Abstract
Chronic granulomatous disease (CGD), an immunodeficiency with recurrent pyogenic infections and granulomatous inflammation, results from loss of phagocyte superoxide production by recessive mutations in any 1 of 4 genes encoding subunits of the phagocyte NADPH oxidase. These include gp91phox and p22phox, which form the membrane-integrated flavocytochrome b, and cytosolic subunits p47phox and p67phox. A fifth subunit, p40phox, plays an important role in phagocytosis-induced superoxide production via a phox homology (PX) domain that binds to phosphatidylinositol 3-phosphate (PtdIns(3)P). We report the first case of autosomal recessive mutations in NCF4, the gene encoding p40phox, in a boy who presented with granulomatous colitis. His neutrophils showed a substantial defect in intracellular superoxide production during phagocytosis, whereas extracellular release of superoxide elicited by phorbol ester or formyl-methionyl-leucyl-phenylalanine (fMLF) was unaffected. Genetic analysis of NCF4 showed compound heterozygosity for a frameshift mutation with premature stop codon and a missense mutation predicting a R105Q substitution in the PX domain. Parents and a sibling were healthy heterozygous carriers. p40phoxR105Q lacked binding to PtdIns(3)P and failed to reconstitute phagocytosis-induced oxidase activity in p40phox-deficient granulocytes, with premature loss of p40phoxR105Q from phagosomes. Thus, p40phox binding to PtdIns(3)P is essential for phagocytosis-induced oxidant production in human neutrophils and its absence can be associated with disease.
Introduction
Superoxide production by the phagocyte NADPH oxidase during the respiratory burst is an essential component of the innate immune response. The active enzyme is assembled from a membrane-bound flavocytochrome b, a heterodimer composed of gp91phox and p22phox subunits, and cytosolic regulatory components p47phox, p67phox, p40phox, and the Rac GTPase.1,2 Genetic defects in the NADPH oxidase cause chronic granulomatous disease (CGD), characterized by absent or markedly reduced enzyme activity, recurrent bacterial and fungal infections, and granulomatous inflammation that can involve multiple organs, including the genitourinary and gastrointestinal tracts.1,3 Recent retrospective studies report clinical and genetic findings on more than 900 patients.4-7 Although there is some ethnic and regional variation, approximately two-thirds of CGD patients have recessive mutations in the X-linked CYBB gene encoding gp91phox, and the remainder have autosomal recessive defects in CYBA, NCF1, or NCF2, encoding p22phox, p47phox, or p67phox, respectively. Mutations involving p40phox or Rac have not been reported as causes of CGD. An infant with a dominant-negative mutation in the hematopoietic-specific Rac2 GTPase was reported, with partial oxidase defects, markedly impaired leukocyte migration and adhesion, and a clinical picture that resembled leukocyte adhesion deficiency rather than CGD.8,9
In the majority of CGD cases, the gene defect leads to the absence of the encoded protein and O2− production. Exceptions include rare variant forms of X-linked CGD with low levels of oxidase activity and patients with autosomal recessive NCF1 mutations who have trace amounts of detectable activity even in the absence of p47phox expression, and a milder clinical course is often seen in each of these groups.1,4-7 However, there is considerable variability in the range and severity of clinical manifestations, even within a given genetic subgroup, likely reflecting the influence of genetic variation in other genes involved in innate immunity and inflammation (eg, Foster et al10 ).
Although the role of p40phox in the NADPH oxidase has been poorly understood,2 recent studies in p40phox-deficient cell lines and in gene-targeted mice established that p40phox stimulates phagocytosis-induced NADPH oxidase activity via a phox homology (PX) domain at its N-terminus that binds to phosphatidylinositol 3-phosphate (PtdIns(3)P), a phosphoinositide that accumulates on phagosomes through the action of class III PtdIns(3)P kinase.11-20 In mice either lacking p40phox or expressing p40phoxR58A, a PX domain mutant that prevents binding to PtdIns(3)P, in vitro killing of Staphylococcus aureus by neutrophils was reduced to an extent similar to that seen in the complete absence of NADPH oxidase activity, and elimination of S aureus after intraperitoneal injection was impaired.16,17 In contrast, PtdIns(3)P binding to p40phox plays a minimal, if any, role in regulating superoxide release elicited by the chemoattractant formyl-methionyl-leucyl-phenylalanine (fMLF) or phorbol ester.15-17,19,20 As mentioned, mutations in p40phox have not been described in CGD,1,4-7 but genome-wide association studies identified an intronic polymorphism associated with Crohn disease in the NCF4 gene (NC_000022.9) encoding p40phox,21,22 and a second polymorphism linked to a subgroup of rheumatoid arthritis.23
Here we describe the first case of autosomal recessive CGD involving NCF4 in a boy who presented initially with granulomatous colitis. Whereas extracellular oxidant production in response to soluble agonists was normal, his neutrophils exhibited a selective deficiency in phagocytosis-induced NADPH oxidase activity. Genetic analysis identified 2 different mutant NCF4 alleles, each inherited from a parent. The paternal allele contains an internal duplication and premature stop codon, and a point mutation in the maternal allele results in a nonfunctional form of p40phox that has defective PtdIns(3)P binding.
