Abstract
The HIV-1 transactivating factor Tat accumulates on the surface of endothelium by interacting with heparan sulfate proteoglycans (HSPGs). Tat also interacts with B-lymphoid Namalwa cells but only when these overexpress HSPGs after syndecan-1 cDNA transfection (SYN-NCs). Accordingly, SYN-NCs, but not mock-transfected cells, adhere to endothelial cells (ECs) when Tat is bound to the surface of either one of the 2 cell types or when SYN-NCs are transfected with a Tat cDNA. Moreover, endogenously produced Tat bound to cell-surface HSPGs mediates cell adhesion of HIV+ ACH-2 lymphocytes to the endothelium. This heterotypic lymphocyte-EC interaction is prevented by HSPG antagonist or heparinase treatment, but not by integrin antagonists and requires the homodimerization of Tat protein. Tat tethered to the surface of SYN-NCs or of peripheral blood monocytes from healthy donors promotes their transendothelial migration in vitro in response to CXCL12 or CCL5, respectively, and SYN-NC extravasation in vivo in a zebrafish embryo model of inflammation. In conclusion, Tat homodimers bind simultaneously to HSPGs expressed on lymphoid and EC surfaces, leading to HSPG/Tat-Tat/HSPG quaternary complexes that physically link HSPG-bearing lymphoid cells to the endothelium, promoting their extravasation. These data provide new insights about how lymphoid cells extravasate during HIV infection.
Introduction
Tat protein, the main HIV transactivating factor, is released by HIV+ cells.1 Extracellular Tat targets different uninfected cells causing a variety of biologic effects related to various AIDS-associated pathologies.1 Extracellular Tat can be found in body fluids,2 but it has the strong tendency to adhere to the surface of HIV+ cells and of nearby uninfected cells.3 In addition, it accumulates in the extracellular matrix,4 where it promotes the adhesion of different cell types.5-7
Extracellular Tat induces inflammatory pathways8 and vascular permeability9 in endothelial cells (ECs), whereas it causes oncogene activation10 and cytokine up-regulation11 in B lymphocytes. These effects may concur to the increased frequency of B-cell lymphomas observed in mice after Tat overexpression12 and to the increased incidence of lymphadenopathies, polyclonal B-cell activation, Kaposi sarcoma, and non-Hodgkin lymphoma in seropositive individuals.13
Heparan sulfate proteoglycans (HSPGs) are expressed on the surface of most cell types at 105 to 106 molecules per cell. They consist of a core protein and of glycosaminoglycan chains, unbranched anionic polysaccharides composed of repeating disaccharide units formed by differently sulfated uronic acid and hexosamine residues.14 Syndecans, the most represented HSPGs on eukaryotic cells, possess a core protein composed of an extracellular domain, a single membrane-spanning domain, and a short cytoplasmic domain that interacts with the cytoskeleton. At the surface of macrophages and B cells, HSPGs act as receptors that mediate chemotaxis to a variety of molecules.15 Moreover, syndecans regulate the growth of B-lymphoid malignancies.16 Interestingly, the expression levels of HSPGs on the surface of CD4+ cells are regulated by viral infection and immune activation.17-19 Accordingly, syndecan-1 expression has been proposed as a marker functional to the diagnosis of AIDS-related lymphomas.20 HSPGs are also expressed at the EC surface where they act as receptors for several heparin-binding growth factors, including HIV-1 Tat,21 thus regulating crucial events, such as inflammation, coagulation, tissue remodeling, angiogenesis, and tumor growth.
Here we describe a novel, alternative mechanism of lymphocyte adhesion and migration across the endothelium in which extracellular Tat homodimers mediate a heterotypic lymphocyte–endothelial cell interaction by binding simultaneously the HSPGs expressed on the surface of both cell types.
Methods
Reagents
A total of 86 amino acid HIV-1 Tat was expressed and purified by Escherichia coli as glutathione-S-transferase (GST-Tat) or green fluorescent protein (Tat-GFP)22,23 fusion proteins. GST and GFP moieties do not interfere with the heparin-binding, transactivating, and cell-adhesive capacity of Tat.24 Different recombinant mutant forms of GST-Tat were purified as described22 and used: GST-TatC→A (cysteine 22, 25, and 27 within the cysteine-rich domain mutated to alanine), GST-TatR→A (arginine 49, 52, 53, 55, 56, and 57 within the basic domain mutated to alanine), and GST-Tat-1e (deletion of the C-terminal amino acid sequence containing the RGD integrin recognition motif). Synthetic peptides representing the amino acid sequences 1 to 20, 41 to 60, and 71 to 85 of Tat were from Medical Research Council AIDS Reagent Project (Potters Bar, United Kingdom); heparan sulfate (HS) from M. Del Rosso (Florence University); anti-VEGFR-2/kinase insert domain receptor (KDR) antibody from H. A. Weich (Max Planck Institute); pCEP-Tat expression vector harboring the HIV-1 Tat cDNA from A. Gualandris (Turin University, Italy); synthetic, LPS-free 86-amino acid form of Tat (s-Tat) and its biotinylated form (b-Tat) were from Tecnogen; heparinase II and III, hyaluronidase, and cycloheximide from Sigma-Aldrich; heparin and Escherichia coli unsulfated K5 polysaccharide from Glycores 2000; suramin from Bayer AG; integrin antagonist linear peptide GRGDSPK and its inactive analog GRADSPK from Neosystem Laboratoire; high molecular weight tetramethylrhodamine dextran (R-HMW dextran, 2 × 106 Da) from Invitrogen; CXCL12 and CCL5 from PeproTech EC; tumor necrosis factor α (TNF-α) from R&D Systems; and polyclonal anti-Tat–FITC antibody from Diatheva.
Real-time biomolecular interaction assay
A BIAcore X apparatus (GE Healthcare) was used. Surface plasmon resonance (SPR) was exploited to measure changes in refractive index caused by the binding of GST-Tat to s-Tat immobilized to a BIAcore CM5 sensor chip. Briefly, s-Tat (40 μg/mL) was allowed to react with the sensor chip as described,25 allowing the immobilization of 6500 resonance units (corresponding to ∼ 0.8 pmol of s-Tat). Similar results were obtained for the immobilization of GST, used as a negative control and for blank subtraction. Next, s-Tat, GST-Tat, or its mutant forms were injected over the s-Tat surfaces for 4 minutes (to allow association with immobilized protein) and then washed until dissociation occurred.25
Cell cultures
Burkitt lymphoma–derived Namalwa cells were grown as described.26 Namalwa clones transfected with the empty vector or with a vector containing syndecan-1 cDNA (EV-NCs and SYN-NCs cells, respectively) were prepared and cultured as described.26 For Tat transfection, 107 cells were incubated with 30 μg of pCEP-Tat expression vector in 0.5 mL of calcium/magnesium-free phosphate-buffered saline (PBS) at 4°C for 10 minutes. Cells were then electroporated at 322 V and 950 microfarads. Selection was started 48 hours later with 50 μg/mL hygromicin. Stable transfection was achieved after 2 weeks. The selected populations were characterized by Western blot analysis with anti-Tat antibodies.
Fetal bovine aortic GM7373 ECs were grown as described.27 Fixation of GM7373 cells was achieved by 2 hours of incubation at 4°C with PBS/3% glutaraldehyde, 5 minutes of incubation with 0.1 M glycine and washes with PBS.
