Abstract
Here we show that endothelial cells (EC) require matrix type 1-metalloproteinase (MT1-MMP) for the formation of lumens and tube networks in 3-dimensional (3D) collagen matrices. A fundamental consequence of EC lumen formation is the generation of vascular guidance tunnels within collagen matrices through an MT1-MMP-dependent proteolytic process. Vascular guidance tunnels represent a conduit for EC motility within these spaces (a newly remodeled 2D matrix surface) to both assemble and remodel tube structures. Interestingly, it appears that twice as many tunnel spaces are created than are occupied by tube networks after several days of culture. After tunnel formation, these spaces represent a 2D migratory surface within 3D collagen matrices allowing for EC migration in an MMP-independent fashion. Blockade of EC lumenogenesis using inhibitors that interfere with the process (eg, integrin, MMP, PKC, Src) completely abrogates the formation of vascular guidance tunnels. Thus, the MT1-MMP-dependent proteolytic process that creates tunnel spaces is directly and functionally coupled to the signaling mechanisms required for EC lumen and tube network formation. In summary, a fundamental and previously unrecognized purpose of EC tube morphogenesis is to create networks of matrix conduits that are necessary for EC migration and tube remodeling events critical to blood vessel assembly.
Introduction
Much progress has occurred in our understanding of the molecular events controlling the processes underlying vascularization of tissues in the context of development and disease.1-7 Work that is receiving increasing attention focuses on identifying specific steps required for vascular morphogenesis, including those involving endothelial cell (EC) lumen formation.8-12 In addition to the identification of specific molecules required for these events, it is important to determine how different cell types such as endothelial cells, pericytes, and vascular smooth muscle cells interact and assemble to form the different characteristic blood vessel types.1,6,13,14
Recent work from our laboratory reveals that ECs form lumens in 3-dimensional (3D) collagen matrices through a signaling cascade involving integrins, Rho GTPases, and membrane-type matrix metalloproteinases (MT-MMPs).8-12 These signaling events stimulate EC intracellular vacuole formation and coalescence that controls EC lumen formation in vitro and in vivo.8,10,12 A variety of integrins have been described to be relevant in regulating angiogenesis and tube formation including both β1 and αv integrins. The relevance of any particular integrin appears to be primarily dependent on the matrix environment (eg, adult, embryonic, wound, tumor) where the EC tube morphogenic process takes place.3,9,15-19
Extracellular matrix (ECM) proteolysis is thought to be an important step in how cells move through 3D matrix environments20-27 and has been implicated in vessel formation11,21,28-32 as well as vessel regression.33-36 Recently, we reported that pericyte recruitment to EC tubes induced stabilization by affecting the production and function of EC-derived tissue inhibitor of metalloproteinases (TIMP)–2 and pericyte-derived TIMP-3, which led to inhibition of both tube morphogenic and regression events.11
In this study, we present novel information revealing a previously unrecognized step in vascular tube morphogenesis, namely, the creation of vascular guidance tunnel networks within the ECM (ie, physical ECM spaces) as a consequence of MT1-MMP proteolysis during EC lumen formation. The formation of these tunnel spaces are directly coupled to signaling events necessary to control EC tube and network assembly. Thus, blockade of EC lumen and tube formation by various means completely abrogates vascular guidance tunnel formation. The generation of these matrix conduits during vascular morphogenesis allows for rapid MMP-independent migration of ECs within 3D collagen matrices which regulate tube remodeling and maturation events.
Methods
Reagents
VEGF and bFGF were purchased from Millipore. Purified TIMP-1 and -2 were obtained from Millipore Bioscience Research Reagents as well as the integrin blocking antibodies α1: MAB1973Z, α2: MAB1950Z, α3: MAB1952Z, α5: MAB1956Z, αV: MAB1953Z, αVβ3: MAB1976Z, and αVβ5: MAB1961Z. α6 (GoH3, ab19765-100) blocking antibodies were purchased from Abcam. Recombinant human TIMP-3 and -4 were purchased from R&D Systems. GM6001, thrombin, and calyculin A were from Calbiochem as well as the inhibitors Go6976 (365250), Go6983 (365251), and PP2 (529573). A rabbit monoclonal antibody to MT1-MMP was purchased from Epitomics (32 010-1). Antibodies for immunostaining include anti-collagen type I (C2456; Sigma-Aldrich).
Cell culture
Human umbilical vein ECs (HUVECs) were purchased from Cambrex/Lonza, used from passages 2 through 6 and cultured on gelatin-coated flasks. bEnd3 cells were obtained from ATCC.