Methods
Written informed consent following an Indiana University School of Medicine–approved protocol was obtained in accordance with the Declaration of Helsinki from the parents and control subjects for the described studies. Neutrophils were isolated from heparin-anticoagulated venous blood using Polymorphprep (AXIS-SHIELD PoC AS). Oxidant production by neutrophils stimulated with 3.3-μm latex beads opsonized with human immunoglobulin G (IgG-beads), serum-opsonized zymosan (SOZ), phorbol myristate acetate (PMA), or formyl-methionyl-leucyl-phenylalanine (fMLF) was measured by luminol- or isoluminol-enhanced chemiluminescence (for intracellular or extracellular oxidant production, respectively).19 PMA-induced activity was also measured by cytochrome c reduction.24 Phagocytosis of IgG-opsonized red cells was quantitated as described.15 Neutrophil bactericidal activity was evaluated25 using serum-opsonized S aureus strain ALC 1435.26
p40phox-knockdown promyelocytic PLB-985 cells19 were transduced with MSCVNeo-p40phox or MSCVNeo-p40phoxR105Q retrovirus, prepared as described,19 and selected with G418. After granulocyte differentiation, NADPH oxidase activity was assayed.19 Lines expressing yellow fluorescent protein (YFP)–tagged derivatives of wild-type or p40phoxR105Q were similarly generated for confocal videomicroscopy studies.19 MSCV retroviral vectors were also used to express the p40phox PX domain tagged with YFP (YFP-PX40) and an R105Q mutant (YFP-PX40R105Q) in COS-7 and PLB-985 cells for videomicroscopy. A protein lipid-overlay assay (Echelon Biosciences Inc) was performed using histidine-tagged PX40, PX40R105Q, and PX40R105A. Cell lysates were analyzed by immunoblotting as described.19,24,27,28
Diagnostic genetic testing for mutations in the CYBB, NCF1, NCF2, and CYBA genes was performed at a commercial laboratory. For analysis of genetic defects in NCF4 and RAC2, genomic DNA and RNA from peripheral blood mononuclear cells were isolated using Blood & Cell Culture DNA Mini Kit (QIAGEN) and Trizol (Invitrogen), respectively. SuperScript III First-Strand Synthesis SuperMix (Invitrogen) was used to synthesize first-strand cDNA. Sequencing of Taq PCR Master (Roche Applied Science)–amplified products from NCF4 exons and flanking introns and from NCF4 and RAC2 cDNAs was performed using a BigDye terminator V3.1 Kit and ABI 3100 Genetic Analyzer (Applied Biosystems). p40phox cDNA polymerase chain reaction (PCR) products were also cloned in pGEM-T Easy (Promega) and sequenced. Sequences were analyzed with AlignX (Invitrogen) and Chromas (Technelysium Pty Ltd). Oligonucleotide primers are in supplemental Table 2 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
For computational modeling, Sybyl Version 8.0 from the Tripos program suite (Tripos Inc) was used to perform the R105Q mutation and then add hydrogen atoms to energy minimize the mutant structure around the mutation site using the Tripos forcefield with Gasteiger charges.
Results
Patient history
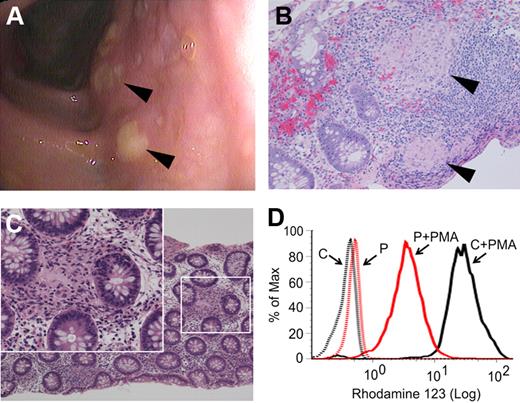
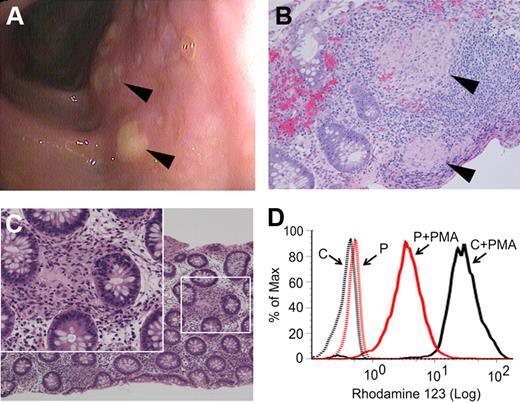
The patient presented at age 3 1/12 years with a 10-week history of perianal rash, 1 month of daily nonhemorrhagic diarrhea, intermittent abdominal pain, low-grade fever, and a 1 kg weight loss. Perioral eczema and several aphthous ulcers were also present. Laboratory studies included a modestly elevated erythrocyte sedimentation rate, C-reactive protein, and platelet count, and reduced serum albumin (supplemental Table 1). Past medical history was remarkable for eczema, sinusitis, and recurrent episodes of croup. Family history included asthma and hypothyroidism but no intestinal disease or immunodeficiency. Endoscopy revealed erosions in the gastric fundus and colonic mucosal edema with diffuse aphthous ulcerations (Figure 1A). Colonic biopsies showed an unusually high number of small epithelioid granulomata near lymphoid aggregates or interspersed between crypts (Figure 1B-C). The finding of granulomatous colitis in such a young patient prompted further investigation for underlying immunodeficiency. His complete blood count and differential T-, B-, and natural killer–cell populations, response to childhood immunizations, and serum immunoglobulin levels were normal. However, although phorbol ester–stimulated neutrophil oxidant production detected by the nitroblue tetrazolium test resembled controls (supplemental Figure 1A), dihydrorhodamine 123 (DHR123) assay on multiple occasions demonstrated partial reduction of intracellular oxidant production (Figure 1D), suggestive of CGD. Because of the abnormal DHR123 assays and the granulomas found on colonic biopsies, diagnostic genetic testing for the 4 known forms of CGD was performed. However, no mutations in X-linked CYBB, or in the autosomal NCF1, NCF2, or CYBA genes, were found.
Findings of granulomatous colitis and partial reduction in neutrophil oxidase activity as measured by DHR123 assay. (A) Diffuse aphthous ulcerations ( ) were visible throughout the colon at the time of presentation. Biopsies showed prominent epithelioid granulomata in lymphoid aggregates (B,
) were visible throughout the colon at the time of presentation. Biopsies showed prominent epithelioid granulomata in lymphoid aggregates (B,  ) and in lamina propria (C) with giant cells (inset). Four-μm sections were stained using hematoxylin and eosin prior to microscopy and photomicrographs were taken with a Nikon Eclipse 80i microscope equipped with a Nikon Digital Sight DS-U1 camera using 20×/0.50NA and 40×/0.75NA objectives. (D) DHR123 fluorescence in control (black line) and patient (red line) neutrophils before and after stimulation with 0.6 μg/mL PMA (representative of 5 assays).