Primary cultures of human adrenal gland capillary ECs (HACECs) were grown as described.28
ACH-2 cells, a chronically HIV infected T-cell line derived from CEM A3.01 cells, produce fully competent Tat. They were grown in RPMI 1640, 10% fetal calf serum (FCS), 1% glutamine, and 1% penicillin/streptomycin as described.29 ACH-2 cells were left untreated or stimulated for 2 days with 1 ng/mL TNF-α at the density of 2 × 105 cells/mL.29 Compared with CEM A3.01 cells, here used as a negative control, ACH-2 cells show a significant basal retro-transcriptase activity that is increased (6-fold) by TNF-α stimulation (data not shown). For adhesion assays, cells were resuspended at 7.5 × 105/mL of culture medium containing 1% FCS and treated as described for Namalwa cells.
Flow cytometry
EV-NCs and SYN-NCs (5 × 106 cells/mL) were incubated for 8 hours in complete medium containing recombinant Tat-GFP protein (15 nM) and washed twice with PBS. In some experiments, 2 additional washes with PBS containing 1.5 M NaCl (PBS/NaCl) were performed to remove surface-bound Tat.23 Cells were then analyzed by flow cytometry using a FACScan flow cytometer (BD Biosciences).
b-Tat binding assay
GM7373 EC monolayers in 96-well plates were incubated for 2 hours at 37°C with heparinase II or III or with hyaluronidase. Then, EC monolayers were incubated for 2 hours at 37°C or 4°C with b-Tat (510 nM) in PBS/1% gelatin with different competitors. After cell washing, the amount of cell-associated b-Tat was detected with horseradish peroxidase–labeled avidin (1/1500) and chromogen substrate ABTS (Kirkgaard & Perry Laboratories).
Cell adhesion to immobilized Tat
Proteins were immobilized to nontissue culture plastic as described.30 Then, EV-NCs and SYN-NCs (100 000 cells/200 μL of RPMI 1640/1% FCS) were added to the wells containing the coated proteins and incubated for 2 hours at 37°C. Then, wells were washed 3 times with 2 mM ethylenediaminetetraacetic acid in PBS and photographed using an inverted microscope (Olympus IX51 connected to an Olympus Camedia C4040Z digital camera, Olympus Optical). The images were digitalized and stored in the computer memory. For each photograph, the number of adherent Namalwa cells was counted in 6 microscopic fields (×400 magnification) chosen randomly.
B-lymphoid-EC adhesion assay
GM7373 EC monolayers were preincubated with heparinase or hyaluronidase in 96-well plates, washed with PBS, incubated for 2 hours at 37°C with Dulbecco modified Eagle medium/1% FCS containing 550 nM GST-Tat, and washed with PBS to remove unbound Tat or with PBS/NaCl to remove surface-bound proteins.24 EV-NCs and SYN-NCs were seeded on the EC monolayers (100 000 cells/well) and allowed to adhere for 2 hours at 37°C. Then, adherent cells were photographed and counted as described in adhesion assay section above. In some experiments, SYN-NCs were pretreated for 2 hours at 37°C with heparinase III and further incubated on a orbital shaker with culture medium containing 550 nM GST-Tat before the adhesion assay. To remove unbound Tat, cells were centrifuged at 500g for 10 minutes, resuspended in culture medium without FCS, centrifuged again (twice), and resuspended in culture medium containing 1% FCS.
Transmigration assay
HACECs (50 000 cells) were seeded on collagen-coated 24-well Transwell culture inserts (6.5-mm diameter clear polycarbonate membrane, 3-μm pores; Biocoat, BD Biosciences) and cultured in complete endothelial growth medium (EGM Bullet Kit, BioWhittaker) for 3 days. Under these conditions, HACECs did not cross the membrane and formed a complete monolayer only on the upper surface of the filter. HACEC monolayers were washed twice with RPMI 1640/0.2% bovine serum albumin (BSA; assay medium). Heparinase III–treated or untreated Namalwa cells were preincubated for 45 minutes at 37°C on an orbital shaker with culture medium alone (naive cells) or containing s-Tat (550 nM; Tat-coated cells). To remove unbound Tat, cells were centrifuged and resuspended as described for adhesion assay. Namalwa cells (250 000) were then placed in the upper chamber of the insert before Transwell immersion. Assay medium (500 μL) containing CXCL12 (25 nM) was added in the lower chamber. After 4 hours at 37°C, migrated cells were collected by centrifugation and counted in a Burker chamber.
Zebrafish tail transection assay
Transgenic VEGFR2:G-RCFP zebrafish line31 (provided by A. Rubinstein, Zygogen) was maintained at 28°C as described.32 Dechorionated embryos at 72 hours after fertilization were anesthetized with 0.04 mg/mL Tricaine, and complete transection of the tail was performed with a sterile pyrogene-free 18-G needle. SYN-NCs were stained with PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich) and preincubated with s-Tat as described for transmigration assay. Six hours after tail transection, approximately200 naive or Tat-coated SYN-NCs were injected into the sinus venous of anesthetized transgenic embryos using borosilicate glass capillary needles and a Picospritzer microinjector (Eppendorf).33 After 24 hours, double-fluorescence images of injected embryos were acquired using an epifluorescence Zeiss Axiovert 200M microscope equipped with a Hal 100 digital camera and AxioVisionLE software.
The extent of SYN-NC extravasation in the injured area was measured by computerized image analysis (Image-Pro Plus; MediaCybernetics).
Results
HSPGs mediate extracellular Tat binding to endothelial and B-lymphoid cells
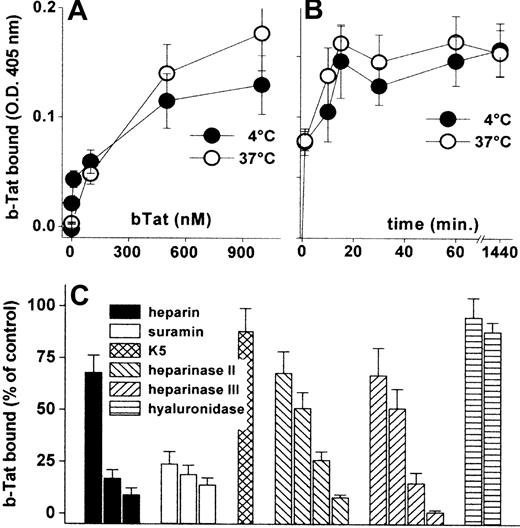
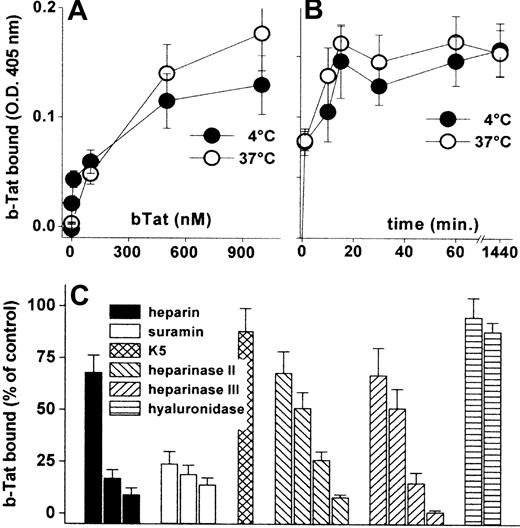
Cytokines/chemokines accumulate on the EC surface where they recruit leukocytes/lymphocytes.34 Accordingly, b-Tat binds to an EC monolayer in a dose- and time-dependent but temperature-independent manner (Figure 1A-B). The binding was very rapid (being already appreciable after 1 minute of incubation) and lasted for at least 24 hours (Figure 1B). b-Tat–EC interaction could be inhibited by the sulfated HSPG antagonists suramin and heparin,35 but not by the unsulfated E coli K5 polysaccharide. In addition, pretreatment of ECs with heparinase II or III, but not with hyaluronidase, prevented the binding of b-Tat in a dose-dependent manner (Figure 1C). Relevant to this point, heparinase III treatment leads to an almost complete removal of HSPGs from the EC surface (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The capacity of Tat to bind to EC monolayers was confirmed in immunofluorescence studies using Tat-GFP (supplemental Table 1). Thus, Tat stably accumulates on the EC surface by binding to the sulfated groups of HSPGs.
