration has been expressed in molarity of VWF monomeric subunits (250 kDa).
Competition binding assays
Either 30 or 50 nM VWFA2, VWFA1A2A3, VWFA1CK, or VWFD4CK was coated onto a plate. Different concentrations (range, 0-600 nM) of the competitor (VWFA2, VWFA1A2A3, or full-length VWF) were preincubated with 12 to 50 nM ADAMTS13 in PBS–1% BSA, 10 mM EDTA, at 37°C for 40 to 60 minutes and subsequently added to the wells, previously blocked with PBS–2.5% BSA. A monoclonal antibody directed against the VWF D4 domain (mAb RU8)31,32 or a polyclonal antibody against the VWF CK domain (pAb C-20) (Santa Cruz) was also investigated as competitive inhibitors for the binding between immobilized VWFA1CK and soluble ADAMTS13. The specificity of the mAb RU8 for the VWF D4 domain was confirmed by its ability to recognize plate-immobilized VWFD4, but not VWFB1CK and VWFA1A2A3.
In all the competition binding experiments, the bound ADAMTS13 was detected using the biotinylated anti–TSP2-4 polyclonal antibody. The curves were fitted to the one-site competition model using GraphPad Prism 4 software and IC50 values obtained. The Ki constants were calculated from the IC50 values, using the equation: Ki = IC50/(1 + [immobilized VWF substrate/KD(app)]).
Binding studied by surface plasmon resonance
The binding between ADAMTS13 and VWF fragments (VWFD4CK, VWFA3CK, VWFD4, VWFB1CK, and VWFA1A2A3) under laminar flow was analyzed by surface plasmon resonance (SPR) using a BIAcore T100 system (BIAcore). The shear rates inside the injection tube and in the microfluidic cells were in the range of 1500 to 2500 s−1.24 The surface of a carboxymethylated dextran (CM5) sensor chip (BIAcore) was activated with 0.4 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 0.1 M N-hydroxysuccinimide. ADAMTS13 was immobilized by amine coupling to one flow cell. To control for nonspecific binding, BSA was immobilized onto the other flow cell. All free reactive surface groups were blocked using 1 M ethanolamine. Different concentrations (0-3.5 μM) of VWF fragments in HBS-P buffer (BIAcore) were injected over both flow cells at 30 μL/min (contact time, 2 minutes). After each injection, any bound protein was stripped with 10 mM NaOH (5 seconds). Data analysis was performed using the BIAevaluation software 3.0 (BIAcore), which allowed determination of ka, kd, and KD values by fitting the derived sensograms to 1:1 Langmuir model. A separate determination of the equilibrium constant KD was performed to validate curve fitting by plotting the maximal responses (RUmax) against substrate concentration and fitting to one-binding site model.
Cleavage of VWFA1CK and VWFA1A2A3 under static conditions
Recombinant ADAMTS13, 10 nM, was preincubated at 37°C for 1 hour in 20 mM Tris-HCl (pH 7.8) and 5 mM CaCl2. Simultaneously, 500 nM VWFA1A2A3 and VWFA1CK were preincubated at 37°C for 1 hour in 20 mM Tris-HCl (pH 7.8), in the absence or presence of 1.5 M urea. Reactions were started by adding the substrate, and subsamples were taken (0, 0.5, 1, 2, and 3 hours), stopped with EDTA, and analyzed by SDS-PAGE and Western blotting using a mouse monoclonal anti-PolyHistidine antibody (Sigma). HRP-conjugated anti–mouse IgG (Dako) was used as a secondary antibody and detected using the chemiluminescent HRP substrate (Millipore).
Inhibition of VWF proteolysis under static and flow conditions
VWFA3CK (0-500 nM) was preincubated with 20 nM ADAMTS13 in 20 mM Tris-HCl (pH 7.8) and 5 mM CaCl2 at 37°C for 45 minutes. VWF (5 μg/mL) was preincubated with 1.5 M urea at 37°C for 45 minutes. The reactions were started by adding VWF to each reaction mix, incubated at 37°C for 40 minutes, and then stopped by EDTA.
For flowing conditions, a mini-vortex Maxi Mix II (Sigma) was used.24 VWFA3CK (0-500 nM) was preincubated with 40 nM ADAMTS13 in 20 mM Tris-HCl (pH 7.8) and 5 mM CaCl2 at 37°C for 1 hour. VWF (5 μg/mL) was added and vortexed for 5 minutes at a rotation rate of 2500 rpm and the reaction was stopped by EDTA. The inhibition of VWF proteolysis under shear by RU8 antibody was similarly assessed.
The cleavage reactions were analyzed by VWF multimer gel analysis on a 1.5% agarose gel, followed by Western blotting using HRP-conjugated anti-VWF IgG (Dako). Alternatively, a rabbit polyclonal antibody directed against VWF D′D328 followed by HRP-conjugated anti–rabbit IgG (Dako) were used for detection. The cleavage reactions were also analyzed by SDS-PAGE on a NuPAGE 3% to 8% Tris-Acetate gel (Invitrogen). The homodimeric 176- to 176-kDa cleavage product was detected by Western blotting using HRP-conjugated anti-VWF IgG (Dako). Alternatively, the rabbit polyclonal antibody directed against VWF D′D3 was used for detection of the homodimeric 140- to 140-kDa cleavage product. The intensity of the bands was estimated using ImageJ software (National Institutes of Health, http://rsbweb.nih.gov/ij).
Results
Recombinant VWF and ADAMTS13 fragments
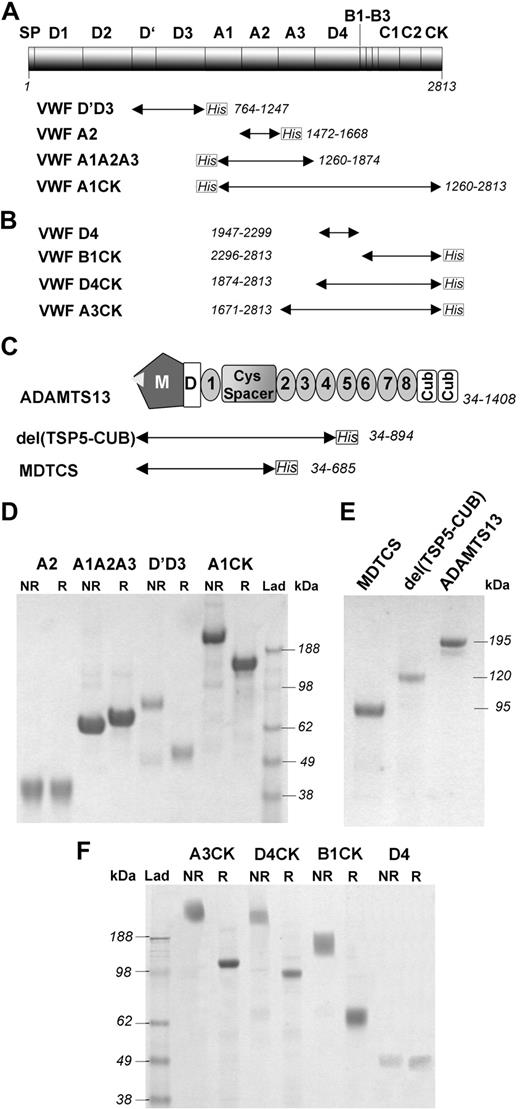
The purified VWF proteins first prepared (Figure 1A) were analyzed for purity using SDS-PAGE (Figure 1D). VWFA2 and VWFA1A2A3 were visualized as single bands of approximately 32 and 64 kDa, respectively. VWFD′D3 was expressed as a dimer, which was reduced to its predicted approximately 55-kDa monomeric size under reducing conditions. VWFA1CK was also expressed as a dimer (∼ 348 kDa) due to disulphide bond formation in the CK dimerization domain.