Tube assembly (vasculogenic) and lumen formation assays
ECs were suspended in 3.75 mg/mL collagen type I matrices as described.37,38 Cultures were allowed to assemble over time and fixed at predetermined time points with 3% glutaraldehyde in phosphate-buffered saline (PBS), pH 7.5 for at least 30 minutes. Cultures that were to be immunostained were fixed in 2% paraformaldehyde in PBS, pH 7.5. Some cultures were then stained with 0.1% toluidine blue in 30% methanol and were destained before visualization and photography, while others were processed for immunostaining. To collapse networks after assembly, 25 nM calyculin A was used. Collapse of vessels was observed within 15 minutes. For collapse and regrowth experiments, 5 μg/mL thrombin was used to induce network collapse and its antagonist hirudin was added to reverse the effects.
Transfection of ECs with siRNA
Smartpool or single siRNAs for human or mouse MT1-MMP, MT3-MMP, MMP-1, and luciferase controls were purchased from Dharmacon RNA Technologies, and the transfections were performed as described previously.37
Viral vectors, stable cell lines, and recombinant adenovirus production
Lentiviral cell lines were prepared using the methods as previously described.11 Confluent T-25 flasks of HUVECs were infected with recombinant adenoviruses encoding MT1-MMP, MMP-1, or green fluorescent protein (GFP) control genes following methods as previously described.8,11 The MT1-MMP cDNA was amplified from a clone obtained from Origene using the primers MT1-MMP HindIII 5′Up-AGAAGCTTGCCACCATGTCTCCCGCCCCAAGACCC and MT1-MMP EcoRV 3′ DN-AGGATATCTCAGACCTTGTCCAGCAGGGAAC. The amplified cDNA was cloned into the pAdTrack vector carrying GFP.8 Mutagenesis of MT1-MMP to create a catalytically inactive proteinase, E240A, was performed using the QuickChange kit from Stratagene. The primers used for this mutagenesis were 5′UP MT1-EAmut-CTGGTGGCTGTGCACGCGCTGGGCCATGCCCTG and 3′DN MT1-EAmut-CAGGGCATGGCCCAGCGCGTGCACAGCCACCAG. Clones were confirmed by sequencing and then were recombined with pAdEasy-1 as described.8
Immunostaining of cultures
Cultures for immunostaining were fixed in 2% paraformaldehyde for 1 hour and solubilized with detergent. Blocking reagents for the secondary antibody were added for 1 hour and cultures incubated with primary antibodies overnight at 4°C. Cultures were washed the following morning in PBS and incubated for 2 hours with secondary antibodies. Final washes were done to remove background, and the cultures were examined by immunofluorescence microscopy.
Microscopy and imaging
Time-lapse videomicroscopy and fluorescence still photography were performed using a fluorescence inverted microscope (Eclipse TE2000-E; Nikon) and the analysis software MetaMorph (Molecular Devices). A temperature-controlled chamber (Solent Scientific) set to 37°C with continuous flow of 5% CO2 was used. For microinjection experiments, pipettes were positioned using a Leitz micromanipulator (E. Leita) while observing the collagen gel under brightfield illumination on the stage of a Zeiss ACM upright microscope (Carl Zeiss Microimaging Inc). Lenses used included a Plan-Fluor 10× with an NA of 0.30, and a Plan-Fluor 20× with an NA of 0.45; immunofluorescence images also used a Plan-Fluor 40× with an NA of 0.60. For time-lapse studies, images were obtained every 10 minutes in a single z-plane with a monochromatic camera (CoolSNAP HQ; Photometrics) and a 6.45 × 6.45-μm pixel pitch (Photometrics). After image acquisition, stacks of each stage position were assembled using the MetaMorph software. Still photography was performed using an inverted microscope (CKX41; Olympus) with 10× NA 0.25 Luc Plan-N lens, associated camera (DP70; Olympus), and DP manager software version 2.1.1.163 (Olympus).
Vascular guidance tunnel microperfusion with silicone oil
Micropipettes were pulled from borosilicate glass capillary tubing (1.0 mm O.D.; 0.5 mm I.D.; Frederick Haer & Co) using a Sutter P-97 puller (Sutter Instruments). The pipettes were sharpened to a tip diameter of 2 μm using a Sutter BV-10 pipette beveller and filled with low-viscosity silicone fluid (dimethylpolysiloxane, 5 csp; Sigma-Aldrich). Pipette positioning was controlled using a Leitz micromanipulator while observing the collagen gel under brightfield illumination on the stage of a Zeiss ACM upright microscope. The pipette was pressurized from its back end using a 1-mL syringe at a pressure of approximately 10 mmHg. Because tunnels were not easily visible under brightfield illumination, the pipette tip was usually positioned near collapsed EC tubes just under the surface of the gel. Tunnel perfusion was recorded using a digital video recorder (Panasonic Corporation) through a CCTV system (IK-C30 color CCD camera; Toshiba).