Interaction of Tat with the endothelium. GM7373 ECs were incubated for 2 hours at 37°C or 4°C with increasing concentrations of b-Tat (A) or for the indicated periods of time with 510 nM b-Tat (B). (C) GM7373 ECs were incubated with b-Tat (510 nM) in the presence of (from left to right) heparin (0.073, 0.73, or 7.3 nM), suramin (2.1, 21, or 210 nM), or unsulfated K5 polysaccharide (7.3 nM). Alternatively, GM7373 ECs were treated with heparinase II or III (1.0, 2.5, 5.0, or 10 mU/mL) or with hyaluronidase (5.0 or 10 mU/mL) before incubation with b-Tat. Then, the amount of EC-associated b-Tat was measured. Each point is the mean ± SEM of 3 to 5 duplicate determinations.
Interaction of Tat with the endothelium. GM7373 ECs were incubated for 2 hours at 37°C or 4°C with increasing concentrations of b-Tat (A) or for the indicated periods of time with 510 nM b-Tat (B). (C) GM7373 ECs were incubated with b-Tat (510 nM) in the presence of (from left to right) heparin (0.073, 0.73, or 7.3 nM), suramin (2.1, 21, or 210 nM), or unsulfated K5 polysaccharide (7.3 nM). Alternatively, GM7373 ECs were treated with heparinase II or III (1.0, 2.5, 5.0, or 10 mU/mL) or with hyaluronidase (5.0 or 10 mU/mL) before incubation with b-Tat. Then, the amount of EC-associated b-Tat was measured. Each point is the mean ± SEM of 3 to 5 duplicate determinations.
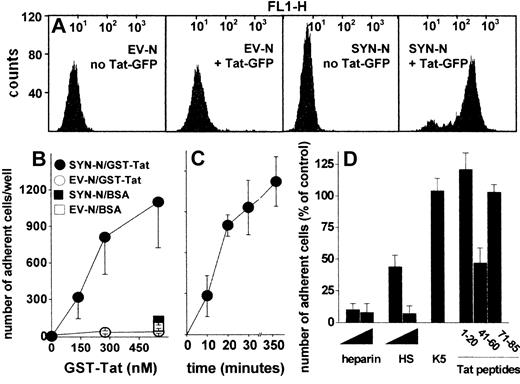
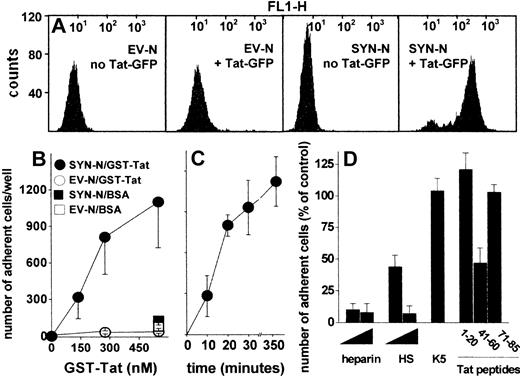
To assess whether HSPGs play a role also in the interaction of extracellular Tat with B-lymphoid cells, we took advantage of an experimental model in which HSPG-deficient Namalwa cells had been transfected with an expression vector harboring the human syndecan-1 cDNA.26 The levels of HSPG expression on the surface of SYN-NCs are at least 10 times higher than those expressed by EV-NCs, here used as negative control.26 On this basis, SYN-NCs and EV-NCs were subjected to fluorescence-activated cell sorter analysis after incubation with Tat-GFP. As shown in Figure 2A, Tat-GFP binds to SYN-NCs but not to EV-NCs. Bound Tat-GFP is completely removed from SYN-NCs by a PBS/NaCl wash (data not shown) indicating its cell surface localization. Indeed, SYN-NCs are unable to internalize the HSPG-bound Tat-GFP (supplemental Figure 2A). To better characterize the binding of extracellular Tat to Namalwa cells, we evaluated the capacity of SYN-NCs and EV-NCs to adhere to GST-Tat–coated plastic. As shown in Figure 2B-C, SYN-NCs, but not EV-NCs, adhere to immobilized GST-Tat in a dose- and time-dependent manner. SYN-NC adhesion was prevented by the sulfated HSPG antagonists heparin and HS,35 but not by the unsulfated K5 polysaccharide (Figure 2D). Accordingly, peptide Tat (41-60) (corresponding to the basic domain of Tat that mediates heparin/HS interaction)22 inhibited SYN-NC adhesion to immobilized GST-Tat, whereas peptides Tat (1-20, corresponding to the acidic domain of Tat, here used as a negative control) and Tat (71-85, containing the RGD motif responsible for Tat-integrin interaction)25 were ineffective (Figure 2D). Taken together, the results demonstrate that extracellular Tat binds to B-lymphoid Namalwa cells only when these express significant amounts of HSPGs, as it occurs in SYN-NCs. In these cells, Tat interaction is mediated by the sulfated HS chains of the transduced syndecan-1 and is independent of integrin engagement.
Interaction of Tat with Namalwa cell surface. (A) Flow cytometry profiles of Namalwa cells before or after treatment with Tat-GFP. (B) Namalwa cells were allowed to adhere to plastic coated with BSA or GST-Tat. Alternatively, SYN-NCs were allowed to adhere on plastic coated with GST-Tat (550 nM) for different periods of time (C), or for 2 hours in the presence of heparin, HS (29, 290 nM), unsulfated K5 polysaccharide (290 nM), or the indicated synthetic peptides of Tat (32 μM) (D). Then, adherent lymphocytes were counted. Each point is the mean ± SEM of 3 to 8 duplicate determinations. In panel D, data are expressed as percentage of adherent cells with respect to wells treated without competitors.
Interaction of Tat with Namalwa cell surface. (A) Flow cytometry profiles of Namalwa cells before or after treatment with Tat-GFP. (B) Namalwa cells were allowed to adhere to plastic coated with BSA or GST-Tat. Alternatively, SYN-NCs were allowed to adhere on plastic coated with GST-Tat (550 nM) for different periods of time (C), or for 2 hours in the presence of heparin, HS (29, 290 nM), unsulfated K5 polysaccharide (290 nM), or the indicated synthetic peptides of Tat (32 μM) (D). Then, adherent lymphocytes were counted. Each point is the mean ± SEM of 3 to 8 duplicate determinations. In panel D, data are expressed as percentage of adherent cells with respect to wells treated without competitors.
