Abstract
Artificial Toll-like receptor 7/8 (TLR7/8) ligands can endow plasmacytoid dendritic cells (pDCs) with tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–dependent lytic properties. Keeping in mind that ssRNA serves as natural TLR7/8 ligand, we searched for TRAIL-expressing cells in persons infected with HIV and identified TRAIL+ pDCs in HIV-1 viremic persons, but not in nonviremic and healthy persons. TRAIL expression on pDCs was directly correlated with individual viral loads. Conversely, HIV-1 viremia was found to be associated with the up-regulation of the apoptosis-transmitting receptor TRAIL R1 on activated CD4+ T cells. As a consequence, the latter became susceptible to TRAIL-dependent pDC-mediated killing. In contrast, initiation of antiretroviral therapy led to the up-regulation of apoptosis-inhibiting TRAIL R4 on CD4+ T cells, which subsequently became resistant against pDC-mediated cellular injury. Definition of pDCs as killers of CD4+ T cells implies a new mechanism of disease progression in HIV infection.
Introduction
Persistent immune activation and loss of CD4+ T cells are hallmarks of chronic, HIV-1 viremic infection.1 Different hypotheses exist to explain the phenomenon of CD4+ T-cell depletion in this disease. The direct cytopathic effect of HIV-1 has been considered to be primarily responsible in this regard.2 An argument against this theory is the sharp decline of CD4+ T cells observed in the early period of HIV-1 infection whereby only a low percentage of CD4+ T cells are infected with the virus.3,4 It has therefore been suggested that HIV-induced indirect mechanisms (eg, T-cell cytotoxicity,5 apoptosis of uninfected bystander cells6 by CXCR4-dependent autophagy7 ) result in the destruction of both infected and noninfected CD4+ T cells.
Several recent reports imply a role for tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) in the induction of CD4+ T-cell apoptosis in persons infected with HIV-1, because (1) plasma levels of TRAIL are elevated in such patients,8 (2) HIV-1–exposed CD4+ T cells are sensitive to TRAIL-mediated apoptosis,9,10 and (3) the death receptor TRAIL R2 is expressed on CD4+ T cells of patients infected with HIV-1 and might be involved in apoptosis of such cells.11 In this regard it is noteworthy that TRAIL, a TNF superfamily member, ligates 2 types of receptors: death receptors triggering TRAIL-induced apoptosis (TRAIL R1 and TRAIL R2) and decoy receptors that possibly inhibit this pathway (TRAIL R3 and TRAIL R4).12 Binding of TRAIL to the death-promoting receptors TRAIL R1 or TRAIL R2 results in receptor oligomerization and, finally, in apoptosis by caspase 3 activation.13
We have reported that plasmacytoid dendritic cells (pDCs), activated by artificial Toll-like receptor 7/8 (TLR7/8) ligands in vitro, can induce TRAIL-dependent apoptosis in permissive tumor cell lines.14 A similar phenomenon was observed not only with certain synthetic TLR7 and TLR9 agonists15 but also with natural TLR7 ligands, such as influenza virus15 and HIV.16,17 TLR7 ligands are potent inducers of IFN-α, and autologous IFN-α production can lead to surface expression of TRAIL on pDCs, endowing them to induce apoptosis in a TRAIL-sensitive tumor cell line.15,18
Here, we sought to explore the in vivo relevance of this in vitro phenomenon by searching for and by phenotypically and functionally characterizing TRAIL-expressing cells in persons infected with HIV-1.
Methods
Patients and tissue material
Blood samples for phenotypic analysis were obtained from 3 different HIV-infected patient groups based on their treatment status and viral load (VL), as well as from patients infected with hepatitis C virus (HCV), patients infected with varicella zoster virus (VZV), and from healthy controls. The following cohorts were investigated: (1) persons with HIV-1 naive to antiretroviral therapy (ART) with greater than 5 × 104 RNA copies/mL plasma (12 men, 8 women; median age, 35.8 years; range, 25-56 years; median CD4+ cell count, 358.4 cells/μL; range, 16-795 cells/μL; median VL: 194 829 copies/mL; range, 652-645 654 copies/mL); 13 patients thereof were classified as noncontrollers with greater than 105 RNA copies/mL plasma (9 men, 4 women; median age, 37.8 years; range, 25-56 years; median CD4+ cell count, 320.4 cells/μL; range, 16-795 cells/μL; median VL, 260 374 copies/mL; range, 107 152-645 654 copies/mL); (2) persons with HIV-1 naive to ART with less than 104 RNA copies/mL plasma (viremic controllers; 5 men, 5 women; median age, 32.8 years; range, 20-42 years; median CD4+ cell count, 535.6 cells/μL; range, 442-726 cells/μL; median VL, 3513 copies/mL; range, 107-6761 copies/mL); (3) patients with HIV-1 on combination ART (cART) with a stable VL less than 50 RNA copies/mL plasma for more than 6 months (7 men, 4 women; median age, 47.9 years; range, 34-59 years; median CD4+ cell count, 543.8 cells/μL; range, 196-883 cells/μL); (4) persons chronically infected with HCV naive to therapy (4 men, 6 women; median age, 43 years; range, 32-63 years); and (5) persons with a primary VZV infection as determined by the clinical picture and positive lesional polymerase chain reaction (PCR) testing for VZV (0 men, 4 women; median age, 28 years; range, 18-39 years). In 6 HIV-1 viremic patients (3 men, 3 women; median age, 42.2 years; range, 31-51 years), pDCs were analyzed before and after the initial 8 to 12 weeks of cART. Coinfections of the study population with HIV and HCV or other acute infections (eg, syphilis, hepatitis B, pneumonia) were excluded by clinical and serologic investigation. HIV-1– and HCV-seronegative healthy adult volunteers (n = 13) served as controls. For cytotoxicity assays, cell sorting was performed with peripheral blood mononuclear cells (PBMCs) of (1) persons infected with HIV-1 naive to ART with greater than 5 × 104 RNA copies/mL plasma, (2) patients with HIV-1 on cART with a stable VL less than 50 RNA copies/mL plasma, and (3) healthy HIV-1– and HCV-seronegative peripheral blood samples purchased as buffy coats from the Vienna Red Cross Center. Biopsies of enlarged lymph nodes were taken from 8 viremic patients infected with HIV-1 (6 men, 2 women; median age, 40.3 years; range, 30-61 years; median CD4+ cell count, 312.1 cells/μL; range, 45-567 cells/μL; median VL, 158 229 copies/mL; range, 105 499-220 086 copies/mL) and 5 persons not infected with HIV for controls (3 men, 2 women; median age, 41 years; range, 28-54 years). These biopsies were performed because of suspected infections and/or neoplasms but failed to disclose such pathologies.
All study subjects participated voluntarily and gave informed consent in accordance with the Declaration of Helsinki; the study was approved by the Ethics Committee of the Medical University of Vienna (EK 719/2007).