) and in lamina propria (C) with giant cells (inset). Four-μm sections were stained using hematoxylin and eosin prior to microscopy and photomicrographs were taken with a Nikon Eclipse 80i microscope equipped with a Nikon Digital Sight DS-U1 camera using 20×/0.50NA and 40×/0.75NA objectives. (D) DHR123 fluorescence in control (black line) and patient (red line) neutrophils before and after stimulation with 0.6 μg/mL PMA (representative of 5 assays).
Findings of granulomatous colitis and partial reduction in neutrophil oxidase activity as measured by DHR123 assay. (A) Diffuse aphthous ulcerations ( ) were visible throughout the colon at the time of presentation. Biopsies showed prominent epithelioid granulomata in lymphoid aggregates (B,
) were visible throughout the colon at the time of presentation. Biopsies showed prominent epithelioid granulomata in lymphoid aggregates (B,  ) and in lamina propria (C) with giant cells (inset). Four-μm sections were stained using hematoxylin and eosin prior to microscopy and photomicrographs were taken with a Nikon Eclipse 80i microscope equipped with a Nikon Digital Sight DS-U1 camera using 20×/0.50NA and 40×/0.75NA objectives. (D) DHR123 fluorescence in control (black line) and patient (red line) neutrophils before and after stimulation with 0.6 μg/mL PMA (representative of 5 assays).
) and in lamina propria (C) with giant cells (inset). Four-μm sections were stained using hematoxylin and eosin prior to microscopy and photomicrographs were taken with a Nikon Eclipse 80i microscope equipped with a Nikon Digital Sight DS-U1 camera using 20×/0.50NA and 40×/0.75NA objectives. (D) DHR123 fluorescence in control (black line) and patient (red line) neutrophils before and after stimulation with 0.6 μg/mL PMA (representative of 5 assays).
His chronic colitis and perianal disease were poorly responsive to glucocorticoids, sulfasalazine, and azathioprine, but he had mild improvement with alternating courses of ciprofloxacin and metronidazole. Exacerbations, which included perianal infections and nondraining perianal fistula, required intermittent bowel rest and intensified antibiotic therapy. Endoscopy after 18 months revealed extensive granulomatous colitis with new ileal involvement. Infliximab and methotrexate afforded minimal improvement. A diversion ileostomy at age 4 years and 11 months was complicated in the immediate postoperative period by intermittent fevers, abdominal pain, and intra-abdominal fluid collections, which were treated with broad spectrum antibiotics and drainage, although only a small amount of culture-negative fluid was obtained. After the ileostomy, he had complete resolution of perianal disease but scant mucous rectal discharge persisted. He has been maintained on oral 5-aminosalicylates.
Neutrophil dysfunction has been supported clinically with prophylactic trimethoprim-sulfamethoxazole and itraconazole and subsequently granulocyte colony-stimulating factor (G-CSF) at 3 micrograms per kilogram per day 3 times a week and intravenous immunoglobulin 1 gram every 4 weeks as empiric adjunctive therapy. The rationale for these latter agents includes reports describing beneficial effects from G-CSF or granulocyte-macrophage CSF for inflammatory bowel disease in the setting of chronic granulomatous disease, glycogen storage disease Ib, and Crohn disease,29-33 and reports that G-CSF may be beneficial as adjunctive therapy for infections in nonneutropenic patients, including those with abnormal neutrophil function such as diabetics.34 It should also be noted that short-term (1-7 days) G-CSF administration to healthy volunteers is reported to increase expression of phagocytic receptors, phagocytosis, oxidative activity, and microbial killing by neutrophils34 ; however, little information is available as to its effects on neutrophil function when used on a chronic basis other than for patients with inherited neutropenia syndromes,35,36 who are not a comparable group.
Neutrophil studies
To further characterize the NADPH oxidase defect in this patient, we assayed neutrophil oxidant production in response to a variety of agonists. Extracellular release of superoxide elicited by either PMA or fMLF was similar to controls (Figure 2A). However, intracellular oxidant production during phagocytosis of SOZ, IgG-beads, or serum-opsonized S aureus was markedly reduced (Figure 2A and supplemental Figure 1B). Phagocytosis of IgG-opsonized particles was quantitatively normal (supplemental Table 3). The selective defect in phagocytosis-induced oxidase activity was observed on 4 occasions over a 12-month period; all were obtained when he was on G-CSF 3 times a week. Phagocytosis-induced oxidant production in neutrophils from his parents and brother was approximately one-half of controls, although PMA-stimulated superoxide release was normal (Figure 2A and supplemental Figure 1B). Reduced detection of intracellular superoxide by luminol or DHR123 can occur in myeloperoxidase deficiency.27 However, immunoblots showed normal levels and size of myeloperoxidase in patient neutrophils (not shown).
NADPH oxidase function and expression in patient and family members. (A) Neutrophil NADPH oxidase activity in response to PMA (200 ng/mL), fMLF (10 μM), or SOZ (2 mg/mL) as indicated, as monitored by cytochrome reduction or isoluminol for extracellular activity (PMA and fMLF, respectively) or luminol for intracellular activity (SOZ). RLU indicates relative light units. Color-coded symbols and bars indicate patient (P, red); mother (M, purple); father (F, green); brother (B, blue); or control (C, black). (Top row) Representative kinetic data; (bottom row) NADPH oxidase activity as the mean ± SD relative to control samples; results of all controls are shown in one bar. Dashed red line indicates background signal for patient neutrophils stimulated with PMA or fMLF in the presence of SOD, or for SOZ, unstimulated cells. PMA and SOZ assays were performed in patient (n = 3), mother (n = 3), father (n = 2), brother (n = 2), and controls (n = 6). *P < .05 NADPH oxidase activity versus controls. For fMLF, superoxide production was measured in the patient (n = 3) and controls (n = 6). (B) Results of a 1-step bactericidal assay at a neutrophil-bacteria ratio = 1:5. Mean ± SD of quadruplicate measurements of bacteria colony-forming units (CFUs) in the absence of neutrophils (blue line), or the presence of control (black line) or patient (red line) neutrophils. *P < .05 for the control versus patient at 20 minutes and 60 minutes. Results for neutrophils treated with the NADPH oxidase inhibitor diphenyliodonium (10 μM) are shown by dashed lines. (C) Immunoblot of NADPH oxidase subunits and densitometry of p40phox and p67phox expression (representative of 2 independently obtained sample sets).