Extracellular Tat-HSPG interaction mediates B-lymphoid cell adhesion to the endothelium
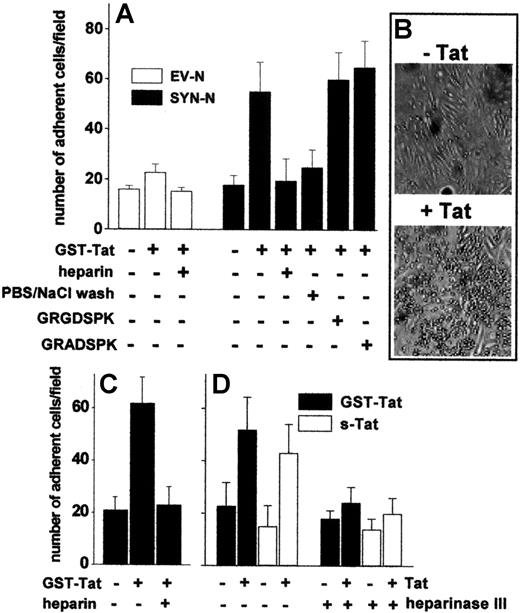
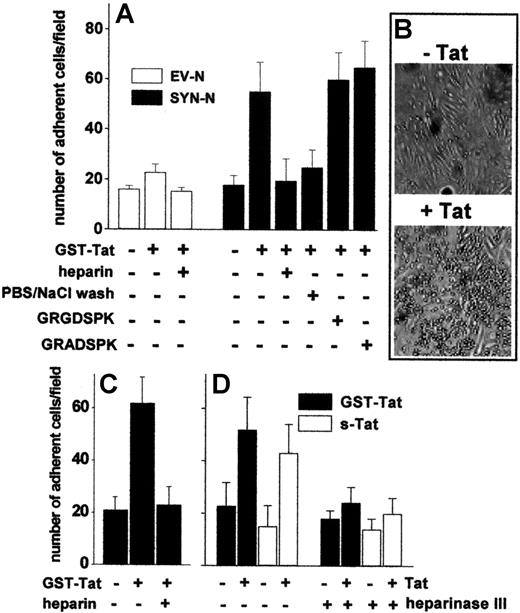
To assess the capacity of extracellular Tat to promote the adhesion of B-lymphoid cells to the endothelium, an EC monolayer was incubated for 2 hours at 37°C with GST-Tat, washed with PBS to remove the unbound protein, and evaluated for its ability to sustain EV-NC or SYN-NC adhesion. Pretreatment with GST-Tat caused a dose-dependent increase in the capacity of ECs to mediate the adhesion of SYN-NCs. Similar results were obtained with an s-Tat protein devoid of the GST moiety (Figure 3A-B, supplemental Figure 2C). In contrast, EV-NCs adhered poorly to both naive and GST-Tat–treated endothelium. A significant increase in SYN-NC adhesion was observed also when GST-Tat was added to a fixed EC monolayer (Figure 3C), ruling out the possibility that a de novo synthesis of cell-adhesive molecule(s) may mediate this effect. In keeping with the ability of extracellular Tat to accumulate on the EC surface by binding to HSPGs (see Figure 1C), the adhesion of SYN-NCs to GST-Tat–treated EC monolayers was prevented by removing bound extracellular Tat with a PBS/NaCl wash or by competition with free heparin, but not with the integrin antagonist GRGDSPK peptide or its inactive GRADSPK mutant (Figure 3A). In addition, the capacity of GST-Tat to promote SYN-NC adhesion to ECs was abolished when the EC monolayer was digested with heparinase III (Figure 3D). These observations indicate that extracellular Tat bound to HSPGs expressed on the EC surface mediates the adhesion of HSPG-bearing Namalwa cells, but not of HSPG-deficient cells.
Tat tethered to the EC surface promotes Namalwa cell adhesion. Namalwa cells were allowed to adhere to naive or Tat-coated living (A,B,D) or fixed (C) GM7373 EC monolayers in the presence of heparin (73 nM), of GRGDSPK or GRADSPK peptides (10 μΜ), or after a wash with PBS/NaCl. (D) GM7373 EC monolayers were left untreated or treated with heparinase III (10 mU/mL), washed, further incubated with GST-Tat or s-Tat (550 nM), and assayed for their capacity to promote SYN-NC adhesion. Each point is the mean ± SEM of 4 to 7 duplicate determinations. (B) Microphotographs of SYN-NCs adherent to naive (−Tat) or Tat-coated (+Tat) GM7373 EC monolayer.
Tat tethered to the EC surface promotes Namalwa cell adhesion. Namalwa cells were allowed to adhere to naive or Tat-coated living (A,B,D) or fixed (C) GM7373 EC monolayers in the presence of heparin (73 nM), of GRGDSPK or GRADSPK peptides (10 μΜ), or after a wash with PBS/NaCl. (D) GM7373 EC monolayers were left untreated or treated with heparinase III (10 mU/mL), washed, further incubated with GST-Tat or s-Tat (550 nM), and assayed for their capacity to promote SYN-NC adhesion. Each point is the mean ± SEM of 4 to 7 duplicate determinations. (B) Microphotographs of SYN-NCs adherent to naive (−Tat) or Tat-coated (+Tat) GM7373 EC monolayer.
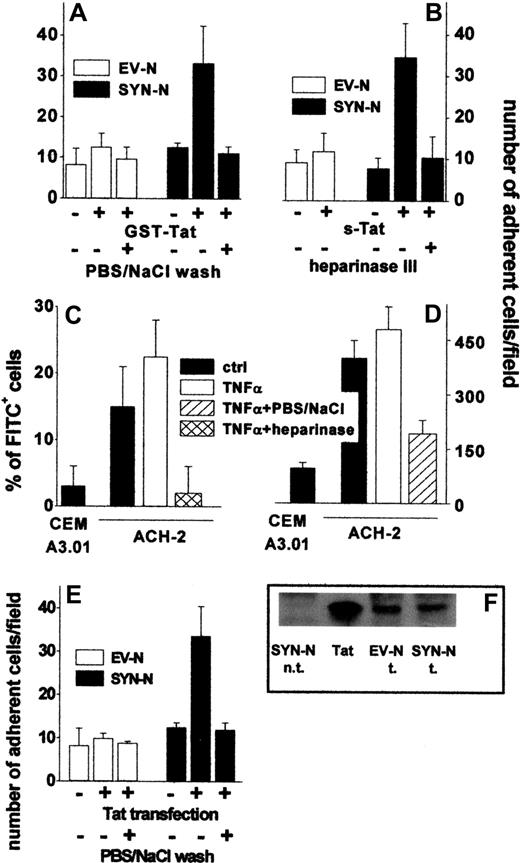
To assess whether extracellular Tat is able to sustain EC-Namalwa cell interaction also when tethered on the B-lymphoid cell surface, EV-NCs and SYN-NCs were preincubated with GST-Tat, washed to remove the unbound protein, and evaluated for their adhesion to an untreated EC monolayer. As shown in Figure 4A, preincubation with GST-Tat significantly increased the adhesion of SYN-NCs, but not EV-NCs, to a “naive” EC monolayer. Again, a PBS/NaCl wash (Figure 4A), as well as cell-surface SYN-NC digestion with heparinase III (Figure 4B), prevented the adhesion of Tat-pretreated SYN-NCs to “naive” ECs. Similar results were obtained using s-Tat instead of the GST-Tat fusion protein (Figure 4B). It should be pointed out that even a very short exposure of SYN-NCs to GST-Tat (10 minutes) and even the addition of GST-Tat to the adhesion medium were sufficient to allow Tat association to the surface of SYN-NCs and their consequent adhesion to the endothelium. At variance, no cell-cell interaction was observed after stimulation with GST-Tat for 2 hours at 37°C followed by its complete removal by PBS/NaCl wash (supplemental Figure 2B).