Flow cytometry
TRAIL and its receptors were analyzed on PBMC subsets by fluorescence-activated cell sorter (FACS) stainings from the patient cohorts described in the previous section. Surface TRAIL, TRAIL R1, TRAIL R2, and TRAIL R3 expressions were visualized with anti-TRAIL (clone 75402; R&D Systems), anti-TRAIL R1 (clone 69036; R&D Systems), anti-TRAIL R2 (clone 71908; R&D Systems), or anti-TRAIL R3 (clone 90906; R&D Systems) mAbs after labeling the purified antibody with a protein labeling kit (FluoReporter Oregon Green 488; Invitrogen), according to the manufacturer's instructions. TRAIL R4 was detected by anti-TRAIL R4 PE (clone 104918; R&D Systems) staining. For analysis, TRAIL and TRAIL receptor stainings were adapted to matched isotype controls. The following antibodies were used: anti–BDCA-2 (CD303) FITC (clone AC144; Miltenyi Biotec); anti-CD123 PE (clone 9F5; BD Biosciences); anti–HLA-DR FITC, PerCp, APC (clone L243; BD Biosciences); anti-CD11c PE, APC (clone B-ly6; BD Biosciences); anti-CD11c FITC (clone BU15; AbD Serotec); anti-CD14 PerCP (clone MΦP9; BD Biosciences); anti-CD19 PerCP (clone SJ25C1; BD Biosciences); anti-CD56 PE-Cy5 (clone B159; BD Biosciences); anti-CD3 PerCP (clone SK-7; BD Biosciences); and anti-CD4 FITC, PE (clone SK3; BD Biosciences). The apoptosis rate was determined with an Annexin V kit (Bender MedSystems) and 7-amino-actinomycin D (7-AAD; BD PharMingen) according to the manufacturer's instructions. For FACS analysis, cells staining positive with the substituting isotype-matched IgG antibodies were always subtracted from the primary antibody-positive cell fraction.
Immunofluorescence staining
Paraffin sections from lymph nodes were used for immunofluorescence analysis after paraffin dewaxing. Double immunofluorescence staining procedures with biotinylated anti–BDCA-2 (CD303; clone 104C12.08; Dendritics) and purified anti–HLA-DR (clone L243; BD Biosciences), anti-CD45RA (clone L48; BD Biosciences), anti-CD123 (clone 9F5; BD Biosciences), or anti-CD80 FITC (clone MAB104; Beckman Coulter) mAbs were performed as previously described.14 Apoptotic cells were detected with a kit from Roche Molecular Biochemicals to visualize DNA breaks as TUNEL+ according to the manufacturer's guidelines and counterstained with purified anti-CD4 (clone 1F6; Novocastra Laboratories), anti-CD3 (clone UCHT1; DAKO), anti-TRAIL (clone 75402; R&D Systems), anti-TRAIL R1 (clone 69036; R&D Systems), or anti-TRAIL R2 (clone 71903; R&D Systems) mAbs. After visualization of purified antibody binding with rhodamine (TRITC)–conjugated goat anti–mouse IgG (Jackson ImmunoResearch), an anti–BDCA-2 (CD303) APC (clone AC144; Miltenyi Biotec), anti-CD14 APC (clone RM052; Beckman Coulter), or anti-CD3 APC (clone SK-7; BD Biosciences) mAb was applied to the sections. Negative controls were obtained in all staining experiments by substituting isotype-matched IgG or IgM (purified, APC-, or FITC-labeled) for the primary antibody. The evaluation of immunofluorescence results was performed as previously described.14
Cell preparations from peripheral blood
As depicted in “Patients and tissue material,” blood samples for cell-sorting experiments were obtained from persons infected with HIV-1 naive to ART with greater than 5 × 104 RNA copies/mL plasma, from patients with HIV-1 on cART with a stable VL less than 50 RNA copies/mL plasma, and from peripheral blood samples of healthy persons purchased from the Vienna Red Cross Center. pDCs and CD4+ T cells were isolated consecutively as follows: PBMCs were obtained by Ficoll-Paque density gradient centrifugation (Histopaque-1077; Sigma-Aldrich) and depleted of B cells, natural killer (NK) cells, hemopoietic stem cells, monocytes, myeloid DCs (mDCs), platelets, and erythrocytes by anti-CD19/CD56/CD34/CD14/CD11c/CD16/CD41/CD235a (3 μg/mL each) immunolabeling and anti–mouse IgG immunomagnetic depletion (MACS). The remaining cell fraction was divided into pDCs and CD4+ T cells by positive selection using anti-CD304 (BDCA-4) microbeads (MACS) and, thereafter, CD4 labeling and anti–mouse IgG1 magnetic beads (MACS). The purity of isolated pDCs and T cells was 90% to 99%, as determined by flow cytometric analysis (FACSCalibur; BD Biosciences) with either anti–BDCA-2 (CD303)/HLA-DR or anti-CD4/anti-CD3/anti-CD8 stainings. None of the other leukocyte subpopulations tested individually (CD8+ T cells, B cells, NK cells, and monocytes) accounted for greater than 1% of the sorted cell population. Viability of the cells was measured with trypan blue (Sigma-Aldrich) after sorting, and dead cells constituted less than 5%.
In another set of experiments (for Figure 4B), we isolated CD14+ monocytes, CD8+ T cells, CD56+ NK cells, and CD4+ T cells by consecutive immunolabeling with anti-CD14, anti-CD3, anti-CD4, and anti-CD56 and anti–mouse IgG immunomagnetic sorting after each individual labeling step (MACS; Miltenyi Biotec). Depletion of CD14+ monocytes was followed by positive selection of CD4+ T cells by CD4 labeling after depletion of pDCs. Thereafter, NK cells were isolated by CD56 labeling and CD8+ T cells by CD3 labeling. Purity and viability controls were performed as described previously.14
Stimulation of pDCs and T cells with HIV-1
Isolated pDCs and CD4+ T cells were cultured with noninfectious aldrithiol-2–treated (AT)–HIV-1/H9 CL.4 virus and AT H9 CL.4 microvesicles (National Institute for Biological Standards and Control) at 300 ng/mL p24CA equivalent for 12 hours.
Cytotoxicity assays
The ability of pDCs, monocytes, NK cells, and CD8+ T cells to exert cytotoxicity against T cells was assessed in a conventional 2-hour europium-TDA release assay (PerkinElmer), as previously described,14,19 according to the manufacturer's instructions. We used freshly isolated CD4+ T cells from different patient cohorts, CD4+ T cells isolated after 48-hour stimulation with 2 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich), and the HIV-infected and noninfected human T-cell line H9 (LGC Standards) as target cells. The activation status of T cells was checked by CD69 (clone FN50; BD PharMingen) FACS stainings. In a 96-well plate 5 × 103 target cells/well were incubated with pDCs from healthy controls, non–HIV-1 viremic, and HIV-1 viremic patients at different effector/target ratios (starting at 10:1) in duplicates. For inhibition of TRAIL-, TRAIL R1–, IFN-α–, and Fas ligand–dependent lysis, 5 μg/mL azide-free neutralizing anti-TRAIL (clone 75411; R&D Systems), anti-TRAIL R1 (clone B-N36; Abcam plc), anti–IFN-α (clone MMHA-1; PBL InterferonSource), anti-Fas ligand (clone NOK-1; BD Biosciences), and an IgG1 isotype (Sigma-Aldrich) as negative control, respectively, were added to effector cells.
Apoptosis rate in an ex vivo situation was determined by Annexin V/7-AAD FACS stainings of CD4+ T cells after 12-hour culture of PBMCs. Before culture pDCs, monocytes, or CD8+ T cells were removed by BDCA-4 beads, CD14 labeling plus anti–mouse IgG beads, and CD8 labeling plus anti–mouse IgG1 beads, respectively. For inhibition of different cytotoxicity pathways, we added 5 μg/mL neutralizing anti-TRAIL, anti–IFN-α, anti-Fas ligand mAbs, or IgG1 isotype control to PBMCs before culture. For inhibition of perforin-based cytotoxicity PBMCs were pretreated with 100nM concanamycin A (Sigma-Aldrich).
Statistical analysis
The significance of the differences in the expression patterns of TRAIL and TRAIL receptors was verified by the nonparametric Mann-Whitney U test. A P value of less than .05 was considered as statistically significant. The correlation of TRAIL-expressing pDCs with the individual CD4+ T cells and the VL was determined by the Pearson correlation coefficient.