NADPH oxidase function and expression in patient and family members. (A) Neutrophil NADPH oxidase activity in response to PMA (200 ng/mL), fMLF (10 μM), or SOZ (2 mg/mL) as indicated, as monitored by cytochrome reduction or isoluminol for extracellular activity (PMA and fMLF, respectively) or luminol for intracellular activity (SOZ). RLU indicates relative light units. Color-coded symbols and bars indicate patient (P, red); mother (M, purple); father (F, green); brother (B, blue); or control (C, black). (Top row) Representative kinetic data; (bottom row) NADPH oxidase activity as the mean ± SD relative to control samples; results of all controls are shown in one bar. Dashed red line indicates background signal for patient neutrophils stimulated with PMA or fMLF in the presence of SOD, or for SOZ, unstimulated cells. PMA and SOZ assays were performed in patient (n = 3), mother (n = 3), father (n = 2), brother (n = 2), and controls (n = 6). *P < .05 NADPH oxidase activity versus controls. For fMLF, superoxide production was measured in the patient (n = 3) and controls (n = 6). (B) Results of a 1-step bactericidal assay at a neutrophil-bacteria ratio = 1:5. Mean ± SD of quadruplicate measurements of bacteria colony-forming units (CFUs) in the absence of neutrophils (blue line), or the presence of control (black line) or patient (red line) neutrophils. *P < .05 for the control versus patient at 20 minutes and 60 minutes. Results for neutrophils treated with the NADPH oxidase inhibitor diphenyliodonium (10 μM) are shown by dashed lines. (C) Immunoblot of NADPH oxidase subunits and densitometry of p40phox and p67phox expression (representative of 2 independently obtained sample sets).
Killing of complement-opsonized S aureus by patient neutrophils was significantly impaired compared with controls (Figure 2B). This decreased bactericidal activity was similar to that seen for control neutrophils treated with the NADPH oxidase inhibitor diphenyliodonium (Figure 2B).
Immunoblot analysis of neutrophil oxidase subunits revealed similar levels of p67phox, p47phox, Rac2, gp91phox, and p22phox in controls, the patient, his parents, and his brother (Figure 2C). However, p40phox expression was reduced by approximately 50% in both the patient and his father.
Genetic analysis of NCF4 and RAC2
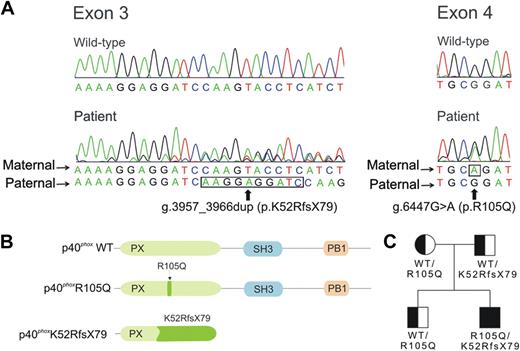
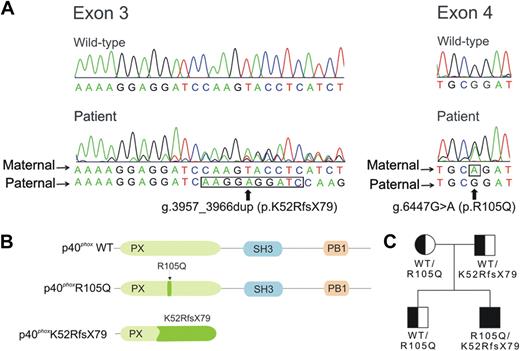
Because earlier studies of CYBB, CYBA, NCF1, and NCF2 were normal, we analyzed RAC2 and NCF4 for mutations. The RAC2 cDNA was normal. However, we identified 2 different mutant NCF4 alleles in patient DNA. The first, found in both the patient and his father, was a duplication 3957_3966dup in exon 3 based on the gDNA sequence, reported as the National Center for Biotechnology Information (NCBI) Reference Sequence number NC_000022.37 This duplication causes a frame shift with Lysine-52 as the first affected residue and a new reading frame ending in a premature stop 79 codons later (K52RfsX79) (Figure 3A). The frameshift predicts a p40phox protein with a theoretical molecular weight of 14 kDa, a truncated PX domain and absent SH3 and PB1 domains (Figure 3B). This defect may explain the reduced amount of p40phox detected in the patient and the father (Figure 2C); whether the 14 kDa mutant is expressed is uncertain, as a suitable antibody is lacking. The second NCF4 allele contained a substitution 6447G>A in exon 4 that predicts a missense R105Q mutation in the PX domain (Figure 3A and 3B). The patient, his mother, and his brother were heterozygous for this allele (Figure 3C). Reverse-transcription–PCR was performed using mononuclear cell RNA, and PCR products were cloned and sequenced. In 19 clones analyzed from the patient, 13 were the R105Q allele and 6 were the K52RfsX79 allele. The patient lacked previously identified NCF4 polymorphisms associated with inflammatory bowel disease (IBD) and rheumatoid arthritis (supplemental Table 4).