Recombinant VWF and ADAMTS13 fragments. (A) A first series of recombinant VWF fragments (VWFD′D3, VWFA2, VWFA1A2A3, and VWFA1CK) were expressed in mammalian cells and purified as described. They all had a polyhistidine epitope tag (C-terminal for VWFD'D3 and VWFA2; N-terminal for VWFA1A2A3 and VWFA1CK) to facilitate purification. The fragments are represented as labeled arrows underneath the diagram of VWF domains. The VWF numbering for each fragment is given. (B) An additional series of recombinant VWF C-terminal fragments (VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK) were expressed in mammalian cells and purified as described. They all had a C-terminal polyHis epitope tag, with the exception of VWFD4 in which no tag was present. (C) Full-length ADAMTS13 consists of a metalloprotease domain (M), a disintegrin-like domain (D), a thrombospondin type 1 repeat, (1) a Cys-rich and spacer domain (Cys Spacer), 7 additional thrombospondin type 1 repeats (2-8), and 2 complement Clr/Cls sea urchin epidermal growth and bone morphogenic protein 1 or CUB domains (Cub). ADAMTS13 variants truncated after the spacer domain, MDTCS, and after the fourth thrombospondin repeat, del(TSP5-CUB), were also generated and are represented as labeled arrows underneath the diagram of ADAMTS13 domains. (D) The VWF fragments depicted in panel A were purified and analyzed by SDS-PAGE followed by Coomassie staining, under both nonreducing (NR) and reducing (R) conditions. VWFD′D3 and VWFA1CK were expressed as dimers, which were reduced to their expected monomeric size under reducing conditions. (E) The wild-type ADAMTS13 and its truncated variants were analyzed by SDS-PAGE followed by Coomassie staining, under reducing conditions. ADAMTS13, del(TSP5-CUB), and MDTCS proteins were visualized on the gel as single bands of approximately 195, 120, and 95 kDa, respectively. (F) The VWF fragments depicted in panel B were purified and analyzed by SDS-PAGE followed by Coomassie staining, under both nonreducing (NR) and reducing (R) conditions. VWFD4 was visualized as a band of the expected molecular weight of approximately 40 kDa. VWFA3CK, VWFD4CK, and VWFB1CK were expressed as dimers (∼ 254, 200, and 132 kDa, respectively) since they all included the CK region.
Recombinant VWF and ADAMTS13 fragments. (A) A first series of recombinant VWF fragments (VWFD′D3, VWFA2, VWFA1A2A3, and VWFA1CK) were expressed in mammalian cells and purified as described. They all had a polyhistidine epitope tag (C-terminal for VWFD'D3 and VWFA2; N-terminal for VWFA1A2A3 and VWFA1CK) to facilitate purification. The fragments are represented as labeled arrows underneath the diagram of VWF domains. The VWF numbering for each fragment is given. (B) An additional series of recombinant VWF C-terminal fragments (VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK) were expressed in mammalian cells and purified as described. They all had a C-terminal polyHis epitope tag, with the exception of VWFD4 in which no tag was present. (C) Full-length ADAMTS13 consists of a metalloprotease domain (M), a disintegrin-like domain (D), a thrombospondin type 1 repeat, (1) a Cys-rich and spacer domain (Cys Spacer), 7 additional thrombospondin type 1 repeats (2-8), and 2 complement Clr/Cls sea urchin epidermal growth and bone morphogenic protein 1 or CUB domains (Cub). ADAMTS13 variants truncated after the spacer domain, MDTCS, and after the fourth thrombospondin repeat, del(TSP5-CUB), were also generated and are represented as labeled arrows underneath the diagram of ADAMTS13 domains. (D) The VWF fragments depicted in panel A were purified and analyzed by SDS-PAGE followed by Coomassie staining, under both nonreducing (NR) and reducing (R) conditions. VWFD′D3 and VWFA1CK were expressed as dimers, which were reduced to their expected monomeric size under reducing conditions. (E) The wild-type ADAMTS13 and its truncated variants were analyzed by SDS-PAGE followed by Coomassie staining, under reducing conditions. ADAMTS13, del(TSP5-CUB), and MDTCS proteins were visualized on the gel as single bands of approximately 195, 120, and 95 kDa, respectively. (F) The VWF fragments depicted in panel B were purified and analyzed by SDS-PAGE followed by Coomassie staining, under both nonreducing (NR) and reducing (R) conditions. VWFD4 was visualized as a band of the expected molecular weight of approximately 40 kDa. VWFA3CK, VWFD4CK, and VWFB1CK were expressed as dimers (∼ 254, 200, and 132 kDa, respectively) since they all included the CK region.
Additional fragments spanning the C-terminal domain of VWF were prepared (Figure 1B,F). VWFD4 was visualized as a band of the expected molecular weight of approximately 40 kDa (Figure 1F). VWFA3CK, VWFD4CK, and VWFB1CK were all expressed as dimers of approximately 254, 200, and 132 kDa, respectively (Figure 1F).
Evidence for an ADAMTS13 binding site in the C-terminal region of VWF
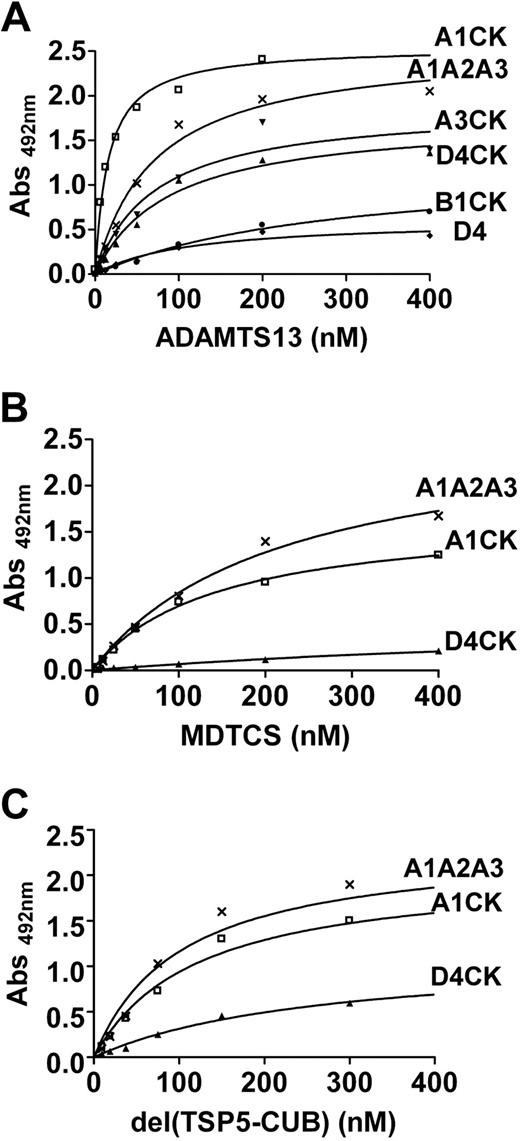
The VWF fragments in Figure 1A were directly immobilized on a microtiter plate and incubated with increasing concentrations of ADAMTS13 (Figure 2A). The KD(app) values derived are listed in Table 1. No binding was observed for VWFD′D3 (Figure 2A). VWFA2 bound to ADAMTS13 with a KD(app) of approximately 33 nM, as expected.12,17 The approximately 3-fold reduction in binding affinity for VWFA1A2A3 suggested that the A1 and A3 domains may partially shield the VWF A2 binding site. Interestingly, an approximately 8-fold increase in ADAMTS13 affinity for VWFA1CK was observed compared with VWFA1A2A3, the first suggestion of a potential ADAMTS13 binding site distal to the VWF A2 domain, in the region spanned by residues 1875 to 2813.