Results
Up-regulation of TRAIL on pDCs from the peripheral blood of HIV-1 viremic patients
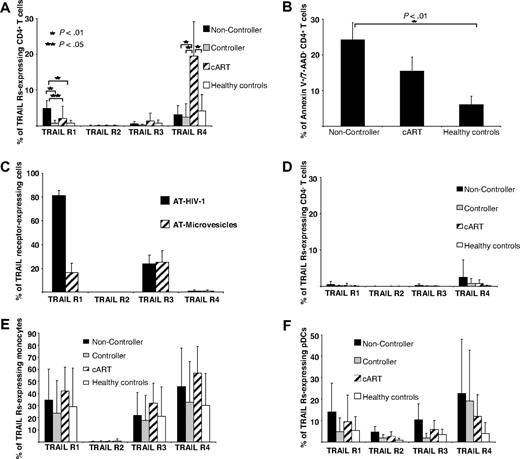
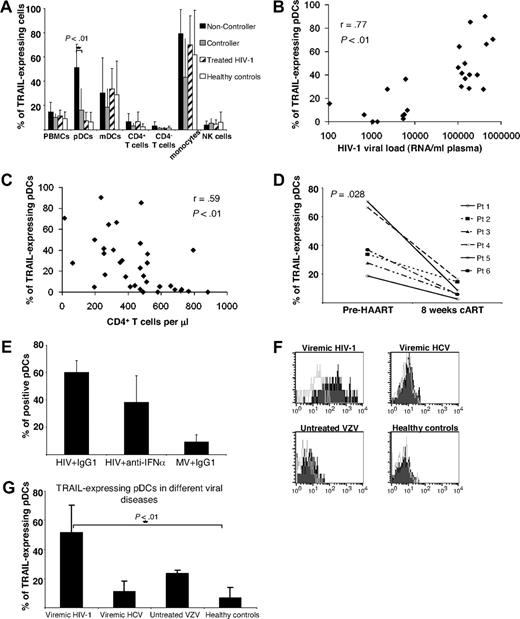
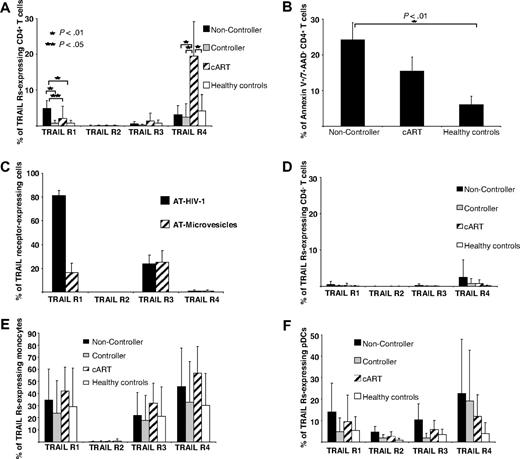
In a first series of experiments we determined TRAIL expression on bulk PBMCs of persons infected with HIV and healthy controls by flow cytometry. By doing so, we observed a trend to increased numbers of TRAIL-expressing cells in viremic patients infected with HIV-1 in contrast to cART-suppressed patients infected with HIV-1 or healthy volunteers (Figure 1A). To pursue this phenomenon further, we classified patients infected with HIV-1 into 3 categories according to different levels of plasma HIV-1 RNA: untreated patients with either HIV-1 VL (1) greater than 105 RNA copies/mL plasma (noncontrollers; n = 13) or (2) less than 104 RNA copies/mL plasma (viremic controllers; n = 10) and (3) patients with HIV-1 treated with cART (“nonviremic”; n = 11) and a stable VL less than 50 RNA copies/mL plasma for more than 6 months. When TRAIL expression was assessed on different cell types, significant differences between the groups were selectively observed on pDCs (Figure 1A). In noncontrollers these cells expressed TRAIL at significantly higher frequencies than those of controllers, cART recipients, or healthy controls (Figure 1A). In absolute numbers, peripheral blood pDCs in noncontroller patients with HIV occurred slightly less frequently than in the other groups studied, but these differences were not statistically significant (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast to pDCs, monocytes and mDCs from patients infected with HIV and healthy controls displayed TRAIL constitutively at similar rates (Figure 1A). CD4+ and CD4− T cells as well as NK cells expressed TRAIL at low rates only (Figure 1A) irrespective of HIV-1 VLs. Importantly, we found, at a high level of significance, the percentage of TRAIL-expressing pDCs to be positively correlated with the individual HIV-1 VLs (Figure 1B) and negatively with the patients' CD4+ cell counts (Figure 1C).
Up-regulated TRAIL surface expression on pDCs from HIV-1 viremic patients. (A) Percentage of various TRAIL+ cell types in subgroups of patients infected with HIV-1. Healthy HIV-1–seronegative persons (n = 13) served as control group. Data are given as mean percentages of TRAIL+ cells minus isotype controls ± SEM. (B) Individual VLs as well as (C) counts of CD4+ T cells were correlated with the percentage of TRAIL-expressing pDCs. (D) Percentages of TRAIL-expressing pDCs were determined in 6 patients infected with HIV-1 before cART initiation and after 8 to 12 weeks, when the VL had fallen below the limit of quantification (< 50 RNA copies/mL plasma) in each case. (E) Isolated pDCs from HIV-negative donors were stimulated with (1) AT-2 HIV-1 plus an isotype control, (2) AT-2 HIV-1 plus a neutralizing anti–IFN-α antibody, and (3) matched microvesicles (MVs) plus isotype control for 12 hours and subsequently FACS stained for surface TRAIL expression. Data are given as mean percentages of TRAIL-positive pDCs minus isotype controls ± SEM of 2 independent experiments. (F) Representative histogram plots of indicated patient groups show the surface expression of TRAIL on freshly isolated pDCs compared with an isotype control (open lines). (G) TRAIL-expressing pDCs in different viral diseases: viremic patients infected with HIV-1 (n = 20), viremic patients infected with HCV (n = 10), patients infected with primary VZV (n = 4), and healthy controls (n = 13). Data are given as mean percentages of TRAIL-positive pDCs minus isotype controls ± SEM.
Up-regulated TRAIL surface expression on pDCs from HIV-1 viremic patients. (A) Percentage of various TRAIL+ cell types in subgroups of patients infected with HIV-1. Healthy HIV-1–seronegative persons (n = 13) served as control group. Data are given as mean percentages of TRAIL+ cells minus isotype controls ± SEM. (B) Individual VLs as well as (C) counts of CD4+ T cells were correlated with the percentage of TRAIL-expressing pDCs. (D) Percentages of TRAIL-expressing pDCs were determined in 6 patients infected with HIV-1 before cART initiation and after 8 to 12 weeks, when the VL had fallen below the limit of quantification (< 50 RNA copies/mL plasma) in each case. (E) Isolated pDCs from HIV-negative donors were stimulated with (1) AT-2 HIV-1 plus an isotype control, (2) AT-2 HIV-1 plus a neutralizing anti–IFN-α antibody, and (3) matched microvesicles (MVs) plus isotype control for 12 hours and subsequently FACS stained for surface TRAIL expression. Data are given as mean percentages of TRAIL-positive pDCs minus isotype controls ± SEM of 2 independent experiments. (F) Representative histogram plots of indicated patient groups show the surface expression of TRAIL on freshly isolated pDCs compared with an isotype control (open lines). (G) TRAIL-expressing pDCs in different viral diseases: viremic patients infected with HIV-1 (n = 20), viremic patients infected with HCV (n = 10), patients infected with primary VZV (n = 4), and healthy controls (n = 13). Data are given as mean percentages of TRAIL-positive pDCs minus isotype controls ± SEM.