Analysis of NCF4 and patient pedigree. (A) Chromatograms corresponding to the wild-type and patient sequences in NCF4 exons 3 and 4. The alleles present in the mother and father are as indicated; nucleotides that are duplicated in 3957_3966dup (exon 3) and the point mutation g.6263G>A (exon 4) are outlined by a black line. Nucleotide positions are based on the gDNA sequence, reported as the NCBI Reference Sequence number NC_000022.37 (B) Location of the NCF4 mutations in a schematic of p40phox. Amino acid positions are based on NCBI Reference Sequence number NP_000622 (from NM_000631; for isoform 1, containing 339 amino acids).37 Predicted functional domains shown include a PX (phagocyte oxidase homology) domain (position 19 to 140), SH3 domain (position 175 to 225), and PB1 (phagocyte oxidase and Bem1p) domain (position 285 to 306).2 (C) Pedigree of the patient's family. Squares represent male family members and circle, the female family member; genotypes for the NCF4 alleles at the protein level are shown.
Analysis of NCF4 and patient pedigree. (A) Chromatograms corresponding to the wild-type and patient sequences in NCF4 exons 3 and 4. The alleles present in the mother and father are as indicated; nucleotides that are duplicated in 3957_3966dup (exon 3) and the point mutation g.6263G>A (exon 4) are outlined by a black line. Nucleotide positions are based on the gDNA sequence, reported as the NCBI Reference Sequence number NC_000022.37 (B) Location of the NCF4 mutations in a schematic of p40phox. Amino acid positions are based on NCBI Reference Sequence number NP_000622 (from NM_000631; for isoform 1, containing 339 amino acids).37 Predicted functional domains shown include a PX (phagocyte oxidase homology) domain (position 19 to 140), SH3 domain (position 175 to 225), and PB1 (phagocyte oxidase and Bem1p) domain (position 285 to 306).2 (C) Pedigree of the patient's family. Squares represent male family members and circle, the female family member; genotypes for the NCF4 alleles at the protein level are shown.
Characterization of p40phoxR105Q mutation
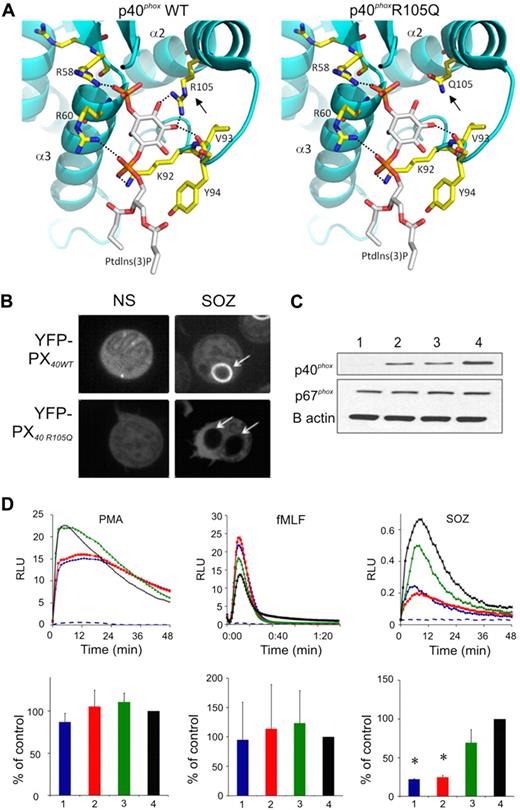
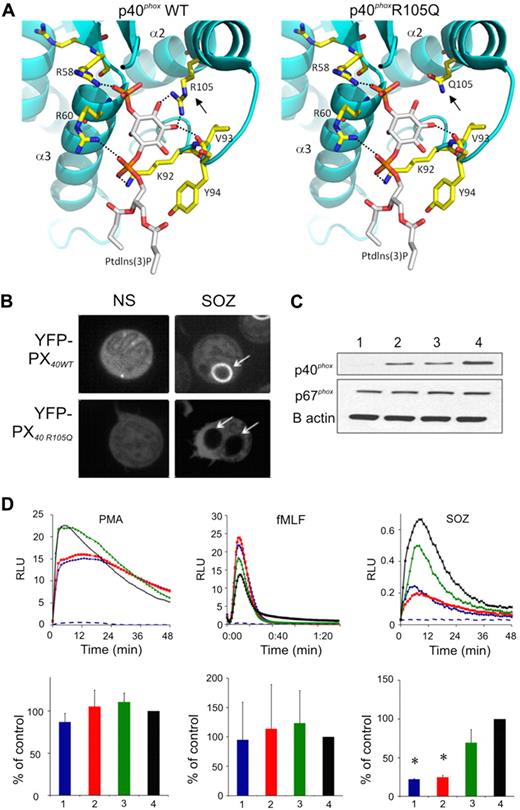
The functional significance of the p40phoxR105Q mutation was evaluated. Prior structural studies38 revealed that the guanidium nitrogen atoms of p40phoxR105 form 2 hydrogen bonds with the inositol moiety in PtdIns(3)P (Figure 4A), and experimental analysis showed that an R105A substitution results in loss of PtdIns(3)P binding.19,38 Computational modeling showed that these interactions were also lost in p40phoxR105Q (Figure 4A). As expected, a fluorescently tagged fragment of p40phox containing the PX domain accumulated on phagosome (Figure 4B) and endosome (supplemental Figure 2A) membranes, both of which are known to be enriched in PtdIns(3)P, unless PtdIns(3)P formation is blocked by the PtdIns3 kinase inhibitor wortmannin (supplemental Figure 2A). In contrast, the R105Q-mutated PX domain remained cytosolic (Figure 4B and supplemental Figure 2A). Furthermore, the R105Q PX domain was unable to bind to PtdIns(3)P in a lipid-binding assay, similar to an R105A mutant (supplemental Figure 2B).