Evidence for an ADAMTS13 binding site on VWF distal to its A2 domain. (A) Of each VWF fragment (depicted in Figure 1A), 30 nM was immobilized on a 96-well microtiter plate and incubated with increasing concentrations of recombinant ADAMTS13 (0-300 nM) in the presence of 10 mM EDTA, for 2 hours at 37°C. The bound ADAMTS13 was detected by a biotinylated anti–TSP2-4 polyclonal antibody followed by streptavidin-HRP and OPD-H2O2. The curves were fitted to the one-binding site model using GraphPad Prism 4. The calculated KD(app) values are listed in Table 1. (B) VWFA2, VWFA1A2A3, and VWFA1CK (50 nM of each) were coated on a microtiter plate and soluble VWFA2 was used as a competitor for their binding to ADAMTS13 (A2/A2, A1A2A3/A2, and A1CK/A2, respectively). In separate experiments, soluble VWFA1A2A3 was tested as an inhibitor for the binding between immobilized VWFA2 and ADAMTS13 (A2/A1A2A3). Different concentrations (range 0-600 nM) of the competitor (VWFA2 or VWFA1A2A3) were preincubated with 12 to 25 nM ADAMTS13, at 37°C for 40 to 60 minutes, and subsequently added to the wells. The detection of the bound ADAMTS13 was performed as described in panel A. The binding in the absence of soluble inhibitor was taken as 100% and the result was plotted as a percentage of this maximal binding against the inhibitor concentration. The curves were fitted using Graph Pad Prism 4 software to the one-site competition model and IC50 values are listed in Table 2. (C) VWFA1CK (50 nM) was immobilized on a microtiter plate and preincubated for 40 to 60 minutes with increasing concentrations (0-400 μg/mL) of either a monoclonal antibody directed against the VWF D4 domain (mAb RU8) or a polyclonal antibody against the VWF CK domain (pAb C-20). ADAMTS13 (12 nM) was applied, incubated for 2 hours at 37°C, and detected as in panel A. The binding in the absence of the inhibitory antibody was taken as 100% binding and the relative binding plotted against the antibody concentration.
Evidence for an ADAMTS13 binding site on VWF distal to its A2 domain. (A) Of each VWF fragment (depicted in Figure 1A), 30 nM was immobilized on a 96-well microtiter plate and incubated with increasing concentrations of recombinant ADAMTS13 (0-300 nM) in the presence of 10 mM EDTA, for 2 hours at 37°C. The bound ADAMTS13 was detected by a biotinylated anti–TSP2-4 polyclonal antibody followed by streptavidin-HRP and OPD-H2O2. The curves were fitted to the one-binding site model using GraphPad Prism 4. The calculated KD(app) values are listed in Table 1. (B) VWFA2, VWFA1A2A3, and VWFA1CK (50 nM of each) were coated on a microtiter plate and soluble VWFA2 was used as a competitor for their binding to ADAMTS13 (A2/A2, A1A2A3/A2, and A1CK/A2, respectively). In separate experiments, soluble VWFA1A2A3 was tested as an inhibitor for the binding between immobilized VWFA2 and ADAMTS13 (A2/A1A2A3). Different concentrations (range 0-600 nM) of the competitor (VWFA2 or VWFA1A2A3) were preincubated with 12 to 25 nM ADAMTS13, at 37°C for 40 to 60 minutes, and subsequently added to the wells. The detection of the bound ADAMTS13 was performed as described in panel A. The binding in the absence of soluble inhibitor was taken as 100% and the result was plotted as a percentage of this maximal binding against the inhibitor concentration. The curves were fitted using Graph Pad Prism 4 software to the one-site competition model and IC50 values are listed in Table 2. (C) VWFA1CK (50 nM) was immobilized on a microtiter plate and preincubated for 40 to 60 minutes with increasing concentrations (0-400 μg/mL) of either a monoclonal antibody directed against the VWF D4 domain (mAb RU8) or a polyclonal antibody against the VWF CK domain (pAb C-20). ADAMTS13 (12 nM) was applied, incubated for 2 hours at 37°C, and detected as in panel A. The binding in the absence of the inhibitory antibody was taken as 100% binding and the relative binding plotted against the antibody concentration.
We also performed competitive inhibition experiments (Figure 2B). VWFA2, VWFA1A2A3, and VWFA1CK were directly coated on the plate and increasing concentrations of soluble VWFA2 or VWFA1A2A3 were used as competitors. Soluble VWFA2 effectively inhibited the binding of ADAMTS13 to immobilized VWFA2, with an IC50 of approximately 72 nM (Table 2), from which a Ki of approximately 28 nM could be calculated. However, the binding site in the VWF A2 domain appeared to be mostly hidden in soluble VWFA1A2A3, as demonstrated by lack of appreciable inhibition when VWFA1A2A3 was used as a competitor for the binding of ADAMTS13 to immobilized VWFA2 (Figure 2B). Furthermore, soluble VWFA2 completely inhibited the interaction between ADAMTS13 and immobilized VWFA1A2A3 (Figure 2B), suggesting that no additional binding sites are localized within this fragment. Interestingly, a significantly reduced inhibition was observed when soluble VWFA2 was used to compete for the binding of VWFA1CK to ADAMTS13 (IC50 ∼ 332 nM).
Inhibition experiments were performed using selected antibodies directed toward different C-terminal regions of VWF (Figure 2C). A polyclonal antibody directed against the CK domain of VWF (pAb C-20) did not inhibit the binding between plate-immobilized VWFA1CK and soluble ADAMTS13. In contrast, a monoclonal antibody directed against the VWF D4 domain (mAb RU8) was able to effectively compete, suggesting that the identified ADAMTS13 binding site may include the VWF D4 domain.