When we compared VLs, CD4+ T-cell counts and TRAIL expression on pDCs in 6 patients infected with HIV-1 before and after the initial 8 to 12 weeks of cART, all of them exhibited a reduction of the VL to less than 50 copies/mL, a remarkable increase of CD4 counts (supplemental Figure 1B) and a highly significant decrease of TRAIL-expressing pDCs (Figure 1D). To examine whether HIV-1 is directly responsible for the up-regulation of TRAIL, we stimulated pDCs from healthy donors with noninfectious HIV-1 or microvesicle controls. Results obtained showed the expression of TRAIL on two-thirds of pDCs on-HIV-1 stimulation (Figure 1E), whereas there was no enhanced surface staining of TRAIL in the microvesicle-incubated pDC fraction. IFN-α blockade with a neutralizing anti–IFN-α antibody resulted in a slight decrease of TRAIL+ pDCs that was statistically not significant (Figure 1E).
To further test whether TRAIL modulation on pDCs occurs in viral diseases other than HIV-1 infection, we analyzed pDCs procured from patients infected with HCV or VZV. Results obtained showed that TRAIL expression levels in VZV infection and, even more pronounced, in persons infected with HCV are far below what is seen in HIV viremia (Figure 1F-G).
Expression of TRAIL receptors on PBMC subsets and apoptosis rate of CD4+ T cells
To determine the potential susceptibility of different PBMC subsets to TRAIL-mediated apoptosis, we used flow cytometry to search for the proapoptotic receptors TRAIL R1 and TRAIL R2, as well as the antiapoptotic receptors TRAIL R3 and TRAIL R4 on PBMC subpopulations from viremic patients infected with HIV-1 and nonviremic patients infected with HIV-1. CD4+ T cells of HIV-1 noncontrollers displayed significantly higher levels of the apoptosis-inducing receptor TRAIL R1 on their surface than controllers, cART recipients, or healthy controls (Figure 2A). CD4+ T cells of cART recipients contained a small TRAIL R1–positive fraction and, interestingly enough, a much larger one carrying the antiapoptotic receptor TRAIL R4 on their surface (Figure 2A). The biologic importance of this finding is underscored by the further observations (1) of a very direct correlation between the percentage of CD4+ T cells expressing Annexin V and those carrying the surface-bound receptor TRAIL R1 (Figure 2A-B) and (2) by the robust and selective induction of TRAIL R1 on CD4+ T cells by AT HIV-1 but not by microvesicle controls (Figure 2C). There was no difference in the apoptosis rate of CD4− T cells of HIV-1 viremic and nonviremic patients or healthy controls (data not shown). Although such an occurrence was reported in the literature,11 we were not able to detect TRAIL R2 on CD4+ T cells of HIV-1 noncontrollers either by FACS immunostaining or by quantitative reverse transcription PCR analysis (data not shown).
TRAIL receptor expression on PBMC subsets and apoptosis rate of CD4+ T cells from patients infected with HIV-1. The expression of the TRAIL receptors R1 to R4 on (A) CD4+ T cells, (D) CD4− T cells, (E) monocytes, and (F) pDCs was analyzed quantitatively in (1) HIV-1 progressors (n = 13), (2) HIV-1 viremic nonprogressors (n = 10), (3) nonviremic patients infected with HIV-1 receiving cART (n = 11), and (4) healthy HIV-1–seronegative persons (n = 13) by FACS. (B) Ex vivo early apoptotic CD4+ T cells were determined by FACS as Annexin V+/7-AAD− T cells in HIV-1 progressors (n = 7), HIV-1 nonprogressors (n = 5), and HIV-1–seronegative healthy controls (n = 5). (C) Isolated CD4+ T cells were stimulated with AT-2 HIV-1 or microvesicle controls for 12 hours and subsequently analyzed for TRAIL receptor expression by FACS stainings in 2 independent experiments. (A-F) Data are given as mean percentages of positive cells minus isotype controls ± SEM.
TRAIL receptor expression on PBMC subsets and apoptosis rate of CD4+ T cells from patients infected with HIV-1. The expression of the TRAIL receptors R1 to R4 on (A) CD4+ T cells, (D) CD4− T cells, (E) monocytes, and (F) pDCs was analyzed quantitatively in (1) HIV-1 progressors (n = 13), (2) HIV-1 viremic nonprogressors (n = 10), (3) nonviremic patients infected with HIV-1 receiving cART (n = 11), and (4) healthy HIV-1–seronegative persons (n = 13) by FACS. (B) Ex vivo early apoptotic CD4+ T cells were determined by FACS as Annexin V+/7-AAD− T cells in HIV-1 progressors (n = 7), HIV-1 nonprogressors (n = 5), and HIV-1–seronegative healthy controls (n = 5). (C) Isolated CD4+ T cells were stimulated with AT-2 HIV-1 or microvesicle controls for 12 hours and subsequently analyzed for TRAIL receptor expression by FACS stainings in 2 independent experiments. (A-F) Data are given as mean percentages of positive cells minus isotype controls ± SEM.
Although only minor proportions of CD4− T cells displayed any of the TRAIL receptors tested for (Figure 2D), monocytes and pDCs expressed TRAIL R1 as well as the antiapoptotic receptors TRAIL R3 and TRAIL R4 (Figure 2E-F). This observation could indicate a mechanism for preventing autocrine TRAIL-mediated killing of cells expressing the corresponding apoptosis-inducing ligand, such as pDCs and monocytes.
Close contact of TRAIL-expressing pDCs to apoptotic T cells in vivo in lymph nodes of patients infected with HIV-1
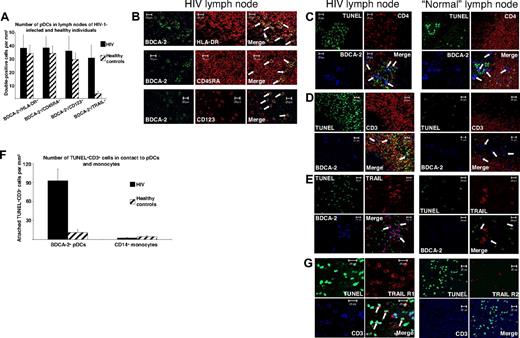
In persons infected with HIV-1 virus replication occurs most actively in lymph nodes and probably other lymphoid tissues,20 ie, sites in which pDCs accumulate preferentially21 and CD4+ T-cell depletion mainly occurs.22 Because TRAIL-mediated apoptosis demands physical interaction of TRAIL and its receptors, we thought that the demonstration of an apoptosis-inducing synapse between TRAIL-expressing pDCs and death receptor–bearing CD4+ T cells in lymph nodes of HIV-1 viremic patients could be a strong argument for the contribution of TRAIL-positive pDCs to T-cell depletion seen during HIV-1 infection. We therefore analyzed lymph nodes of untreated noncontroller persons infected with HIV-1 (n = 8) with a VL greater than 105 RNA copies/mL plasma and healthy controls (n = 5) by immunofluorescence stainings for T cells and pDCs. Double stainings of anti–BDCA-2 with anti–MHC-II, anti-CD45RA, or anti-CD123 disclosed slightly increased numbers of pDCs in lymph nodes of viremic patients infected with HIV-1 in the parafollicular T-cell zones (Figure 3A-B). Transferase-mediated dUTP nick-end labeling (TUNEL) staining showed a higher number of apoptotic cells in lymph nodes of viremic patients infected with HIV-1 compared with those of healthy controls (Figure 3C-E). We further observed (1) that most of these apoptotic cells were CD4+ (Figure 3C) and CD3+ (Figure 3D) cells and (2) that apoptotic T cells were predominantly located in close proximity to BDCA-2+ pDCs in lymph nodes from patients infected with HIV-1 (Figure 3C-D,F). Such a topographic relation was not found for CD14+ monocytes and apoptotic T cells (Figure 3F). In contrast to pDCs of normal lymph nodes, most of those from viremic patients infected with HIV-1 stained positively for TRAIL (Figure 3A,E). These results indicate that the apoptotic synapse between pDCs and T cells takes place in lymphoid tissue of HIV-1 viremic patients. In accordance with our results from peripheral blood, TUNEL+CD3+ T cells from HIV-1 viremic patients stained positively for TRAIL R1, whereas they lacked TRAIL R2 (Figure 3G).