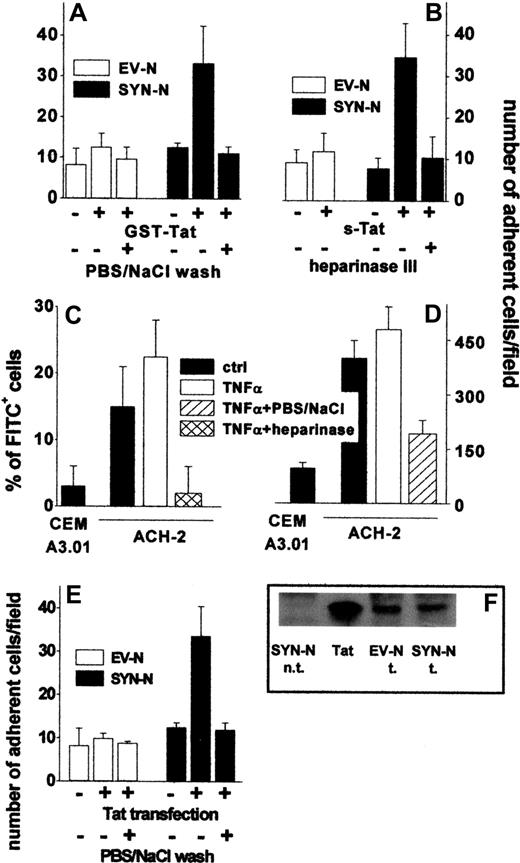
Tat tethered to the surface of HIV+ ACH-2 T lymphocytes and Namalwa cells promotes their adhesion to ECs. (A) Namalwa cells were preincubated for 2 hours with GST-Tat (550 nM) and washed with PBS or with PBS/NaCl. (B) EV-NCs and SYN-NCs were pretreated with heparinase III (10 mU/mL), incubated for 2 hours with s-Tat (550 nM), and washed with PBS. (C) ACH-2 or CEM A3.01 cells were left untreated or subjected to heparinase III treatment (20 mU/mL) and then analyzed by cytofluorimetry using a polycolonal FITC-coupled anti-Tat antibody. (D) The same cells were washed with PBS or with PBS/NaCl and allowed to adhere to naive EC monolayer. (E) Namalwa cells transfected with a cDNA encoding for Tat were washed with PBS or PBS/NaCl. Then, cells were allowed to adhere to naive EC monolayer and adherent lymphocytes counted. (F) Western blot analysis with anti-Tat antibodies of Tat-transfected Namalwa cells (600 000 cells). Tat indicates s-Tat (17 pmol); n.t., not transfected; and t., transfected.
Tat tethered to the surface of HIV+ ACH-2 T lymphocytes and Namalwa cells promotes their adhesion to ECs. (A) Namalwa cells were preincubated for 2 hours with GST-Tat (550 nM) and washed with PBS or with PBS/NaCl. (B) EV-NCs and SYN-NCs were pretreated with heparinase III (10 mU/mL), incubated for 2 hours with s-Tat (550 nM), and washed with PBS. (C) ACH-2 or CEM A3.01 cells were left untreated or subjected to heparinase III treatment (20 mU/mL) and then analyzed by cytofluorimetry using a polycolonal FITC-coupled anti-Tat antibody. (D) The same cells were washed with PBS or with PBS/NaCl and allowed to adhere to naive EC monolayer. (E) Namalwa cells transfected with a cDNA encoding for Tat were washed with PBS or PBS/NaCl. Then, cells were allowed to adhere to naive EC monolayer and adherent lymphocytes counted. (F) Western blot analysis with anti-Tat antibodies of Tat-transfected Namalwa cells (600 000 cells). Tat indicates s-Tat (17 pmol); n.t., not transfected; and t., transfected.
In addition, Tat-dependent SYN-NC adhesion to EC monolayers was not affected when the preincubation with GST-Tat was performed at 4°C or in the presence of the protein synthesis inhibitor cycloheximide or when adhesion to the EC monolayer was allowed to occur at 4°C, indicating that the de novo synthesis of adhesive molecules is not involved in the process (data not shown).
In vivo, HIV+ lymphocytes synthesize and secrete Tat that remains associated to the HSPGs of the cell surface.3 Accordingly, cytofluorimetric analysis demonstrated that Tat was present on the surface of chronically HIV-infected ACH-2 T-lymphocytes both in the absence and in the presence of TNF-α activation, but not on the surface of the uninfected CEM A3.01 cell counterpart. Tat associated to the surface of ACH-2 cells could be completely removed by heparinase III treatment, demonstrating its association to cell-surface HSPGs (Figure 4C). Accordingly, ACH-2 cells adhered to endothelium more efficiently than CEM A3.01 cells, and their adhesion was prevented by a PBS/NaCl wash (Figure 4D).
To further confirm the capacity of endogenously produced Tat protein to mediate cell-cell interaction, EV-NCs and SYN-NCs were permanently transfected with an expression vector encoding for native Tat protein and then assayed for their adhesion to a “naive” endothelium. Despite the similar levels of Tat protein expression (Figure 4F), SYN-NC, but not EV-NC transfectants, showed an increase in their ability to adhere to endothelium (Figure 4E). In addition, adhesion was prevented when transfected lymphocytes were washed with PBS/NaCl before being seeded on the endothelium (Figure 4E).
In conclusion, exogenously added and/or endogenously produced Tat protein can bind to B-lymphoid cells and HIV+ T lymphocytes when these express HSPGs on their surface. Once bound to lymphocytes, extracellular Tat mediates lymphocyte adhesion to EC surface HSPGs, eventually promoting a heterotypic cell-cell interaction.