Statistical analysis
Statistical analysis of selected EC vasculogenic and lumen formation data was performed using SPSS 11.0 (SPSS) or Microsoft Excel (Microsoft). Analysis of variance was used to compare means of 2 or more groups. Statistical significance was set at minimum with P less than .05. Student t tests were used when analyzing 2 groups within a single experiment (with a minimum n = 10).
Results
MT1-MMP controls EC lumen formation in 3D collagen matrices through matrix degradation
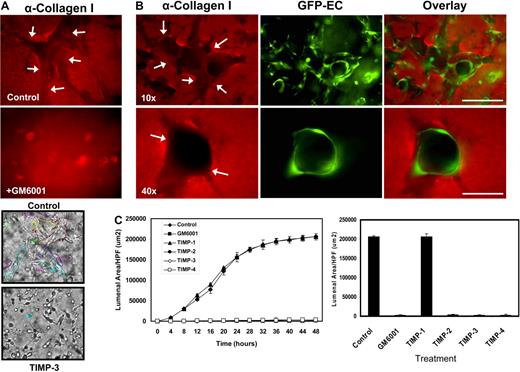
To illustrate the normal process of EC tube formation in our in vitro model, we show a representative time-lapse movie over a 48-hour period (supplemental Video 1; available online on the Blood website; see the Supplemental Materials link at the top of the online article). In this assay system,37,38 ECs are seeded as single cells in the 3D collagen matrix to mimic the developmental process of vasculogenesis,5 and over a 24- to 48-hour period, they develop lumen and tube networks. To examine if modifications to the collagen matrix occur during lumenogenesis, we stained the matrix using anti-collagen type I antibodies (Figure 1A-B). Matrix spaces (ie, vascular guidance tunnels) are readily observed during morphogenesis that directly correspond to the area surrounding ECs (labeled with GFP; Figure 1B).
MMP activity is required for EC lumen formation and generation of vascular guidance tunnels in 3D collagen matrices. (A) EC cultures were established with or without the addition of the proteinase inhibitors GM6001 (5 μM; top panels) or recombinant TIMP-3 (5 μg/mL; bottom panels). Collagen gels were fixed at 24 hours and processed for immunostaining of the collagen type I matrix (top panels) or were fixed after 48 hours (bottom panels). (Top panels) White arrows indicate the outline of vascular guidance tunnels. Bar equals 50 μm. (Bottom panels) Representative light microscopy images are shown demonstrating quantification of EC lumen formation with and without TIMP-3 addition. Bar equals 100 μm. (B) GFP-ECs were seeded within collagen matrices and allowed to form lumens and tube networks. Cultures were fixed at 96 hours and immunostained for the collagen type I matrix using a collagen type I monoclonal antibody and an Alexa Fluor 594 conjugated secondary antibody. Representative fluorescent images are shown which illustrate that ECs undergo morphogenesis within vascular guidance tunnels. Arrows denote the borders of vascular guidance tunnels. 10×, bar equals 100 μm; 40×, bar equals 25 μm. (C) ECs were suspended in 3D collagen gels and allowed to undergo morphogenesis for 48 hours. Lumen areas per field were determined by tracing EC lumens using Metamorph software from time-lapse images at the indicated time points. The effects of exogenous addition of TIMPs 1-4 and GM6001 on EC lumen formation over the time course is shown, with the adjacent bar graph highlighting EC lumen area at the final 48-hour time point. n = 3 fields per time point.
MMP activity is required for EC lumen formation and generation of vascular guidance tunnels in 3D collagen matrices. (A) EC cultures were established with or without the addition of the proteinase inhibitors GM6001 (5 μM; top panels) or recombinant TIMP-3 (5 μg/mL; bottom panels). Collagen gels were fixed at 24 hours and processed for immunostaining of the collagen type I matrix (top panels) or were fixed after 48 hours (bottom panels). (Top panels) White arrows indicate the outline of vascular guidance tunnels. Bar equals 50 μm. (Bottom panels) Representative light microscopy images are shown demonstrating quantification of EC lumen formation with and without TIMP-3 addition. Bar equals 100 μm. (B) GFP-ECs were seeded within collagen matrices and allowed to form lumens and tube networks. Cultures were fixed at 96 hours and immunostained for the collagen type I matrix using a collagen type I monoclonal antibody and an Alexa Fluor 594 conjugated secondary antibody. Representative fluorescent images are shown which illustrate that ECs undergo morphogenesis within vascular guidance tunnels. Arrows denote the borders of vascular guidance tunnels. 10×, bar equals 100 μm; 40×, bar equals 25 μm. (C) ECs were suspended in 3D collagen gels and allowed to undergo morphogenesis for 48 hours. Lumen areas per field were determined by tracing EC lumens using Metamorph software from time-lapse images at the indicated time points. The effects of exogenous addition of TIMPs 1-4 and GM6001 on EC lumen formation over the time course is shown, with the adjacent bar graph highlighting EC lumen area at the final 48-hour time point. n = 3 fields per time point.