Close contact between TRAIL-expressing pDCs and apoptotic T cells in lymph nodes of patients infected with HIV-1. (A) A quantitative analysis of pDCs in lymph nodes of viremic patients infected with HIV-1 (n = 8) and controls (n = 5) was performed by immunofluorescence staining with an anti–BDCA-2 mAb combined with anti–HLA-DR, anti-CD45RA, or anti-CD123 mAbs. The quantity of TRAIL-expressing pDCs was analyzed by anti-TRAIL/anti–BDCA-2 staining. Data are given as absolute numbers of double-positive cells per square millimeter ± SEM. (B) Representative views of anti–BDCA-2/anti–HLA-DR, anti–BDCA-2/anti-CD45RA, and anti–BDCA-2/anti-CD123 stainings of a lymph node of a viremic patient infected with HIV-1. Arrows denote double-positive pDCs. (C-E) Immunofluorescence triple labeling of lymph nodes of patients infected with HIV-1 and HIV-1–seronegative controls with (C) TUNEL (FITC), anti-CD4 (TRITC), and anti–BDCA-2 (Cy5); (D) TUNEL (FITC), anti-CD3 (TRITC), and anti–BDCA-2 (Cy5); or (E) TUNEL (FITC), anti-TRAIL (TRITC), and anti–BDCA-2 (Cy5) showed BDCA-2+, TRAIL-expressing pDCs surrounded by apoptotic T cells in HIV-1 lymph nodes. Arrows denote BDCA-2+ pDCs. (F) To quantify the proximity of pDCs or monocytes to apoptotic T cells, the contacts of BDCA-2+ or CD14+ cells to TUNEL+CD3+ cells were enumerated in lymph nodes of HIV-infected patients and healthy controls. The data are given as absolute numbers of indicated cells in contact to apoptotic T cells ± SEM. (G) Immunofluorescence triple labeling of lymph nodes of viremic patients infected with HIV-1 with TUNEL (FITC), anti-TRAIL R1, or anti-TRAIL R2 (TRITC) and anti-CD3 (APC) denotes apoptotic T cells as TRAIL R1–expressing and TRAIL R2–deficient cells. Arrows denote triple-positive cells. (B-E,G) Original magnification ×400. Pictures were recorded with a Zeiss Axiovert 200M microscope (40×/1.30 oil objective), a HAL100 camera, and LSM510 software (release 3.0, update 3.2) in a 1024 × 1024 solution.
Close contact between TRAIL-expressing pDCs and apoptotic T cells in lymph nodes of patients infected with HIV-1. (A) A quantitative analysis of pDCs in lymph nodes of viremic patients infected with HIV-1 (n = 8) and controls (n = 5) was performed by immunofluorescence staining with an anti–BDCA-2 mAb combined with anti–HLA-DR, anti-CD45RA, or anti-CD123 mAbs. The quantity of TRAIL-expressing pDCs was analyzed by anti-TRAIL/anti–BDCA-2 staining. Data are given as absolute numbers of double-positive cells per square millimeter ± SEM. (B) Representative views of anti–BDCA-2/anti–HLA-DR, anti–BDCA-2/anti-CD45RA, and anti–BDCA-2/anti-CD123 stainings of a lymph node of a viremic patient infected with HIV-1. Arrows denote double-positive pDCs. (C-E) Immunofluorescence triple labeling of lymph nodes of patients infected with HIV-1 and HIV-1–seronegative controls with (C) TUNEL (FITC), anti-CD4 (TRITC), and anti–BDCA-2 (Cy5); (D) TUNEL (FITC), anti-CD3 (TRITC), and anti–BDCA-2 (Cy5); or (E) TUNEL (FITC), anti-TRAIL (TRITC), and anti–BDCA-2 (Cy5) showed BDCA-2+, TRAIL-expressing pDCs surrounded by apoptotic T cells in HIV-1 lymph nodes. Arrows denote BDCA-2+ pDCs. (F) To quantify the proximity of pDCs or monocytes to apoptotic T cells, the contacts of BDCA-2+ or CD14+ cells to TUNEL+CD3+ cells were enumerated in lymph nodes of HIV-infected patients and healthy controls. The data are given as absolute numbers of indicated cells in contact to apoptotic T cells ± SEM. (G) Immunofluorescence triple labeling of lymph nodes of viremic patients infected with HIV-1 with TUNEL (FITC), anti-TRAIL R1, or anti-TRAIL R2 (TRITC) and anti-CD3 (APC) denotes apoptotic T cells as TRAIL R1–expressing and TRAIL R2–deficient cells. Arrows denote triple-positive cells. (B-E,G) Original magnification ×400. Pictures were recorded with a Zeiss Axiovert 200M microscope (40×/1.30 oil objective), a HAL100 camera, and LSM510 software (release 3.0, update 3.2) in a 1024 × 1024 solution.
pDC-mediated TRAIL-dependent killing of HIV-1 viremic CD4+ T cells
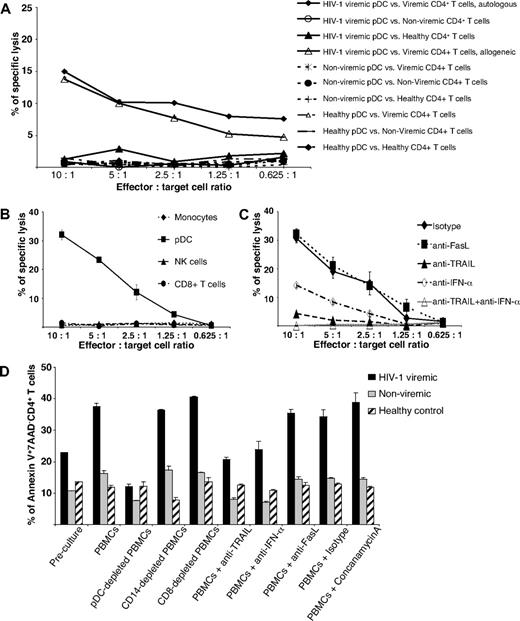
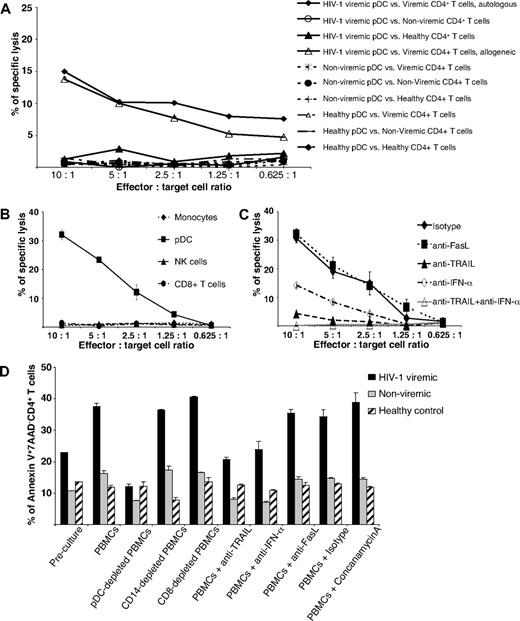
To examine the function of TRAIL-expressing pDCs in HIV-1–related T-cell depletion, we performed cytotoxicity assays using purified peripheral blood–derived pDCs as effector cells and CD4+ T cells as target cells, both isolated from HIV-1 viremic and cART-suppressed nonviremic patients as well as healthy controls. Although freshly isolated pDCs from nonviremic patients with HIV-1 and healthy controls hardly exerted any cytotoxic activity against CD4+ T cells from either cohort, pDCs from HIV-1 viremic patients effectively lysed autologous or allogeneic CD4+ T cells from viremic patients infected with HIV-1 but not those from nonviremic patients with HIV-1 or healthy controls (Figure 4A). In contrast to pDCs, monocytes, CD8+ T cells, and NK cells from viremic patients did not display a noteworthy cytotoxic activity against autologous CD4+ T cells (Figure 4B).