HSPG-mediated B-lymphoid-EC interaction requires Tat homodimerization
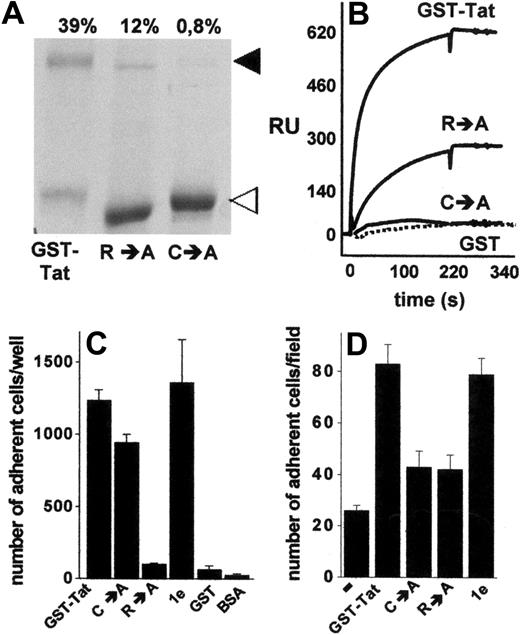
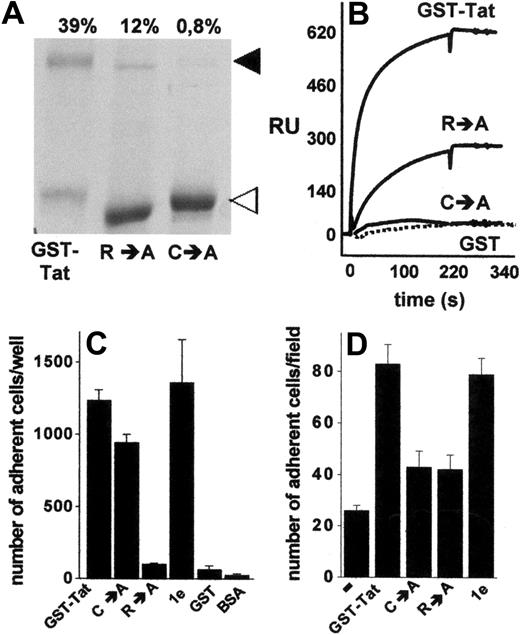
The data shown in Figures 3 and 4 indicate that extracellular Tat mediates cell-cell interaction via a simultaneous binding to HSPGs expressed on the surface of both cell types. The presence of a single heparin-binding site has been demonstrated in Tat protein.22 On the other hand, Tat has the tendency to form stable homodimers,36,37 a property that depends on a cysteine-rich domain distinct from the basic domain involved in heparin interaction.36 Thus, we investigated the possibility that B-lymphoid–EC interaction requires Tat homodimerization, each Tat monomer being involved in a mutually exclusive binding with one of the 2 cell types. To this purpose, we used various Tat muteins characterized by a different capacity to form homodimers or to interact with heparin. Compared with GST-Tat, the GST-TatC→A mutant did not form homodimers, whereas the GST-TatR→A mutant retained a significant, albeit reduced, ability to undergo homodimerization, as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under nonreducing conditions (Figure 5A). Tat homodimers were dissociated by boiling the sample in the presence of β-mercaptoethanol or dithiothreitol (data not shown), thus confirming the role of cysteine bridges in Tat homodimerization. To confirm the capacity of Tat to self-interact, we exploited surface SPR technology. As shown in Figure 5B, s-Tat immobilized to a BIAcore sensor chip was able to bind GST-Tat and, to a lesser extent, GST-TatR→A, but not GST-TatC→A and GST devoid of the Tat moiety (Figure 5B). Interestingly, the kinetic parameters and affinity of s-Tat/GST-TatR→A binding are comparable with those calculated for s-Tat/GST-Tat and s-Tat/s-Tat interactions (supplemental Table 2). Taken together, these data demonstrate the ability of Tat to form homodimers and that this self-interaction requires the cysteine-rich domain but not the heparin-binding basic domain of the protein. Relevant to this point, in keeping with their differential heparin-binding capacity, immobilized GST-TatC→A, but not immobilized GST-TatR→A, retained the ability to promote SYN-NC adhesion. It should be noticed that the GST-Tat-1e mutant (which is characterized by the deletion of the integrin-binding RGD motif but still binds heparin) promoted SYN-NC adhesion, similarly to GST-Tat. No SYN-NC adhesion was instead observed to immobilized GST devoid of the Tat moiety or to BSA (Figure 5C).
Homodimerization of Tat is required to mediate Namalwa cell adhesion to the endothelium. (A) Aliquots (5 μg) of the indicated recombinant forms of GST-Tat were analyzed in nonreducing 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by blue Coomassie staining. ◀ and ◁ point to the 72-kDa homodimer and to the monomeric form of Tat, respectively. (Top of panel) Percentage of Tat that underwent dimerization as calculated by densitometric measurement of the bands. (B) Overlay of blank-subtracted sensograms showing the binding of the indicated proteins (500 nM) injected over a BIAcore sensor chip coated with s-Tat. The SPR signal was expressed in terms of resonance units (RU). (C) SYN-NCs were allowed to adhere to plastic coated with the indicated proteins (550 nM). Then, adherent lymphocytes were counted. Each point is the mean ± SEM of 3 to 8 duplicate determinations. (D) GM7373 EC monolayers were preincubated without (−) or with the indicated proteins (550 nM), washed to remove unbound proteins, and assayed for their capacity to promote SYN-NC adhesion.
Homodimerization of Tat is required to mediate Namalwa cell adhesion to the endothelium. (A) Aliquots (5 μg) of the indicated recombinant forms of GST-Tat were analyzed in nonreducing 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by blue Coomassie staining. ◀ and ◁ point to the 72-kDa homodimer and to the monomeric form of Tat, respectively. (Top of panel) Percentage of Tat that underwent dimerization as calculated by densitometric measurement of the bands. (B) Overlay of blank-subtracted sensograms showing the binding of the indicated proteins (500 nM) injected over a BIAcore sensor chip coated with s-Tat. The SPR signal was expressed in terms of resonance units (RU). (C) SYN-NCs were allowed to adhere to plastic coated with the indicated proteins (550 nM). Then, adherent lymphocytes were counted. Each point is the mean ± SEM of 3 to 8 duplicate determinations. (D) GM7373 EC monolayers were preincubated without (−) or with the indicated proteins (550 nM), washed to remove unbound proteins, and assayed for their capacity to promote SYN-NC adhesion.
On these bases, Tat muteins were evaluated for their ability to promote the interaction of SYN-NCs with an EC monolayer. As anticipated, because of its incapacity to interact with HSPGs22 and to induce SYN-NC adhesion when immobilized to plastic, GST-TatR→A mutant did not promote B-lymphoid–EC interaction (Figure 5D). The ability to promote B-lymphoid–EC interaction was also lost in the GST-TatC→A mutant (Figure 5D), despite that it retained its Namalwa cell-adhesive capacity when assayed on plastic (Figure 5C). On the contrary, GST-Tat-1e mutant retained the capacity to promote B-lymphoid–EC interaction (Figure 5D), thus confirming the lack of a role for integrin engagement in this phenomenon. These data tightly associate the capacity of extracellular Tat to form homodimers to its capacity to promote HSPG-dependent adhesion of B-lymphoid cells to the endothelium.
Tat induces trans-endothelial migration of B-lymphoid cells
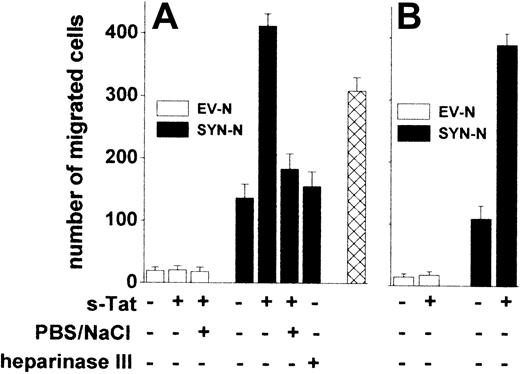
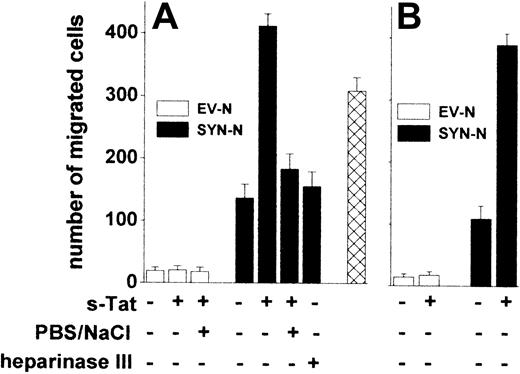
The adhesion of lymphocytes to the endothelium is a prerequisite for their extravasation during inflammation,38 AIDS progression,39 and lymphomas.40 We investigated whether Tat tethered on the surface of B-lymphoid cells was able to promote their trans-migration across a monolayer of HACECs. To this purpose, EV-NCs and SYN-NCs were preincubated with s-Tat, washed with PBS to remove the unbound protein, and evaluated for their migration across an EC monolayer in response to the chemokine CXCL12.41 Preliminary experiments demonstrated that CXCL12 exerts a similar chemotactic response in both cell types when tested in a Boyden chamber assay (data not shown). Preincubation with s-Tat significantly increased the capacity of SYN-NCs, but not of EV-NCs, to trans-migrate across the EC monolayer when stimulated by CXCL12 (Figure 6A). Again, in keeping with the HSPG dependency of this effect, a PBS/NaCl wash, as well as heparinase III pretreatment, inhibited SYN-NC trans-migration. It must be pointed out that Tat retains the capacity to stimulate trans-endothelial migration of SYN-NCs but not of EV-NCs also when tethered to the surface of ECs rather than of lymphoid cells (Figure 6B).