The C-terminal region of VWF interacts with TSP5-CUB domains of ADAMTS13
Additional VWF C-terminal fragments (Figure 1B) were then used (Figure 3A). VWFA3CK and VWFD4CK both bound to ADAMTS13, with KD(app) values of approximately 100 and 143 nM, respectively (Figure 3A and Table 1). This demonstrated that the core of the C-terminal binding site for ADAMTS13 is located within the region spanning residues 1874 to 2813. However, the isolated VWFD4 and VWFB1CK fragments showed appreciable reductions in binding and the KD(app) values could not be precisely calculated (Figure 3A and Table 1).
Evidence for the interaction between ADAMTS13 TSP5-CUBs domains and VWF C-terminal region. (A) VWFA1CK and VWFA1A2A3 (depicted in Figure 1A) and the C-terminal VWF fragments (depicted in Figure 1B; 30 nM of each) were immobilized on a microtiter plate. Increasing concentrations of ADAMTS13 (0-400 nM) were applied and incubated for 2 hours at 37°C. Bound ADAMTS13 was detected by an HRP-conjugated anti-myc antibody and OPD-H2O2. The curves were fitted to the one-binding site model using GraphPad Prism 4. The calculated KD(app) values are listed in Table 1. (B) VWFA1CK, VWFA1A2A3, and VWFD4CK (30 nM of each) were immobilized on a microtiter plate and incubated with increasing concentrations of MDTCS (0-400 nM). Bound MDTCS was detected as in panel A and the KD(app) values are listed in Table 1. (C) VWFA1CK, VWFA1A2A3, and VWFD4CK (30 nM of each) were immobilized on a microtiter plate and incubated with increasing concentrations of del(TSP5-CUB) (0-400 nM). Bound del(TSP5-CUB) was detected as in panel A and the KD(app) values are listed in Table 1.
Evidence for the interaction between ADAMTS13 TSP5-CUBs domains and VWF C-terminal region. (A) VWFA1CK and VWFA1A2A3 (depicted in Figure 1A) and the C-terminal VWF fragments (depicted in Figure 1B; 30 nM of each) were immobilized on a microtiter plate. Increasing concentrations of ADAMTS13 (0-400 nM) were applied and incubated for 2 hours at 37°C. Bound ADAMTS13 was detected by an HRP-conjugated anti-myc antibody and OPD-H2O2. The curves were fitted to the one-binding site model using GraphPad Prism 4. The calculated KD(app) values are listed in Table 1. (B) VWFA1CK, VWFA1A2A3, and VWFD4CK (30 nM of each) were immobilized on a microtiter plate and incubated with increasing concentrations of MDTCS (0-400 nM). Bound MDTCS was detected as in panel A and the KD(app) values are listed in Table 1. (C) VWFA1CK, VWFA1A2A3, and VWFD4CK (30 nM of each) were immobilized on a microtiter plate and incubated with increasing concentrations of del(TSP5-CUB) (0-400 nM). Bound del(TSP5-CUB) was detected as in panel A and the KD(app) values are listed in Table 1.
We next assessed the binding of truncated ADAMTS13 fragments MDTCS (Figure 3B) and del(TSP5-CUB) (Figure 3C) to immobilized VWF fragments. VWFA1A2A3 bound to both MDTCS and del(TSP5-CUB) with an affinity similar to that found for full-length ADAMTS13 (Table 1). Interestingly, VWFA1CK binding to both MDTCS and del(TSP5-CUB) showed a more than 10-fold decrease in affinity compared with that found for full-length ADAMTS13 (Table 1). This affinity was similar to that found for VWFA1A2A3. Together, these results suggested that the ADAMTS13 region TSP5-CUB interacts with the C-terminal domains of VWF, conferring an overall stronger interaction between VWFA1CK and full-length ADAMTS13. As expected, VWFD4CK showed no interaction with MDTCS (Figure 3B). A moderate increase in binding could be noticed when del(TSP5-CUB) was incubated with immobilized VWFD4CK (Figure 3C). However, the presence of the distal TSP repeats (TSP5-8) and CUB domains seemed to be necessary to provide effective binding to VWFD4CK (Figure 3A). Similar results were obtained when the truncated ADAMTS13 fragments were evaluated with immobilized VWFA3CK (data not shown).
Soluble VWF interacts with ADAMTS13 distal domains through its C-terminal region
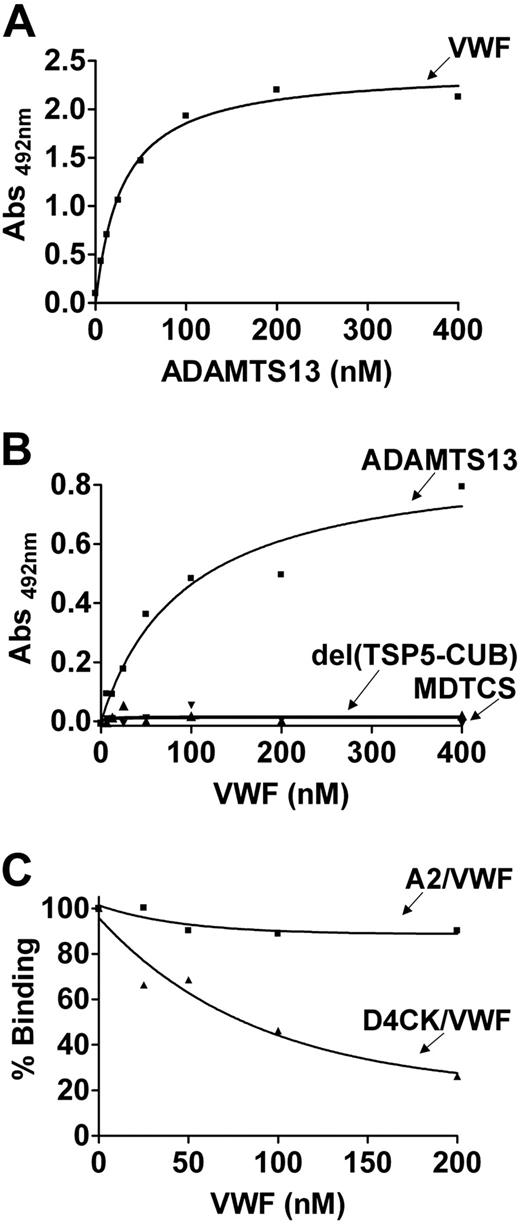
Plate-immobilized VWF bound to full-length ADAMTS13 with high affinity (KD(app) ∼22 nM; Figure 4A). When VWF in solution (in the absence of denaturants or flow and assumed to be globular) was added to plate-immobilized ADAMTS13, it still showed interaction, but with a reduced affinity (KD(app) 85.6 ± 8.0 nM, N = 3; Figure 4B). No binding to soluble VWF was detected with either MDTCS or del(TSP5-CUB) (Figure 4B). These results suggested that the region of ADAMTS13 that mediates the interaction with soluble VWF lies within the distal domains of ADAMTS13 (TSP5-CUB).