Cytotoxic activity of freshly isolated pDCs from viremic patients infected with HIV-1. (A) Isolated pDCs from (1) viremic patients infected with HIV-1 and (2) nonviremic patients infected with HIV-1, and (3) healthy controls were cultured with CD4+ T cells from the respective patient groups for an europium-TDA release assay at the effector/target cell ratios indicated. Data represent means of duplicate wells ± SEM from 1 of 3 independent experiments. (B) Freshly isolated monocytes, pDCs, NK cells, and CD8+ T cells were incubated with autologous CD4+ T cells from viremic patients infected with HIV-1 for an europium-TDA release assay at the effector/target cell ratios indicated. Data represent means of duplicate wells ± SEM from 2 independent experiments. (C) pDCs from a viremic patient infected with HIV-1 as effector cells were preincubated with indicated neutralizing antibodies and, thereafter, with autologous CD4+ T cells. Cytotoxicity was determined by an europium-TDA release assay at the effector/target cell ratios indicated. Data represent means of duplicate wells ± SEM from 4 independent experiments. (D) To elucidate the relevance of different cell populations and cytotoxic molecules for the killing of CD4+ T cells in peripheral blood, we either removed pDCs, monocytes, or cytotoxic T cells or added the inhibitory substances indicated (neutralizing antibodies, concanamycin A) before a 12-hour culture period of PBMCs from (1) viremic patients infected with HIV-1 and (2) nonviremic patients infected with HIV-1 and (3) healthy controls. Early apoptosis of CD4+ T cells was determined by Annexin V/7-AAD/CD4/CD3 quadruple FACS stainings before and after the culture. Data represent means of duplicate wells ± SEM from 1 of 2 independent experiments.
Cytotoxic activity of freshly isolated pDCs from viremic patients infected with HIV-1. (A) Isolated pDCs from (1) viremic patients infected with HIV-1 and (2) nonviremic patients infected with HIV-1, and (3) healthy controls were cultured with CD4+ T cells from the respective patient groups for an europium-TDA release assay at the effector/target cell ratios indicated. Data represent means of duplicate wells ± SEM from 1 of 3 independent experiments. (B) Freshly isolated monocytes, pDCs, NK cells, and CD8+ T cells were incubated with autologous CD4+ T cells from viremic patients infected with HIV-1 for an europium-TDA release assay at the effector/target cell ratios indicated. Data represent means of duplicate wells ± SEM from 2 independent experiments. (C) pDCs from a viremic patient infected with HIV-1 as effector cells were preincubated with indicated neutralizing antibodies and, thereafter, with autologous CD4+ T cells. Cytotoxicity was determined by an europium-TDA release assay at the effector/target cell ratios indicated. Data represent means of duplicate wells ± SEM from 4 independent experiments. (D) To elucidate the relevance of different cell populations and cytotoxic molecules for the killing of CD4+ T cells in peripheral blood, we either removed pDCs, monocytes, or cytotoxic T cells or added the inhibitory substances indicated (neutralizing antibodies, concanamycin A) before a 12-hour culture period of PBMCs from (1) viremic patients infected with HIV-1 and (2) nonviremic patients infected with HIV-1 and (3) healthy controls. Early apoptosis of CD4+ T cells was determined by Annexin V/7-AAD/CD4/CD3 quadruple FACS stainings before and after the culture. Data represent means of duplicate wells ± SEM from 1 of 2 independent experiments.
Because TRAIL expression on pDCs is influenced by IFN-α,18 we performed inhibition experiments by adding neutralizing antibodies against either (1) TRAIL, (2) IFN-α, (3) TRAIL plus IFN-α, or, for control purposes, (4) Fas ligand. As shown in Figure 4C, preincubation of pDCs from a HIV-1 viremic patient with inhibitory anti-TRAIL plus anti–IFN-α antibodies essentially abolished pDC cytotoxicity against CD4+ T cells from a HIV-1 viremic patient, whereas anti-Fas ligand antibodies had no effect. Neutralization of TRAIL inhibited pDC-mediated killing to a higher extent than neutralizing anti–IFN-α (Figure 4C). Keeping in mind that CD4+ T cells of HIV-1 viremic patients are TRAIL R1–expressing target cells, we performed neutralization experiments with anti-TRAIL R1 and anti-TRAIL antibodies and found a similar degree of inhibition in both instances (supplemental Figure 2).
To address the biologic relevance of these findings, we determined the apoptosis rate of Annexin V+/7-AAD− CD4+ T cells of viremic patients infected with HIV-1 or nonviremic patients with HIV-1 and healthy controls after 12 hours of ex vivo cell culture. To define the killing pathways and cell types involved in detail, we either removed specific cell populations from or added neutralizing antibodies to total PBMCs. As a result, we found high rates of early apoptotic CD4+ T cells in viremic patients infected with HIV-1, but moderate and low numbers of early apoptotic CD4+ T cells in nonviremic patients and healthy controls, respectively (Figure 4D). Depletion of pDCs resulted in a significant decrease of apoptotic CD4+ T cells of viremic patients infected with HIV-1 and, to a lesser extent, nonviremic patients infected with HIV-1, whereas removal of CD14+ monocytes or CD8+ cytotoxic T cells had no effect in either patient group. A similar, yet less pronounced, decrease of apoptotic CD4+ T cells was also observed after the addition of anti-TRAIL or anti–IFN-α antibodies to PBMCs from viremic patients infected with HIV-1 or nonviremic patients infected with HIV-1 (Figure 4D). The specificity of this inhibition is also documented by the failure of concanamycin A or anti-Fas ligand antibodies to block the induction of apoptosis in CD4+ T cells. In healthy controls the apoptosis rate of CD4+ T cells was consistent under all conditions tested, ranging between 10% and 15% (Figure 4D). Taken together, our results show that pDCs, TRAIL, and IFN-α play a relevant role in the induction of CD4+ T-cell apoptosis in viremic patients infected with HIV-1.