Modeling and function of the p40phoxR105Q mutant. (A) A model showing a close-up view of the Ptdlns(3)P binding cavity within the PX domain of wild-type p40phox, and the R105Q p40phox mutant (). The protein is shown in ribbon representation and colored in cyan. Residues that interact with Ptdlns(3)P are shown in capped-sticks rendering and color-coded based on atom types (C, N, O, and P in yellow, blue, red, and orange, respectively). Ptdlns(3)P is also color-coded based on atom types except that carbon is colored in white. Dashed lines show hydrogen bond interactions of R58, R60, V93, and R105 with Ptdlns(3)P. The interactions between amino acid 105 and Ptdlns(3)P are lost in the R105Q mutant (), as the distance between the Q105 amide oxygen and nitrogen atoms is greater than 5 Å, which precludes any hydrogen bonding. In addition, Q105 is neutral and therefore cannot compensate for the loss of the positive charge on R105, which contributes to stabilize interactions with Ptdlns(3)P. Images were created with program PyMOL (DeLano Scientific LLC). (B) Representative image collected during confocal videomicroscopy of nonstimulated (NS) PLB-985 granulocytes and during phagocytosis of SOZ, where cells are expressing the wild-type YFP-p40phox PX domain (YFP-PX40WT) or with R105Q mutation (YFP-PX40 R105Q), as indicated. Representative of cells filmed in 3 experiments. (C-D) Results using granulocyte-differentiated PLB-p40 KD cells (1 or blue), PLB-p40 KD cells expressing p40phoxR105Q (2 or red), PLB-p40 KD cells expressing wild-type p40phoxwt (3 or green), and wild-type PLB-985 cells (4 or black). (C) Immunoblots probed with antibodies directed at the indicated proteins. Representative of 3 experiments. (D) PLB-985 granulocyte NADPH oxidase activity in response to PMA (200 ng/mL), fMLF (10 μM), or SOZ (2 mg/mL) as indicated, as monitored by isoluminol for extracellular activity (PMA and fMLF) or luminol for intracellular activity (SOZ). RLU indicates relative light units. (Top row) Representative kinetic data; (bottom row) NADPH oxidase activity as the mean ± SD (n = 3) relative to control wild-type PLB-985 granulocytes. Dashed blue line shows signal from PMA- or fMLF-stimulated PLB-p40 KD cells in the presence of SOD, or in the absence of stimulus for the SOZ data. *P < .05, PLB-p40 KD cells expressing p40phoxR105Q versus PLB KD cells expressing wild-type p40phox.
Modeling and function of the p40phoxR105Q mutant. (A) A model showing a close-up view of the Ptdlns(3)P binding cavity within the PX domain of wild-type p40phox, and the R105Q p40phox mutant (). The protein is shown in ribbon representation and colored in cyan. Residues that interact with Ptdlns(3)P are shown in capped-sticks rendering and color-coded based on atom types (C, N, O, and P in yellow, blue, red, and orange, respectively). Ptdlns(3)P is also color-coded based on atom types except that carbon is colored in white. Dashed lines show hydrogen bond interactions of R58, R60, V93, and R105 with Ptdlns(3)P. The interactions between amino acid 105 and Ptdlns(3)P are lost in the R105Q mutant (), as the distance between the Q105 amide oxygen and nitrogen atoms is greater than 5 Å, which precludes any hydrogen bonding. In addition, Q105 is neutral and therefore cannot compensate for the loss of the positive charge on R105, which contributes to stabilize interactions with Ptdlns(3)P. Images were created with program PyMOL (DeLano Scientific LLC). (B) Representative image collected during confocal videomicroscopy of nonstimulated (NS) PLB-985 granulocytes and during phagocytosis of SOZ, where cells are expressing the wild-type YFP-p40phox PX domain (YFP-PX40WT) or with R105Q mutation (YFP-PX40 R105Q), as indicated. Representative of cells filmed in 3 experiments. (C-D) Results using granulocyte-differentiated PLB-p40 KD cells (1 or blue), PLB-p40 KD cells expressing p40phoxR105Q (2 or red), PLB-p40 KD cells expressing wild-type p40phoxwt (3 or green), and wild-type PLB-985 cells (4 or black). (C) Immunoblots probed with antibodies directed at the indicated proteins. Representative of 3 experiments. (D) PLB-985 granulocyte NADPH oxidase activity in response to PMA (200 ng/mL), fMLF (10 μM), or SOZ (2 mg/mL) as indicated, as monitored by isoluminol for extracellular activity (PMA and fMLF) or luminol for intracellular activity (SOZ). RLU indicates relative light units. (Top row) Representative kinetic data; (bottom row) NADPH oxidase activity as the mean ± SD (n = 3) relative to control wild-type PLB-985 granulocytes. Dashed blue line shows signal from PMA- or fMLF-stimulated PLB-p40 KD cells in the presence of SOD, or in the absence of stimulus for the SOZ data. *P < .05, PLB-p40 KD cells expressing p40phoxR105Q versus PLB KD cells expressing wild-type p40phox.
Wild-type p40phox or mutant p40phoxR105Q was expressed in PLB-985 myeloid cells made deficient in endogenous p40phox by shRNAi19 (PLB-p40 KD cells; Figure 4C). Superoxide release elicited by PMA or fMLF was similar in granulocyte-induced wild-type PLB-985 cells, PLB-p40KD cells, and cells expressing either wild-type p40phox or p40phoxR105Q (Figure 4D). In contrast, phagocytosis-induced production of intracellular oxidants was markedly impaired in PLB-p40KD cells stimulated with either SOZ (Figure 4D) or IgG-beads (data not shown). Expression of wild-type p40phox rescued this defect, but p40phoxR105Q did not (Figure 4D). These results reproduced the NADPH oxidase defect observed in the patient's neutrophils.