Soluble VWF interaction with ADAMTS13 distal domains. (A) VWF (30 nM) was immobilized on a 96-well plate and incubated with increasing concentrations of ADAMTS13 (0-400 nM) for 2 hours at 37°C. Bound ADAMTS13 was detected by biotinylated anti–TSP2-4 polyclonal antibody, followed by streptavidin-HRP and OPD-H2O2. (B) ADAMTS13, MDTCS, and del (TSP5-CUB) (30 nM of each) were coated into corresponding wells in a microtiter plate. Soluble VWF (0-400 nM) was then added and incubated for 2 hours at 37°C. Bound VWF was detected using HRP-conjugated anti-VWF IgG. (C) VWFA2 or VWFD4CK (30 nM of either) was immobilized on a plate. Increasing concentrations of soluble VWF (0-200 nM) were preincubated for 60 minutes with 12 and 50 nM ADAMTS13, respectively. Bound ADAMTS13 was detected as in panel A. The result was plotted as relative inhibition against VWF concentration, with the binding in the absence of inhibitor taken as 100%.
Soluble VWF interaction with ADAMTS13 distal domains. (A) VWF (30 nM) was immobilized on a 96-well plate and incubated with increasing concentrations of ADAMTS13 (0-400 nM) for 2 hours at 37°C. Bound ADAMTS13 was detected by biotinylated anti–TSP2-4 polyclonal antibody, followed by streptavidin-HRP and OPD-H2O2. (B) ADAMTS13, MDTCS, and del (TSP5-CUB) (30 nM of each) were coated into corresponding wells in a microtiter plate. Soluble VWF (0-400 nM) was then added and incubated for 2 hours at 37°C. Bound VWF was detected using HRP-conjugated anti-VWF IgG. (C) VWFA2 or VWFD4CK (30 nM of either) was immobilized on a plate. Increasing concentrations of soluble VWF (0-200 nM) were preincubated for 60 minutes with 12 and 50 nM ADAMTS13, respectively. Bound ADAMTS13 was detected as in panel A. The result was plotted as relative inhibition against VWF concentration, with the binding in the absence of inhibitor taken as 100%.
We have shown in Figure 3 that TSP5-CUB domains participate in direct binding with the C-terminal region of VWF. To investigate whether the same region is exposed on the surface of the globular VWF and mediating the interaction with ADAMTS13, we performed inhibition experiments. As shown in Figure 4C, soluble VWF did not inhibit the binding of ADAMTS13 to immobilized VWFA2, suggesting that the binding site for the spacer domain located in the VWF A2 domain is hidden in the soluble VWF. In contrast, soluble VWF effectively competed for the binding of ADAMTS13 to immobilized VWFD4CK. The IC50 was approximately 156 nM and the calculated Ki, approximately 129 nM, which is comparable with the KD(app) approximately of 143 nM found for the interaction between VWFD4CK and ADAMTS13.
Binding of VWF variants to ADAMTS13 under flow studied using SPR
Full-length ADAMTS13 was covalently bound to the SPR sensor chip and increasing concentrations of VWF C-terminal fragments were injected over it. We observed a concentration-dependent binding response for VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK (Figure 5A,C,E,G). VWFA1A2A3 (up to a concentration of 1.5 μM) was also injected over the chip, but no response was detected (data not shown). The derived sensograms were fitted to the 1:1 Langmuir model (fitting shown in Figure 5A,C,E,G) and ka, kd, and KD determined (Table 3). VWFA3CK and VWFD4CK bound to ADAMTS13 with a KD of approximately 120 and 125 nM. Furthermore, it was possible to quantify the weaker binding of VWFD4 and VWFB1CK to ADAMTS13 (KD ∼ 619 and ∼ 643 nM).
Binding between ADAMTS13 and VWF fragments under flow conditions determined by SPR. Different concentrations (0-3.5 μM) of VWF fragments (VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK) were injected at a flow rate of 30 μL/min over flow cell 1 and flow cell 2 of a sensor chip, on which BSA (∼ 2000-3000 response units [RU]) and ADAMTS13 (∼ 4000-8000 RU) were immobilized, respectively. The curves representing the binding of ADAMTS13 to VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK are shown in panels A, C, E, and G, respectively. The curves are the subtraction between the signals registered in flow cell 2 and in flow cell 1. The association and dissociation phases of the derived sensograms were fitted to 1:1 Langmuir model, giving values for ka, kd, and KD. The plotting of the RUmax at each substrate concentration of VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK is shown in panels B, D, F, and G, respectively. These curves were fitted to the one-binding site model, giving values for KD constants.
Binding between ADAMTS13 and VWF fragments under flow conditions determined by SPR. Different concentrations (0-3.5 μM) of VWF fragments (VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK) were injected at a flow rate of 30 μL/min over flow cell 1 and flow cell 2 of a sensor chip, on which BSA (∼ 2000-3000 response units [RU]) and ADAMTS13 (∼ 4000-8000 RU) were immobilized, respectively. The curves representing the binding of ADAMTS13 to VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK are shown in panels A, C, E, and G, respectively. The curves are the subtraction between the signals registered in flow cell 2 and in flow cell 1. The association and dissociation phases of the derived sensograms were fitted to 1:1 Langmuir model, giving values for ka, kd, and KD. The plotting of the RUmax at each substrate concentration of VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK is shown in panels B, D, F, and G, respectively. These curves were fitted to the one-binding site model, giving values for KD constants.
Cleavage analysis under static and flow conditions
We initially analyzed the proteolysis of VWFA1A2A3 and VWFA1CK fragments by ADAMTS13, under static conditions, in the absence (Figure 6A) and presence (Figure 6B) of 1.5 M urea. No proteolysis occurred for either fragment in the absence of urea (Figure 6A). In the presence of urea (Figure 6B), the approximately 42-kDa band corresponding to the N-terminal cleavage product is visible for both fragments after 30 minutes, but the reaction reached a plateau and did not go to completion, even after 3-hour incubation. This resistance to cleavage has been previously described for VWFA2, VWFA1A2, VWFA2A3, and VWFA1A2A3.12,30,33 No difference in rate or extent of proteolysis could be observed between the 2 VWF fragments.
Analysis of VWF proteolysis under static and flow conditions. (A) VWFA1A2A3 and VWFA1CK (500 nM of each) were incubated with 10 nM ADAMTS13 in the absence of any denaturants. Subsamples were taken at various time points (0-3 hours) and analyzed by SDS-PAGE under reducing conditions, followed by Western blotting using an anti-His antibody. No cleavage was apparent and the uncleaved VWFA1A2A3 and VWFA1CK were visualized as bands of approximately 64 and 174 kDa, respectively. (B) VWFA1A2A3 and VWFA1CK were incubated with ADAMTS13, as in panel A, in the presence of 1.5 M urea. A band of approximately 42 kDa corresponding to the N-terminal cleavage product was detected for both VWFA1A2A3 and VWFA1CK, demonstrating proteolysis. No difference was evident in the rate of cleavage of the 2 fragments. (C) Increasing concentrations of VWFA3CK (0-500 nM) were preincubated with 20 nM ADAMTS13, before being added to 5 μg/mL VWF in the presence of 1.5 M urea. The reactions were stopped after 40 minutes and analyzed on a 1.5% agarose gel followed by Western blotting using an anti-D′D3 polyclonal antibody. In the absence of inhibitor, VWF was efficiently cleaved. No inhibition is observed in the presence of VWFA3CK. (D) Increasing concentrations of VWFA3CK (0-250 nM) were preincubated with 40 nM ADAMTS13, before being added to 5 μg/mL VWF and vortexing for 5 minutes. The reaction was analyzed on a 1.5% agarose gel. VWF was extensively cleaved after 5 minutes. In the presence of VWFA3CK, the amount of cleavage of high- and intermediate-molecular-weight multimers was reduced. (E) RU8 antibody (0-100 μg/mL) was preincubated with VWF, before being added to ADAMTS13 and vortexing as in panel D. The antibody significantly inhibited the cleavage of multimeric VWF. (F) A static experiment similar to the one described in panel C was prepared. The samples were analyzed on a 3% to 8% Tris-acetate gel and detected by an anti-D′D3 polyclonal antibody. The 140- to 140-kDa cleavage product was detected and no inhibition was observed in the presence of VWFA3CK. (G) A cleavage reaction under flow was prepared as in panel D, analyzed on a 3% to 8% Tris-acetate gel and detected by the anti-D′D3 polyclonal antibody. The intensity of the band corresponding to the 140- to 140-kDa homodimer gradually decreased when increasing concentrations of VWFA3CK were present in the reaction. (H) A cleavage reaction under flow was prepared as in panel E and analyzed on a 3% to 8% Tris-acetate gel, and the 176- to 176-kDa homodimer was detected by a polyclonal anti-VWF antibody HRP. RU8 antibody inhibited VWF proteolysis.