Activation of CD4+ T cells makes them susceptible to TRAIL-mediated killing by pDCs
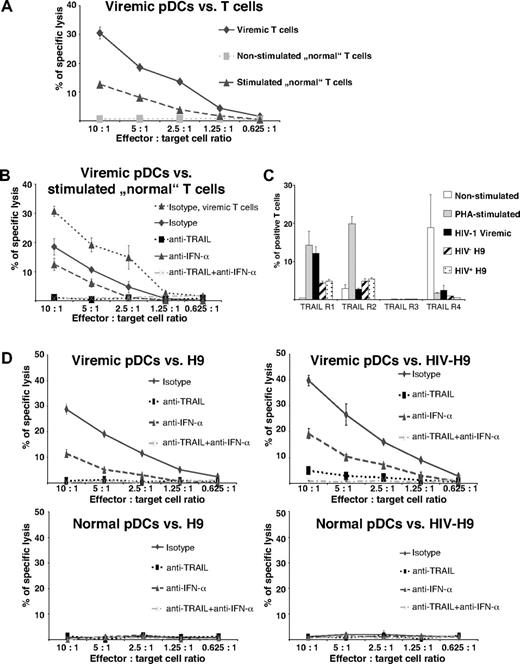
Because chronic immune activation in HIV infection is accompanied by a progressive CD4+ T-cell loss, we tested whether activation makes T cells susceptible to pDC-mediated killing. We therefore stimulated PBMCs with PHA for 48 hours, isolated CD4+ T cells, and subsequently performed cytotoxicity assays with stimulated and nonstimulated CD4+ T cells as target cells. pDCs from HIV-1 viremic patients were used as effector cells. As shown in Figure 5A, pDCs from viremic patients infected with HIV-1 lysed not only CD4+ T cells from viremic patients but also, albeit to a lesser extent, PHA-prestimulated CD4+ T cells derived from noninfected donors. Unstimulated cells, in contrast, were not targeted by pDCs (Figure 5A).
Susceptibility of activated T cells to killing by pDCs from HIV-1 viremic patients. (A) Cytotoxic activity of isolated pDCs from viremic patients infected with HIV-1 was tested against nonstimulated and PHA-stimulated CD4+ T cells from healthy HIV-1–seronegative persons, and CD4+ T cells from a viremic patient infected with HIV-1 served as positive control target cells. Data represent means of duplicate wells ± SEM from 4 independent experiments of europium-TDA release assays. (B) pDCs derived from a viremic patient infected with HIV-1 were incubated with anti-TRAIL, anti-Fas ligand, anti–IFN-α, anti-TRAIL plus anti–IFN-α, or of an IgG1 isotype mAbs and, thereafter, with PHA-stimulated CD4+ T cells from HIV-1–seronegative persons. CD4+ T cells from a HIV-1 viremic patient served as positive control. Data represent means of duplicate wells ± SEM from 2 independent experiments of europium-TDA release assays. (C) TRAIL receptors were analyzed on nonstimulated and PHA-stimulated CD4+ T cells from healthy HIV-1–seronegative persons, HIV-1 viremic CD4+ T cells, and HIV-negative and HIV-positive H9 cells by FACS staining. The data are given as mean percentages of positive cells ± SEM from 2 independent experiments. (D) pDCs from a viremic patient infected with HIV-1 (viremic pDCs) or healthy HIV-1–seronegative control (normal pDCs) were preincubated with IgG1 isotype, anti-TRAIL, anti-Fas ligand, anti–IFN-α, or anti-TRAIL plus anti–IFN-α mAbs. HIV-1–infected or –uninfected H9 cell lines served as target cells. Data represent means of duplicate wells ± SEM from 1 of 2 independent experiments of europium-TDA release assays.
Susceptibility of activated T cells to killing by pDCs from HIV-1 viremic patients. (A) Cytotoxic activity of isolated pDCs from viremic patients infected with HIV-1 was tested against nonstimulated and PHA-stimulated CD4+ T cells from healthy HIV-1–seronegative persons, and CD4+ T cells from a viremic patient infected with HIV-1 served as positive control target cells. Data represent means of duplicate wells ± SEM from 4 independent experiments of europium-TDA release assays. (B) pDCs derived from a viremic patient infected with HIV-1 were incubated with anti-TRAIL, anti-Fas ligand, anti–IFN-α, anti-TRAIL plus anti–IFN-α, or of an IgG1 isotype mAbs and, thereafter, with PHA-stimulated CD4+ T cells from HIV-1–seronegative persons. CD4+ T cells from a HIV-1 viremic patient served as positive control. Data represent means of duplicate wells ± SEM from 2 independent experiments of europium-TDA release assays. (C) TRAIL receptors were analyzed on nonstimulated and PHA-stimulated CD4+ T cells from healthy HIV-1–seronegative persons, HIV-1 viremic CD4+ T cells, and HIV-negative and HIV-positive H9 cells by FACS staining. The data are given as mean percentages of positive cells ± SEM from 2 independent experiments. (D) pDCs from a viremic patient infected with HIV-1 (viremic pDCs) or healthy HIV-1–seronegative control (normal pDCs) were preincubated with IgG1 isotype, anti-TRAIL, anti-Fas ligand, anti–IFN-α, or anti-TRAIL plus anti–IFN-α mAbs. HIV-1–infected or –uninfected H9 cell lines served as target cells. Data represent means of duplicate wells ± SEM from 1 of 2 independent experiments of europium-TDA release assays.
Preincubation of pDCs with an inhibitory anti-TRAIL or anti-TRAIL plus anti–IFN-α antibody essentially abolished pDC cytotoxicity against PHA-stimulated CD4+ T cells. Similar to the situation in HIV viremic patients, inhibition of IFN-α only partially influenced CD4+ T-cell killing by pDCs (Figure 5B), suggesting that similar mechanisms are operative in pDC-mediated killing of stimulated and viremic CD4+ T cells. Comparison of TRAIL receptors of nonstimulated, PHA-stimulated and HIV-1 viremic CD4+ T cells elucidated similar expression patterns of PHA-stimulated and viremic CD4+ T cells with an up-regulation of the proapoptotic receptors TRAIL R1 and TRAIL R2 on their surface (Figure 5C).
To directly compare the cytotoxic activity of pDCs from viremic patients with standardized HIV-infected or HIV-uninfected target cells, we performed killing assays with HIV-expressing and -nonexpressing H9 cells as targets. HIV-positive and HIV-negative H9 cells exhibited a similar surface pattern of TRAIL receptors, expressing TRAIL R1 and TRAIL R2 at low percentages but lacking the apoptosis-inhibiting receptors TRAIL R3 and TRAIL R4 (Figure 5C). Viremic pDCs substantially killed HIV-infected H9 cells and, to a lesser extent, uninfected H9 cells, whereas pDCs from a healthy person showed no cytotoxic activity against either cell type (Figure 5D). Anti-TRAIL pretreatment inhibited killing of HIV-negative and HIV-infected H9 cells (Figure 5D), whereas it was not completely abolished by anti–IFN-α antibodies alone (Figure 5D), suggesting that pDC-mediated killing of virus-infected and virus-uninfected activated target cells relies on TRAIL-dependent mechanisms and, to a lesser extent, IFN-α–dependent mechanisms.
Discussion
The mechanism of how HIV mediates CD4+ T-cell depletion remains one of the most intensively studied, yet still unresolved questions in HIV pathophysiology. There is clear evidence that HIV replicates in and subsequently destroys activated CD4+ T cells, be it by induction of syncytium formation,23 alteration of membrane permeability,24,25 or mitochondrial dysfunction.26 HIV-infected CD4+ T cells can also be killed by HIV-specific cytotoxic T cells,5 or by Fas/FasL interactions.27 The reason why we must assume that other modes of cellular injury are also operative in CD4+ T-cell depletion of HIV-infected persons is that the number of apoptotic cells greatly exceeds the number of HIV-infected cells,4,28 and apoptosis in lymph nodes is observed primarily in the HIV-negative cell fraction.6 It is conceivable though that the elimination of such noninfected CD4+ T cells is accomplished by HIV proteins released from infected cells acting on neighboring noninfected cells,29,30 by CXCR4-dependent autophagy of noninfected bystander cells,7 or by activation-induced cell death.31,32 In several reports death receptor–mediated apoptosis, eg, by Fas33 or TRAIL receptors,11 was made responsible for activation-induced cell death.