Tat tethered to the surface of Namalwa cells or of endothelium promotes lymphoid cell trans-endothelial migration. Namalwa cells (A) or HACEC monolayers (B) were left untreated or pretreated with heparinase III and/or preincubated with s-Tat (550 nM). After washing with PBS or PBS/NaCl, Namalwa cells were assayed for migration across HACEC monolayer in response to CXCL12 (25 nM). Alternatively, SYN-NCs were subjected to the PBS/NaCl wash before preincubation with s-Tat and assayed for transmigration ( ). Each point is the mean ± SEM of 2 or 3 duplicate determinations.
). Each point is the mean ± SEM of 2 or 3 duplicate determinations.
Tat tethered to the surface of Namalwa cells or of endothelium promotes lymphoid cell trans-endothelial migration. Namalwa cells (A) or HACEC monolayers (B) were left untreated or pretreated with heparinase III and/or preincubated with s-Tat (550 nM). After washing with PBS or PBS/NaCl, Namalwa cells were assayed for migration across HACEC monolayer in response to CXCL12 (25 nM). Alternatively, SYN-NCs were subjected to the PBS/NaCl wash before preincubation with s-Tat and assayed for transmigration ( ). Each point is the mean ± SEM of 2 or 3 duplicate determinations.
). Each point is the mean ± SEM of 2 or 3 duplicate determinations.
Peripheral blood monocytic cells (PBMCs) isolated from healthy donors express surface HSPGs (supplemental Figure 3A). Similarly to SYN-NCs, also PBMCs increased their migration across a “naive” HACEC monolayer in response to the chemokine CCL5 when preincubated with s-Tat. In addition, a PBS/NaCl wash of Tat-pretreated PBMCs prevented this effect (supplemental Figure 3B). These data rule out the possibility that the ability of extracellular Tat to mediate EC interaction and trans-migration is limited to syndecan-1 transfectants.
Tat tethered to B-lymphoid cells favors their extravasation in the zebrafish embryos
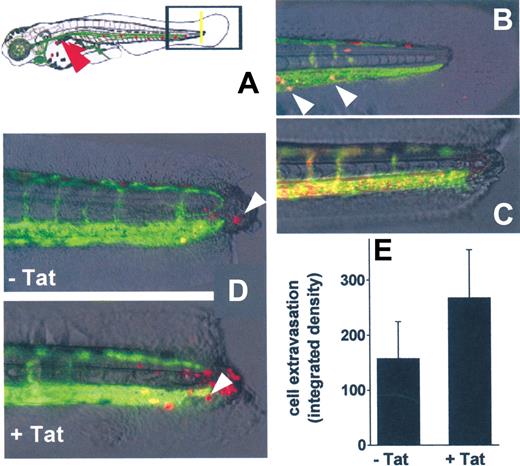
To investigate whether Tat engaged on the surface of B-lymphoid cells is able to promote cell extravasation in vivo, we used a zebrafish embryo model of tail transection (Figure 7A) already used to investigate inflammation-mediated neutrophil extravasation.42 To better visualize cell extravasation, we used VEGFR2:G-RCFP zebrafish embryos, in which GFP expression is driven by the endothelial-specific VEGFR2 promoter,31 and SYN-NCs preloaded with the red fluorescent dye PKH26.43
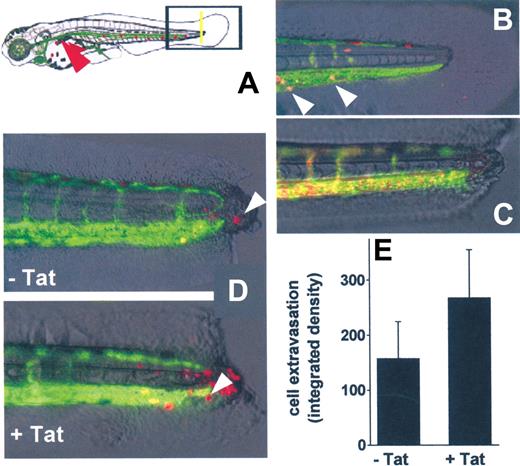
In vivo SYN-NC extravasation in a zebrafish embryo tail transection model. (A) Schematic representation of the tail transection model in VEGFR2:G-RCFP transgenic zebrafish embryos transplanted with red-stained SYN-NCs. Yellow line represents the site of the cut of the tail; red arrowhead points to the site of cell injection. (B) Detail of an intact tail of a zebrafish embryo 12 hours after injection of red-stained SYN-NCs. White arrowheads point to red-stained cells inside the GFP+ blood vessels. (C) Detail of a cut tail of a zebrafish embryo injected with R-HMW dextran showing no vessel leakiness. (D) Details of cut tails of zebrafish embryos injected with naive (−Tat) or Tat-coated (+Tat) red-stained SYN-NCs. White arrowheads point to extravasated cells. (E) Quantification of SYN-NC extravasation by computerized image analysis. Each point is the mean ± SEM of 6 or 7 embryos from 2 independent experiments.
In vivo SYN-NC extravasation in a zebrafish embryo tail transection model. (A) Schematic representation of the tail transection model in VEGFR2:G-RCFP transgenic zebrafish embryos transplanted with red-stained SYN-NCs. Yellow line represents the site of the cut of the tail; red arrowhead points to the site of cell injection. (B) Detail of an intact tail of a zebrafish embryo 12 hours after injection of red-stained SYN-NCs. White arrowheads point to red-stained cells inside the GFP+ blood vessels. (C) Detail of a cut tail of a zebrafish embryo injected with R-HMW dextran showing no vessel leakiness. (D) Details of cut tails of zebrafish embryos injected with naive (−Tat) or Tat-coated (+Tat) red-stained SYN-NCs. White arrowheads point to extravasated cells. (E) Quantification of SYN-NC extravasation by computerized image analysis. Each point is the mean ± SEM of 6 or 7 embryos from 2 independent experiments.
In preliminary experiments, PKH26-loaded SYN-NCs were injected into the blood flow of intact zebrafish embryos. Twenty-four hours after injection, SYN-NCs were still viable and circulate inside the GFP+ vessels (Figure 7B). In addition, microangiography of the zebrafish embryo vasculature performed 6 hours after tail transection did not show any extravasation of injected R-HMW dextran in the injured area, demonstrating the integrity of blood vessels (Figure 7C).
On this basis, untreated or Tat-pretreated SYN-NCs were injected into the blood flow of zebrafish embryos 6 hours after tail transection and evaluated for their extravasation at the site of injury. As assessed by epifluorescence microscopy followed by computerized image analysis of the extravasated cells, Tat pretreatment caused a significant increase of the capacity of SYN-NCs to extravasate, compared with naive cells (Figure 7D-E).