Analysis of VWF proteolysis under static and flow conditions. (A) VWFA1A2A3 and VWFA1CK (500 nM of each) were incubated with 10 nM ADAMTS13 in the absence of any denaturants. Subsamples were taken at various time points (0-3 hours) and analyzed by SDS-PAGE under reducing conditions, followed by Western blotting using an anti-His antibody. No cleavage was apparent and the uncleaved VWFA1A2A3 and VWFA1CK were visualized as bands of approximately 64 and 174 kDa, respectively. (B) VWFA1A2A3 and VWFA1CK were incubated with ADAMTS13, as in panel A, in the presence of 1.5 M urea. A band of approximately 42 kDa corresponding to the N-terminal cleavage product was detected for both VWFA1A2A3 and VWFA1CK, demonstrating proteolysis. No difference was evident in the rate of cleavage of the 2 fragments. (C) Increasing concentrations of VWFA3CK (0-500 nM) were preincubated with 20 nM ADAMTS13, before being added to 5 μg/mL VWF in the presence of 1.5 M urea. The reactions were stopped after 40 minutes and analyzed on a 1.5% agarose gel followed by Western blotting using an anti-D′D3 polyclonal antibody. In the absence of inhibitor, VWF was efficiently cleaved. No inhibition is observed in the presence of VWFA3CK. (D) Increasing concentrations of VWFA3CK (0-250 nM) were preincubated with 40 nM ADAMTS13, before being added to 5 μg/mL VWF and vortexing for 5 minutes. The reaction was analyzed on a 1.5% agarose gel. VWF was extensively cleaved after 5 minutes. In the presence of VWFA3CK, the amount of cleavage of high- and intermediate-molecular-weight multimers was reduced. (E) RU8 antibody (0-100 μg/mL) was preincubated with VWF, before being added to ADAMTS13 and vortexing as in panel D. The antibody significantly inhibited the cleavage of multimeric VWF. (F) A static experiment similar to the one described in panel C was prepared. The samples were analyzed on a 3% to 8% Tris-acetate gel and detected by an anti-D′D3 polyclonal antibody. The 140- to 140-kDa cleavage product was detected and no inhibition was observed in the presence of VWFA3CK. (G) A cleavage reaction under flow was prepared as in panel D, analyzed on a 3% to 8% Tris-acetate gel and detected by the anti-D′D3 polyclonal antibody. The intensity of the band corresponding to the 140- to 140-kDa homodimer gradually decreased when increasing concentrations of VWFA3CK were present in the reaction. (H) A cleavage reaction under flow was prepared as in panel E and analyzed on a 3% to 8% Tris-acetate gel, and the 176- to 176-kDa homodimer was detected by a polyclonal anti-VWF antibody HRP. RU8 antibody inhibited VWF proteolysis.
We next performed a functional inhibition assay of VWF proteolysis under static conditions in the presence of 1.5 M urea. Because of its high expression level, VWFA3CK could be used for this purpose. No inhibition of VWF proteolysis could be observed even at the highest concentration of VWFA3CK (500 nM; Figure 6C). The same result was obtained by analyzing a similar cleavage reaction by SDS-PAGE followed by Western blotting to detect the homodimeric 140- to 140-kDa cleavage product (Figure 6F).
We repeated the inhibition of VWF proteolysis under shear stress using a mini-vortex. VWF was efficiently cleaved by ADAMTS13 and only the low-molecular-weight multimers were visible on the gel within 5 minutes of proteolysis (Figure 6D), faster than expected from results reported elsewhere,24 possibly due to higher shear rate generated in our setting. VWFA3CK was able to block the cleavage of VWF by ADAMTS13, as shown by the gradual reappearance of the intermediate- and high-molecular-weight multimers when ADAMTS13 was preincubated with 62.5 to 250 nM VWFA3CK (Figure 6D). The same reaction was assessed by SDS-PAGE and Western blotting to detect the 140- to 140-kDa homodimer. When 250 nM of the inhibitor was used, approximately 80% inhibition of proteolysis was observed (Figure 6G). Next, we performed a similar inhibition experiment in which VWF proteolysis by ADAMTS13 was inhibited by the monoclonal RU8 antibody directed against the VWF D4 domain. RU8 antibody effectively inhibited VWF proteolysis by ADAMTS13 (Figure 6E). This result was confirmed by SDS-PAGE and Western blotting, where the inhibition of proteolysis was estimated to be approximately 45% when 50 μg/mL RU8 was used (Figure 6H).
Discussion
In this study, we evaluated the binding to ADAMTS13 to a series of VWF fragments using 2 different approaches, a plate binding assay and surface plasmon resonance. We identified binding, approximately 86 nM, that could not be attributed to the VWF A2 domain. In initial plate assay experiments, we found an approximately 8-fold increase in ADAMTS13 affinity for VWFA1CK, compared with VWFA1A2A3 (Figure 2A and Table 1), suggesting the presence of an ADAMTS13 binding site in the region spanning residues 1875 to 2813 of VWF. Furthermore, we demonstrated that a VWF C-terminal fragment (VWFD4CK) was able to bind ADAMTS13 with a KD(app) of approximately 143 nM (Figure 4A and Table 1). The inhibition experiment using the RU8 monoclonal antibody against the VWF D4 domain further suggested that this domain is likely to be involved in the binding interaction (Figure 2C). However, the binding of isolated VWFD4 and VWFB1CK fragments to ADAMTS13 was drastically impaired and could not be precisely quantified using this plate-based method. We therefore investigated the binding of the VWF fragments in a BIAcore system in real time and under laminar flow. Our experiments were performed at estimated shear rates of approximately 1500 to 2500 s−1, comparable with those found in the microvasculature. This method allowed us to confirm the KD values previously found for the binding of ADAMTS13 to VWFA3CK and VWFD4CK (Figure 5A-D and Table 3). Furthermore, it was possible to quantify the lower affinity binding of ADAMTS13 to VWFD4 and VWFB1CK (KD ∼ 645 and 622 nM, respectively; Figure 5E-H and Table 3). These results suggested that a cooperative activity between different residues in the 2 regions may be essential to produce maximal interaction. Site-directed mutagenesis of residues in both fragments may lead to a more precise localization of the binding site.