We and others could recently show that after TLR7 stimulation pDCs up-regulate surface TRAIL and can use this molecule to kill target cells expressing proapoptotic TRAIL receptors.14,15,18 This phenomenon is also seen after stimulation of pDCs with ligands for other intracellular TLRs, but not after incubation with agonists for TLR1-6 (Madeleine Kalb, G. Stary, F.K., and G. Stingl, manuscript in preparation: “The molecular players of TLR7/8-triggered dendritic cell cytotoxicity”).15 Keeping in mind that HIV motifs can serve as natural TLR7 agonists,16,17 we screened PBMCs of HIV-infected persons for TRAIL expression and, indeed, found such an occurrence on different leukocyte subsets. Monocytes and mDCs expressed TRAIL on their surface, not only those of HIV-infected persons but also those of healthy persons. The functionality of TRAIL on monocytes has yet to be verified. We were consistently unable to demonstrate TRAIL-dependent killing of CD4+ T cells by monocytes from a viremic patient infected with HIV-1. The reason(s) for this escape(s) us at present. One may argue that certain, yet unknown, costimulatory molecules (eg, IFN-α) or inhibitory receptors are necessary for TRAIL-mediated killing that are expressed by pDCs but not by monocytes and mDCs. One may also speculate that different isoforms or splicing variants of TRAIL with distinct functional properties are expressed by pDCs, monocytes, and mDCs. Collectively, our results argue against an important role of TRAIL-bearing monocytes in CD4+ T-cell depletion seen in viremic persons infected with HIV.
Notably, pDCs were the only cell population whose TRAIL expression correlated positively with the individual HIV-1 VLs. Functionally, TRAIL-expressing pDCs were able to kill CD4+ T cells of viremic patients infected with HIV as well as activated but not naive T cells of HIV-seronegative volunteers in a TRAIL- and IFN-α–dependent manner. Given that pDCs represent only a small fraction of peripheral blood leukocytes, one wonders whether TRAIL+ pDCs could significantly contribute to T-cell apoptosis in HIV infection. One should not forget that pDCs, although numerically reduced in the blood of HIV-infected patients34 and simian immunodeficiency virus (SIV)–infected macaques,35 are increased in lymphoid tissues in HIV and SIV infections (Figure 3A),21,36 and higher numbers of pDCs in lymph nodes of SIV-infected macaques correlate with viremia and IFN-α levels.36 Our studies confirm these observations and further show the occurrence as well as topographical proximity of TRAIL+ pDCs and apoptotic TRAIL R1+ T cells in lymphoid tissue of viremic patients infected with HIV, ie, the predominant site of CD4+ T-cell depletion.22 To the best of our knowledge, this is the first report of the close in vivo contact between probable effector cells and apoptotic T cells expressing proapoptotic receptors in lymph nodes of HIV-infected persons. However, we cannot exclude the possibility that mechanisms other than the TRAIL–TRAIL R1 pathway are also operative in the apoptosis of T cells in lymphoid tissue. The interaction of Fas and FasL, which are both overexpressed in tonsils from HIV-infected patients,37 is certainly a candidate in this regard. Although absent on apoptotic T cells in our lymph node stainings, Herbeuval et al38 found higher levels of TRAIL R2 mRNA in tonsils from HIV progressors than in those from HIV nonprogressors. Because we were able to block pDC-mediated killing by a neutralizing anti-TRAIL R1 antibody, could not detect TRAIL R2 on T cells by either FACS staining, quantitative reverse transcription PCR in the peripheral blood, or immunofluorescence staining of lymph nodes, and could show a strong up-regulation of TRAIL R1 but not TRAIL R2 on CD4+ T cells on in vitro HIV-1 stimulation, we are quite confident that TRAIL R1, and not TRAIL R2, is the relevant receptor in TRAIL-induced T-cell apoptosis in HIV infection.
IFN-α production of pDCs was shown to be critical for immune activation in a macaque model and markedly reduced in nonpathogenic SIV infections.39 Our data support the theory of activation-induced cell death being one mechanism of CD4+ T-cell depletion in HIV infection, because susceptibility to pDC/TRAIL-mediated killing depends on the activation of CD4+ T cells. This is underscored by our observation that the susceptibility of CD4+ T cells depends on HIV viremia in vivo and/or activation of T cells in vitro. In both situations, in vivo and in vitro, we found a dominance of proapoptotic over antiapoptotic TRAIL receptors on CD4+ T cells as a possible explanation for their susceptibility to pDC-mediated apoptosis.13 Although the antiapoptotic receptor TRAIL R4 is expressed on T cells from both viremic patients and healthy controls, TRAIL R1 was selectively found on T cells from HIV-1 viremic patients and on T cells from normal donors on HIV-1 stimulation. These findings indicate that the balance between different TRAIL receptor variants determines the susceptibility of a given cell type to TRAIL-mediated apoptosis. However, we cannot exclude the possibility that TRAIL R4 has a reduced functionality in viremic patients positive for HIV. This expression profile of TRAIL receptors on CD4+ T cells in the different patient groups analyzed could be explained by either HIV-induced or HIV-independent effects on T cells in vivo. Because (1) CD4+ T cells from HIV-negative volunteers were sensitive to TRAIL-mediated cytotoxicity by pDCs after PHA-stimulation and (2) both HIV-infected and HIV-uninfected H9 cells were killed by pDCs, one can argue that additional infections in HIV viremic patients causing activation of noninfected T cells lead to pDC-mediated apoptosis and, ultimately, to the depletion of such cells.
Our results imply that TRAIL-expressing pDCs are part of an innate antiviral immune response. This defense mechanism is probably a double-edged sword. On the one hand, TRAIL+ pDCs could be beneficial by contributing to the elimination of infected host cells,15,40 as long as a pool of noninfected cells would secure appropriate tissue/organ functionality. In the absence of such a reservoir, TRAIL-expressing pDCs may cause widespread cell death (eg, liver, kidney) leading to organ failure or, in the case of HIV infection, profound immunodeficiency. By so outwitting the immune system, TRAIL+ pDCs could contribute to disease progression in HIV infection.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Madeleine Kalb for critically reading the manuscript, Dr Johannes Stary for his support in statistical issues, and Andreas Ebner for helping to prepare the figures.
This work was supported by the EU Program EVA Center for AIDS reagents, National Institute for Biological Standards and Control (NIBSC), United Kingdom (AVIP Contract no. LSHP-CT-2004-503487). Aldrithiol-2–treated HIV-1/H9 CL.4 virus and aldrithiol-2–treated H9 CL.4 microvesicles were from Dr J. Lifson, AIDS Vaccine Cancer Virus Program, National Cancer Institute at Frederick, MD.
Authorship
Contribution: G. Stary designed and performed research, collected data, and drafted the manuscript; I.K., S.K., and F.K. performed research and collected data; T.S. and L.M. provided tissue material and contributed vital new reagents; H.Q. performed research and collected data; and N.K. and G. Stingl designed research and drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.K. is Department of Dermatology, Wilhelminenspital, Vienna, Austria.
Correspondence: Georg Stary, Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases (DIAID), Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; e-mail: georg.stary@meduniwien.ac.at.
References
Author notes
N.K. and G. Stingl contributed equally to this study.