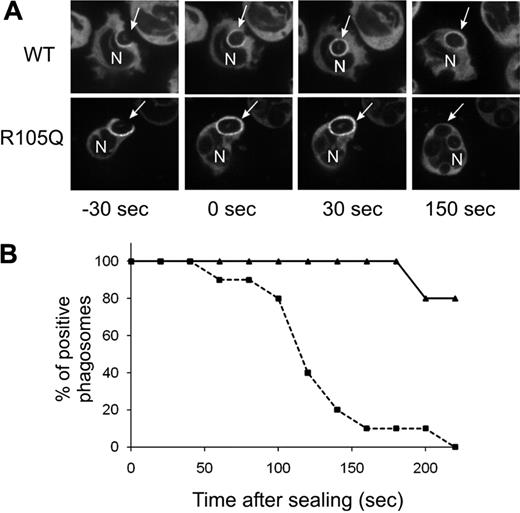
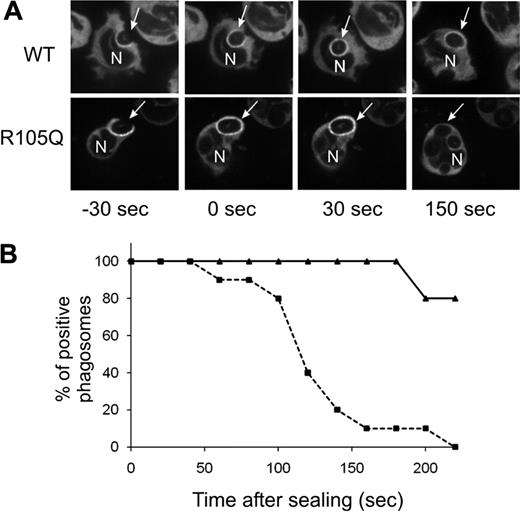
Although PtdIns(3)P binding to p40phox stimulates oxidase activity during phagocytosis, recruitment of p40phox to the cups of forming phagosomes is mediated by an interaction between complementary PB1 domains in p40phox and p67phox.19 Consistent with this, accumulation of YFP-tagged p40phoxR105Q expressed in PLB-p40KD cells was detected on the phagocytic cup and in nascent phagosomes, similar to a YFP-tagged wild-type p40phox probe (Figure 5A-B). As previously reported, YFP-tagged wild-type p40phox remained detectable on phagosomes for many minutes after closure.19 However, p40phoxR105Q disappeared from phagosomes within minutes of internalization (Figure 5A-B).
Translocation of YFP-p40phox or YFP-p40phoxR105Q during phagocytosis of SOZ. Confocal videomicroscopy was used to monitor phagosome translocation of YFP-p40phox and YFP-p40phoxR105Q expressed in PLB-p40 KD granulocytes. (A) Time-lapse images. Times shown are referenced to time of phagosome sealing. Arrow indicates a monitored SOZ phagosome; N, nucleus. (B) Phagosomes that showed accumulation of wild-type or mutant YFP-tagged p40phox during phagosome cup formation were monitored for continued presence of the YFP-tagged proteins for 220 seconds after sealing. Solid line indicates YFP-p40phoxWT; dashed line, YFP-p40phoxR105Q. Data for YFP-p40phoxR105Q and YFP-p40phoxWT were collected from 10 and 5 phagosomes, respectively, as filmed in 3 independent experiments.
Translocation of YFP-p40phox or YFP-p40phoxR105Q during phagocytosis of SOZ. Confocal videomicroscopy was used to monitor phagosome translocation of YFP-p40phox and YFP-p40phoxR105Q expressed in PLB-p40 KD granulocytes. (A) Time-lapse images. Times shown are referenced to time of phagosome sealing. Arrow indicates a monitored SOZ phagosome; N, nucleus. (B) Phagosomes that showed accumulation of wild-type or mutant YFP-tagged p40phox during phagosome cup formation were monitored for continued presence of the YFP-tagged proteins for 220 seconds after sealing. Solid line indicates YFP-p40phoxWT; dashed line, YFP-p40phoxR105Q. Data for YFP-p40phoxR105Q and YFP-p40phoxWT were collected from 10 and 5 phagosomes, respectively, as filmed in 3 independent experiments.
Discussion
To our knowledge, this is the first reported case of a patient with autosomal recessive defects involving the p40phox subunit of the NADPH oxidase. The boy presented at 3 years of age with granulomatous colitis. Neutrophil NADPH oxidase assays showed a substantial but selective defect in intracellular production of oxidants and impaired killing of S aureus. His parents and brother, all healthy, showed a partial reduction in neutrophil intracellular oxidant production. Genetic analysis established that the patient is a compound heterozygote for 2 inherited mutations in NCF4. The paternal allele has an internal duplication resulting in a frameshift and premature stop codon (K52RfsX79), and the maternal allele, also present in his brother, harbors a point mutation encoding a R105Q substitution in the PtdIns(3)P-binding PX domain. The R105Q mutation compromises PtdIns(3)P binding, consistent with prior studies on the importance of R105.19,38 The causal link between p40phoxR105Q and defective neutrophil oxidant production was established by the recapitulation of the patient's defective neutrophil phenotype when p40phoxR105Q was expressed in granulocytes deficient in endogenous p40phox. As for p40phoxR105A,19 the R105Q substitution resulted in premature loss of p40phox from the phagosome. These results reinforce accumulating evidence that generation of PtdIns(3)P by class III PtdIns(3)P kinases and subsequent binding to p40phox are essential prerequisites for stimulating high-level intracellular NADPH oxidase activity in neutrophils undergoing phagocytosis or stimulated with phorbol ester.12-20 To our knowledge, this is also the first example of a human disease associated with a defect in a PX domain, a motif that interacts with specific phosphoinositides to localize and regulate PX domain–containing proteins.39
Because NCF4 encodes a subunit of the NADPH oxidase complex that plays a selective but essential function in regulating enzyme activity and a patient with null mutations had chronic granulomatous inflammation in the gastrointestinal tract, we believe that autosomal recessive defects in NCF4 should be classified as a fifth genetic subgroup of CGD. The overall frequency of this subgroup and the spectrum of clinical manifestations are currently unknown and will require further investigation. Based on the findings presented here and in previous studies,15-17,19,20 p40phox defects will result in a selective but substantial reduction in intracellular NADPH oxidase activity, which is distinct from other forms of CGD where both intracellular and extracellular oxidant production is affected. It is possible that the selective deficiency in oxidase activity could modify the enhanced susceptibility to bacterial and fungal infections characteristic of CGD. It is also important to note that the PB1 domain–dependent association between p40phox and p67phox contributes to their mutual stability in neutrophils, as evidenced by the observation that p40phox protein levels are markedly reduced in CGD patients lacking p67phox due to NCF2 defects.2 The patient described here expressed a nonfunctional p40phox and neutrophil levels of p67phox were unaffected. However, complete absence of p40phox expression is likely to reduce neutrophil p67phox levels as reported for p40phox-null murine neutrophils and that resulted in partial reductions in extracellular oxidant production in response to fMLF or phorbol ester in addition to the marked impairment in phagosome NADPH oxidase activity.16,17
To date, the patient reported here has not had infections with opportunistic pathogens or other organisms characteristic of CGD. He has been on antimicrobial prophylaxis for the 3 years since presentation, making it difficult to assess his predisposition to infection. However, in vitro killing of S aureus by patient neutrophils was markedly impaired, and the patient's history of sinusitis and of intra-abdominal fluid collections with fever complicating his ileostomy could suggest an in vivo bactericidal defect.