Using the plate binding assay, we confirmed high-affinity binding to the isolated VWF A2 domain (KD(app) of ∼ 33 nM), both when it was immobilized on a plate and when in solution (Figure 2A-B and Tables 1–2). When VWFA1A2A3 fragment was tested in solution, the binding site in the VWF A2 domain was, however, not fully exposed (Figure 2B), probably due to masking by the VWF A1 domain.33 Also in the BIAcore system, VWFA1A2A3 did not bind to the chip-immobilized ADAMTS13, suggesting that the flowing conditions used were insufficient to unfold the molecule. It has been shown previously that the combined contribution of shear stress and platelets is necessary to unfold and expose the VWF A2 cleavage site in VWF and allow proteolysis to occur.34 In our experiments, we did not observe any direct contribution of the VWF A3 domain in binding to ADAMTS13 (Figures 2A-B and 4A), although this has been previously reported to be an ADAMTS13 docking site.35 Functional studies have shown that the VWF A3 domain does not play an important role in determining VWF proteolysis, at least under static conditions.12,33
We have also demonstrated that the novel C-terminal binding site of VWF interacts with ADAMTS13 distal domains (TSP5-CUB), as both MDTCS and del(TSP5-CUB) bound to both VWFA1A2A3 and VWFA1CK with a similar affinity but did not show any appreciable interaction with VWFD4CK (Figure 3B-C and Table 1). However, it should be noticed that a minimal binding between del(TSP5-CUB) and VWFD4CK was observed, even if it could not be precisely quantified (Figure 3C). It is possible that some residues in the ADAMTS13 TSP2-4 domains may be involved in the interaction, but the presence of the TSP5-CUB domains is necessary to produce complete recognition. It has been shown elsewhere that isolated ADAMTS13 TSP5-8CUB and TSP2-8CUB domain fragments bind to full-length VWF with KD(app) values of approximately 212 and 140 nM, respectively.24 Interestingly, these affinities are the same order of magnitude of the KD(app) of approximately 140 nM that we found for the binding of VWFD4CK to full-length ADAMTS13.
VWF is thought to circulate in plasma in a folded, globular conformation that is resistant to ADAMTS13 proteolysis. Whether globular VWF can interact with ADAMTS13 (without proteolysis necessarily occurring) has not been fully addressed before. We demonstrated that soluble VWF bound to plate-immobilized ADAMTS13 with a KD(app) of approximately 86 nM (Figure 4B and Table 1). This is consistent with a previously published KD(app) value of approximately 90 nM for the interaction between ADAMTS13 and VWF captured on a plate with a specific monoclonal antibody.30 It must be pointed out that these affinity estimates are based on use of the molarity of the VWF subunit, rather than of the multimer (which would be impractical to estimate because of its heterogeneity). This enables rational comparison when fragments are used to define the role of individual domains. The good agreement in determined affinity constants here over a range the fragments used, even when some were dimeric, supports this use of monomer molarity. The situation in vivo, where VWF multimers are heterogeneous in terms of size and ligand occupancy, represents a challenge to model, but these estimates of affinity constitute a starting point. Interestingly, no interaction occurred in the present study between soluble VWF and either MDTCS or del(TSP5-CUB) (Figure 4B), suggesting that TSP5-8 and CUB domains are responsible for the binding to soluble VWF. Our results also suggest that the residues on the surface of VWF with which these domains interact are located in the region VWF D4CK, whereas the spacer binding site on the VWF A2 domain remains hidden in the core of the VWF molecule (Figure 4C).
The cleavage experiments illustrated in Figure 6 showed that both VWFA3CK and RU8 antibody inhibit VWF proteolysis by ADAMTS13 under the shear forces generated by a mini-vortex, but did not have any effect on VWF cleavage under static conditions. This suggested that the binding between the C-terminal region of VWF and ADAMTS13 distal domains plays a role in VWF proteolysis under flowing conditions, in accordance with previously published results.23,24 Not surprisingly, the effect of denaturants and shear forces can produce divergent results on the unfolding of VWF. It is likely that in the presence of urea, VWF is extensively unfolded and the binding site in the VWF A2 domain is exposed and available for interaction with the ADAMTS13 spacer domain. In contrast, shear forces may unravel VWF only transiently and the C-terminal domain docking mechanism may be necessary to engage the 2 molecules as shear stress attempts to dissociate them. An alternative explanation for the lack of inhibition in the static experiments is that urea may have an inhibitory effect on the binding between VWFA3CK and ADAMTS13.
In contrast to the laminar flow used in the SPR experiments, the mini-vortex generates a turbulent flow (estimated shear rate > 12000 s−1)36,37 that may be found downstream of a partially occluded vessel. We have demonstrated that the binding between the C-terminal region of VWF and ADAMTS13 distal domains is important in determining substrate proteolysis at least under these specific conditions. Although a recent study using murine models has indeed demonstrated that the carboxyl-terminal domains of ADAMTS13 play an important role in regulating the thrombus formation under high shear rate (5000 s−1),38 there may also be subtle yet important differences in sequence specificity between the murine and human proteins that influence dynamic events in thrombus formation.
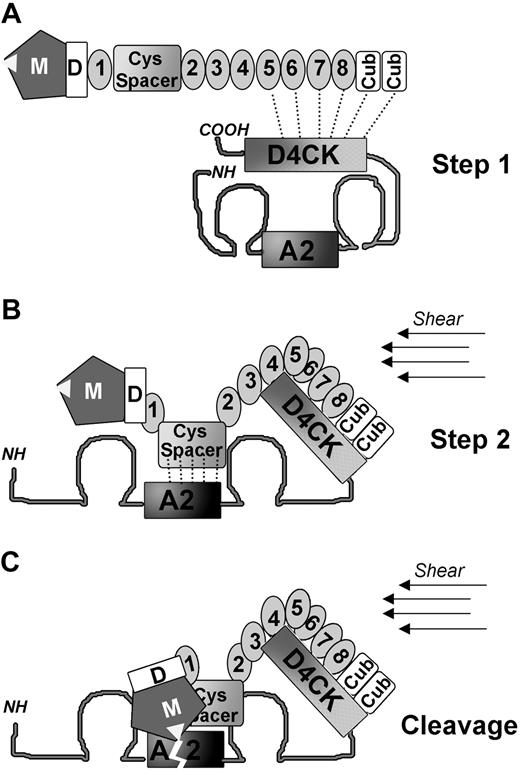
Based on our findings, we propose an initial 2-step model of VWF binding to ADAMTS13 leading to proteolysis. Under normal physiologic conditions, VWF circulates in plasma in a globular conformation where the A2 domain is hidden within the core of the molecule. A modest affinity, approximately 86 nM, binding site in the C-terminal region of VWF (D4CK) is, however, constitutively exposed and can interact with ADAMTS13 distal domains (TSP5-CUB). Both molecules can therefore associate, without cleavage taking place (Figure 7A step 1). The modest affinity suggests that only small amounts of ADAMTS13 will be associated with VWF at any one time, the amount depending on the shear conditions. Under the effect of high shear forces or at the surface of a growing hemostatic plug or thrombus, VWF unravels. The initial docking of ADAMTS13 to the C-terminal region of VWF may facilitate further conformational changes in VWF proximal domains promoting the subsequent higher affinity, approximately 33 nM, binding between the ADAMTS13 spacer domain and VWF A2 domain (Figure 7B, step 2). At this stage, additional binding interactions may occur between domains adjacent to and including ADAMTS13 protease and VWF A2 domains that finally lead to VWF cleavage (Figure 7C). We do not suggest that proteolysis cannot occur without initial tethering, only that it is more efficient with tethering; see also legend to Figure 7. The multistep process, involving several binding and interaction sites of moderate affinity, explains why disruption of a single interaction can appreciably inhibit cleavage, as is shown in Figure 6D. Our model of interaction between VWF and ADAMTS13 is tentative, with further clarification needed of the influence of different shear rates, of the occurrence of possible induced conformational changes in both molecules, and of the role of potential proteins and cellular cofactors.