Discussion
During HIV infection, the abnormal extravasation of HIV+ lymphocytes contributes to the dissemination of the virus39 and to the progression of AIDS-associated leukemia/lymphomas.40 Here we describe, for the first time, the capacity of extracellular Tat protein to promote B-lymphoid cell adhesion to the endothelium and extravasation via heterotypic mechanism of cell-cell interaction. This is the result of the ability of Tat homodimers to engage the HSPGs expressed on the cell surface of both cell types, leading to the formation of a HSPG/Tat-Tat/HSPG quaternary complex that physically links HSPG-bearing lymphoid cell to the endothelium (supplemental Figure 4). This mechanism is independent of de novo synthesis of adhesive molecules by ECs and/or by lymphocytes, being observed also when Tat is added to a fixed EC monolayer or when B-lymphoid cells are treated with Tat at 4°C or in the presence of cycloheximide.
Extracellular Tat binds various cell surface receptors (integrins, KDR, and HSPGs)21 via different domains. Here, much evidence demonstrates that the B-lymphoid–EC interaction is selectively mediated by the interaction of Tat with HSPGs expressed by the 2 cells: (1) B-lymphoid SYN-NCs, but not EV-NCs, adhere to ECs after Tat incubation, although both clones retain the capacity to up-regulate the expression of cytokines (TNF-β and CCL3) when stimulated by extracellular Tat (data not shown). (2) A PBS/NaCl wash, known to remove cationic proteins (including Tat) from cell surface HSPGs,23 prevents Tat-mediated lymphocyte adhesion to EC as well as trans-endothelial migration. Notably, the PBS/NaCl wash per se does not affect trans-endothelial lymphocyte migration (Figure 6A). (3) The HSPG-antagonists heparin, HS, and suramin, as well as HSPG digestion by heparinases, inhibit Tat binding to the EC surface and/or SYN-NC adhesion to immobilized Tat. (4) Experiments performed with recombinant mutated forms or synthetic peptides of Tat or with the integrin-antagonist peptide GRGDSPK demonstrate that the adhesion of SYN-NCs to immobilized Tat requires the basic, heparin-binding domain of Tat22 but not its integrin-recognition RGD motif.44 Similarly, neutralizing anti-KDR antibodies do not affect the binding of b-Tat to ECs (data not shown). Noticeably, previous observations had shown the capacity of extracellular Tat to increase EC adhesion and trans-endothelial migration of Burkitt lymphoma cells from AIDS patients, even though these cells do not express αvβ3, α5β1, αvβ5 integrins, or KDR.39
Tat forms stable homodimers,36,37 a property that depends on a cysteine-rich domain distinct from the basic domain involved in heparin interaction.36,45 Here we have shown that site-directed mutagenesis of Tat protein in either one of the 2 regions destroys the ability of Tat to mediate B-lymphoid–EC interaction. Thus, the cysteine-rich and the basic domains of Tat appear to cooperate in mediating the HSPG-dependent lymphocyte adhesion to the endothelium, the former region promoting the assembly of Tat homodimers that will engage the 2 facing cells by binding cell surface HSPGs via the basic domains of the 2 Tat protomers (supplemental Figure 4). Interestingly, Tat-Tat complexes dissociate very slowly (supplemental Table 2), indicating a very stable interaction between the 2 Tat molecules. Further studies are required to formally demonstrate the formation of HSPG+Tat-Tat, HSPG quaternary complexes during EC–B-lymphoid cell interaction.
Previous observations had shown that Tat activates HIV+ lymphocytes by an autocrine mechanism of action,11 leading to overexpression of various cell adhesive molecules.46 In addition, Tat released by HIV+ cells acts paracrinally on ECs, causing the damage of capillaries,8 the increase of their permeability, the activation of a proinflammatory program,47 and the overexpression of cell-adhesive molecules on the EC surface.48 These effects will eventually promote lymphocyte/leukocyte adhesion to the endothelium and extravasation.39 Our data extend these observations by demonstrating the existence of an additional, direct mechanism of action of extracellular Tat that physically links the 2 cell types in a metabolically independent manner, the only prerequisite being the presence of cell surface HSPGs on both cell types. All these mechanisms may concur to the observed increase in the ability of Tat-pretreated SYN-NCs to extravasate in a zebrafish embryo model of tail transection.
The Tat-dependent, HSPG-mediated EC interaction may contribute to the abnormal extravasation of HIV+ lymphocytes/leukocytes that occurs during HIV infection and that promotes HIV dissemination39,48 and progression of leukemia/lymphomas in AIDS patients.40 Here we show that significant levels of HSPG-associated Tat protein are present in chronically HIV-infected ACH-2 T lymphocytes also in the absence of TNF-α activation. Accordingly, low but significant levels of viral transcription are detectable in nonactivated cells (data not shown). Thus, extracellular Tat may contribute to EC adhesion and extravasation of latently HIV-infected lymphocytes. On the other hand, once released by HIV+ cells in the lymph nodes, Tat may remain entrapped in the HSPG-rich EC surface of high endothelial venules where it may promote extravasation of uninfected lymphocytes in HIV+ tissue, a process that may contribute to CD4+ cell depletion during AIDS progression (Chen et al49 and references therein). Indeed, we have observed that extracellular Tat mediates EC-lymphoid cell interaction either when produced endogenously by Tat-transfected SYN-NCs or when added exogenously as a recombinant protein.
When produced and released by HIV+ cells, extracellular Tat readily associates to the surface of producing cells and retains the capacity to bind HIV-1 gp120 protein, enhancing virus attachment and entry into the cell.3 In addition, the expression of syndecan-1 has been proposed as a marker for the diagnosis of AIDS-related lymphomas.20 Thus, the Tat-dependent, HSPG-mediated B-lymphoid–EC interaction may represent a target for pharmacologic interventions in AIDS and AIDS-associated pathologies. Preliminary observations showed that heparin-mimicking, synthetic sulfonic acid polymers act as multitarget compounds by acting as extracellular Tat and gp120 antagonists50 and by inhibiting Tat-mediated EC/lymphocyte interaction (data not shown). Further studies are required to explore this hypothesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Paola Paioli and Dr Paola Camossi for their skillful technical assistance, Dr Antonella Bugatti for performing BIACORE experiments, and Prof Guido Poli for helpful suggestions and discussions.
This work was supported by the Istituto Superiore di Sanita (AIDS Project) and Associazione Italiana per la Ricerca sul Cancro (M.R., M.G.), Istituto Superiore di Sanita (Oncotechnological Program), Associazione Italiana per la Ricerca sul Cancro, Ministero dell'Istruzione, dell'Universita e della Ricerca (Centro di Eccellenza IDET, Cofin projects), Fondazione Berlucchi, and NOBEL Project Cariplo (M.P.).
Authorship
Contribution: C.U., L.C., S.N., and S.F. designed and performed research; S.N., M.G., A.C., and M.A. contributed analytical tools; G.D. contributed analytical tools and new reagents; M.P. designed research and wrote the manuscript; and M.R. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Rusnati, General Pathology & Immunology, Department of Biomedical Sciences and Biotechnology, viale Europa 11, 25123 Brescia, Italy; e-mail: rusnati@med.unibs.it.


 ). Each point is the mean ± SEM of 2 or 3 duplicate determinations.
). Each point is the mean ± SEM of 2 or 3 duplicate determinations.