The major clinical manifestation in this patient has been chronic granulomatous inflammation in the gastrointestinal tract, particularly the colon and rectum, which has been difficult to manage. Gastrointestinal involvement is frequently seen in CGD, and inflammatory bowel disease (IBD)–like colitis affects between 7% to 20% of CGD patients reported in different series.4,5,40 Of note, gastrointestinal symptoms can be the presenting or only manifestation of CGD, as was the case for 8 of 46 CGD patients identified with gastrointestinal symptoms in one study.40 This same study also found that whereas the incidence of intestinal inflammation was 43% in patients with X-linked CGD, it is relatively uncommon in autosomal recessive CGD, occurring in only 11% of this subgroup of patients.40 It is noteworthy that genome-wide association studies identified a NCF4 polymorphism rs4821544 associated with Crohn disease.21,22 Although this variation was not present in our patient, identification of 2 null alleles for NCF4 in a young child with IBD manifestations strengthens the relationship between NCF4, phagocytosis-induced oxidant production, and predisposition to IBD.
Although the fraction of children with IBD who have CGD or other underlying immunologic defects is small (eg, Liu et al41 ), it important to maintain a high index of suspicion, particularly in very young patients. Overall, 20% to 25% of IBD cases involve children younger than 20 years,42 but less than 14% of pediatric patients present before 6 years of age.43 IBD-like manifestations have been reported in both quantitative and qualitative neutrophil disorders, including chronic and cyclic neutropenia, leukocyte adhesion deficiency-1, and glycogen storage disease-1b, in addition to CGD.44 When screening patients with IBD-like symptoms for NADPH oxidase defects, the DHR123 assay may be better suited than the NBT test because the former is quantitative and also detects only intracellular oxidase activity, expected to be reduced even in the case of NCF4 defects.
In summary, these results show that p40phox and its binding to PtdIns(3)P are essential for high-level intracellular oxidant production in human neutrophils and that this selective impairment in NADPH oxidase activity can be associated with granulomatous colitis, a manifestation also seen in other forms of chronic granulomatous disease. These results additionally suggest that evaluation for NCF4 mutations is warranted in cases of autosomal recessive CGD without identifiable defects in CYBA, NCF1, and NCF2, as well as in other patients with reduced NADPH oxidase activity. The similarities or differences in clinical manifestations with NCF4 mutations compared with other genetic subgroups of CGD remain to be defined. Further exploration of the roles of p40phox and the NADPH oxidase in processes linked to increased risk of inflammatory bowel disease, including antigen presentation, defective innate immunity, and autophagy,21,22,45,46 also promise to be informative.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patient and his family for their participation in this study, Dr Chris Lever for primary care of this patient, and the staff at Alberta Children's Hospital for their care of the patient. We also thank Danuta Stack and the Flow Cytometry Facility at Calgary Lab Services for performing neutrophil oxidase assays, and Mervin Yoder (Department of Pediatrics) and Tatiana Foroud (Department of Medical and Molecular Genetics) at Indiana University School of Medicine for helpful discussions. We thank David Lambeth (Emory University), Gary Bokoch and Ulla Knaus (Scripps Research Institute), and Algirdas Jesaitis (Montana State University) for gifts of antibodies.
This work was supported by National Institutes of Health grants RO1HL45635 (M.C.D.) and RO1AI070958 (W.M.N.); the Riley Children's Foundation (M.C.D.); the American Heart Association Midwest Affiliate Postdoctoral Fellowship (J.D.M.); the Jeffrey Modell Foundation (D.B.L.); and facilities and resources at the Veterans Administration in Iowa City (W.M.N.). Microscopy facilities in the Indiana Center for Biological Microscopy are also supported by a grant (INGEN) from the Lilly Endowment and the Indiana University Simon Cancer Center (P30 CA082709).
National Institutes of Health
Authorship
Contribution: J.D.M. and A.A.A. designed and performed experiments, analyzed the data, prepared the figures, and drafted the paper; N.A.M.W. analyzed and interpreted the clinical data; contributed the case summary, and helped edit the paper; I.W. analyzed and interpreted the clinical data and helped edit the paper; C.C.M.W. analyzed and interpreted the clinical data and contributed the case summary; X.J.L. contributed key reagents and protocols; C.C.M. and N.D.S. performed experiments; D.B.L. analyzed and interpreted the clinical data, provided the initial evaluation for NADPH oxidase defects, and helped edit the paper; M.S., J.D.K., and W.Y. analyzed and interpreted the clinical data; S.O.M. provided structural analysis with computer modeling and the corresponding figure and helped edit the paper; W.M.N. contributed key reagents and protocols and helped edit the paper; and M.C.D. oversaw the design and interpretation of the experiments, preparation of the paper and figures, and editing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary C. Dinauer, Cancer Research Institute R4, Indiana University School of Medicine, 1044 West Walnut St, Rm 402A, Indianapolis, IN 46202; e-mail: mdinauer@iupui.edu.
References
Author notes
*J.D.M. and A.A.A. contributed equally to this work.



 ) were visible throughout the colon at the time of presentation. Biopsies showed prominent epithelioid granulomata in lymphoid aggregates (B,
) were visible throughout the colon at the time of presentation. Biopsies showed prominent epithelioid granulomata in lymphoid aggregates (B,