The proposed 2-site initial binding interaction mechanism between VWF and ADAMTS13. (A) A schematic diagram of ADAMTS13 shows its domains. VWF is represented in its globular conformation. The ADAMTS13 cleavage site in the VWF A2 domain is buried in the center of the molecule and not accessible to be cleaved by the metalloprotease. However, a binding site in the C-terminal region (D4CK) of VWF is constitutively exposed, allowing interaction with the ADAMTS13 distal domains (TSP5-8 and CUBs). How these domains are orientated and how precisely they interact with each other remains to be clarified: the model is indicative only in this respect. (B) Under condition of high shear, VWF unravels. The initial anchoring of the distal domains of ADAMTS13 to the C-terminal region of VWF may help the exposure of the VWF A2 domain binding site and favor the correct positioning of ADAMTS13 spacer domain. (C) Once the higher-affinity interaction between the spacer domain and the A2 domain is established, the ADAMTS13 protease domain can access and cleave the Y1605-M1606 bond in the A2 domain of VWF. Note that we do not suggest that direct cleavage of VWF by ADAMTS13, without the initial tethering step (Step 1), cannot take place. For example, murine investigations with MDTCS demonstrated cleavage of VWF in vivo.39 Such cleavage can arise only when the VWF A2 domain is unfolded by shear and can then attract ADAMTS13. The longer time taken for association to take place, compared with when ADAMTS13 is already associated with VWF, however, may result in less efficient proteolysis. Proteolysis in vivo clearly depends upon several factors, including the location of unfolding, the local flow conditions, and the extent of tethering of VWF onto cellular surfaces.
The proposed 2-site initial binding interaction mechanism between VWF and ADAMTS13. (A) A schematic diagram of ADAMTS13 shows its domains. VWF is represented in its globular conformation. The ADAMTS13 cleavage site in the VWF A2 domain is buried in the center of the molecule and not accessible to be cleaved by the metalloprotease. However, a binding site in the C-terminal region (D4CK) of VWF is constitutively exposed, allowing interaction with the ADAMTS13 distal domains (TSP5-8 and CUBs). How these domains are orientated and how precisely they interact with each other remains to be clarified: the model is indicative only in this respect. (B) Under condition of high shear, VWF unravels. The initial anchoring of the distal domains of ADAMTS13 to the C-terminal region of VWF may help the exposure of the VWF A2 domain binding site and favor the correct positioning of ADAMTS13 spacer domain. (C) Once the higher-affinity interaction between the spacer domain and the A2 domain is established, the ADAMTS13 protease domain can access and cleave the Y1605-M1606 bond in the A2 domain of VWF. Note that we do not suggest that direct cleavage of VWF by ADAMTS13, without the initial tethering step (Step 1), cannot take place. For example, murine investigations with MDTCS demonstrated cleavage of VWF in vivo.39 Such cleavage can arise only when the VWF A2 domain is unfolded by shear and can then attract ADAMTS13. The longer time taken for association to take place, compared with when ADAMTS13 is already associated with VWF, however, may result in less efficient proteolysis. Proteolysis in vivo clearly depends upon several factors, including the location of unfolding, the local flow conditions, and the extent of tethering of VWF onto cellular surfaces.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Tom Vink, University Medical Center Utrecht, for providing VWFD4 protein.
This work was supported by British Heart Foundation grant RG/02/008, now RG/06/007. We are grateful for support from the National Institute for Health Research (NIHR) Biomedical Research Center funding scheme.
National Institutes of Health
Authorship
Contribution: S.Z. designed the research, performed experiments, analyzed results, and wrote the paper; A.C.K.C. performed experiments, analyzed results, and revised the paper; E.G. and P.J.L. performed experiments and revised the paper; T.A.J.M. contributed vital reagents and analytical tools; M.A.L. revised the paper; M.T. performed experiments; and D.A.L. designed the research, analyzed results, and extensively revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sara Zanardelli or David Lane, Department of Haematology, Imperial College London, 5th Fl, Commonwealth Bldg, Hammersmith Hospital Campus, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: sara.zanardelli03@imperial.ac.uk or d.lane@imperial.ac.uk.


![Figure 5. Binding between ADAMTS13 and VWF fragments under flow conditions determined by SPR. Different concentrations (0-3.5 μM) of VWF fragments (VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK) were injected at a flow rate of 30 μL/min over flow cell 1 and flow cell 2 of a sensor chip, on which BSA (∼ 2000-3000 response units [RU]) and ADAMTS13 (∼ 4000-8000 RU) were immobilized, respectively. The curves representing the binding of ADAMTS13 to VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK are shown in panels A, C, E, and G, respectively. The curves are the subtraction between the signals registered in flow cell 2 and in flow cell 1. The association and dissociation phases of the derived sensograms were fitted to 1:1 Langmuir model, giving values for ka, kd, and KD. The plotting of the RUmax at each substrate concentration of VWFA3CK, VWFD4CK, VWFD4, and VWFB1CK is shown in panels B, D, F, and G, respectively. These curves were fitted to the one-binding site model, giving values for KD constants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/13/10.1182_blood-2009-05-224915/4/m_zh89990941300005.jpeg?Expires=1767660654&Signature=aUAxeoVFI6GATnDA~b5rlEf02lmlqVpIAU8-8CYJ6I8-~smAfbBFsV4wxK-2eWtePj8Udn0sleeIK9DBsi4c2SkfFKU1NQ46Q9v1TcZMs-anbeqfyrmhWa-aSemgSTBTQkEXGiOFzgQ5jLQALhHNrIA0ybsRBWVOZ~aDFEAjhZeIpb1-72QVQT27ocg99ZR1HRGBMU9hnlmr3KEbTuU4c0nlJk0tBCDt0nQPF3G5iuFyNnjWnJAhj46opLk8USDpzvXu8qQN~nK4m7Gas9bpOePtc3W987e~2bWKeLBl7sa9n1P8BwiM3cqWQShbKkedw35d0l~meGSPtZpgpZdc9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)