We used the pan-MMP inhibitor GM6001 (Figure 1A) as well as TIMPs 1 through 4 (Figure 1A,C) to address the functional role of MMPs. Formation of lumens is completely blocked by the addition of GM6001, and TIMPs 2 through 4 but not TIMP-1 (Figure 1A,C and supplemental Videos 2-4). Time-lapse movie analysis was done to demonstrate lumen formation over time (Figure 1C), and lumens were traced using Metamorph software (Figure 1A bottom panels). The inability of TIMP-1 to block membrane-type MMPs or EC lumen formation (Figure 1C and supplemental Video 2) strongly implicates a role for MT1-MMP (or possibly other MT-MMPs) during these events. Both GM6001 as well as TIMPs such as TIMP-3 completely inhibit the formation of lumens and tubes (Figure 1A and supplemental Videos 3-4) but also the formation of vascular guidance tunnel networks (Figure 1A-B). A time-lapse series using unlabeled ECs and FITC-labeled collagen type I matrices reveals the codevelopment of EC lumens/tubes and vascular guidance tunnels (supplemental Figure 1A); furthermore, the quantification of lumen versus tunnel formation reveals that the 2 processes happen concurrently (supplemental Figure 1B). Confocal microscopic and quantitative analysis (by tracing the matrix-free spaces) of FITC-collagen matrices in these cultures reveals marked blockade of tunnel formation in the presence of GM6001 (supplemental Figures 1A,2A). Thus, vascular guidance tunnels are directly generated as a consequence of the EC lumen and tube formation process.
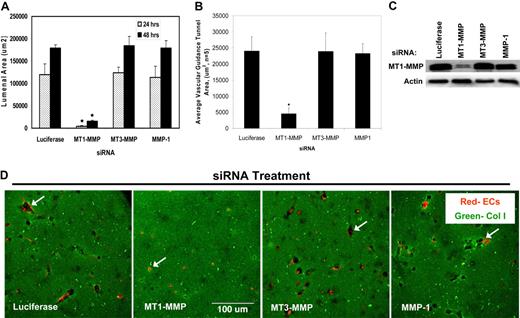
To further investigate the potential role of MT-MMP's during the EC lumen formation process, FITC-labeled 3D collagen type I matrices and mRFP-labeled ECs were used during siRNA suppression experiments of MT1-MMP and related proteins. At both 24 and 48 hours, siRNA suppression of MT1-MMP dramatically attenuated lumen formation (quantitated by tracing black matrix-free zones (ie, tunnels) as a measure of lumenal area, Figure 2A,D) as well as tunnel formation (Figure 2B). This response is not seen with siRNA suppression of other MT-MMP's or MMP-1 (Figure 2) with specificity of MT1-MMP knockdown relative to control shown in Figure 2C. An additional siRNA to MT1-MMP, which we have previously reported to block HT1080 tumor cell invasion of 3D collagen matrices,27 also blocks EC lumen formation further documenting specificity for MT1-MMP (supplemental Figure 3). Time-lapse images also document the ability of MT1-MMP siRNA but not MT3-MMP or MMP-1 siRNAs to block EC lumenogenesis (supplemental Figure 4). Interestingly, although siRNAs to MT3-MMP have no effect on EC lumen formation, tracing of lumen areas during this process reveals that MT2-MMP appears to have a role (supplemental Figure 3). We previously reported that siRNA suppression of MT2-MMP played a co-role with MT1-MMP in controlling EC invasion of 3D collagen matrices, although it has less influence than MT1-MMP11 similar to our findings here.
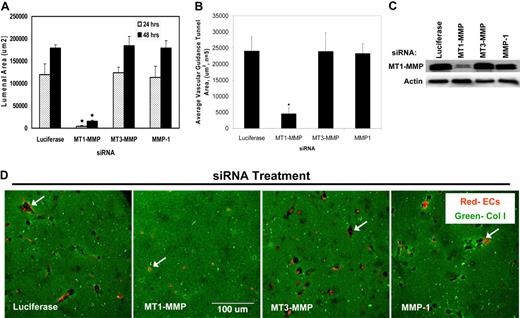
siRNAs directed to MT1-MMP block EC lumen formation and generation of vascular guidance tunnels in 3D collagen matrices. (A) The graph shows the average corresponding lumen area in micrometers2 per high-powered field for each siRNA treatment. Cultures were examined at either 24 or 48 hours of culture. The inability of MT1-MMP siRNA treated cells to form lumens is shown, with the bars representing the average lumenal area ± SD (P < .01; n = 3). (B) The graph shows the average corresponding vascular guidance tunnel area in micrometers2 per high-powered field for each siRNA treatment. Individual fields were photographed under fluorescence after immunostaining of gels (24-hour cultures) with anti-collagen type I antibodies. Vascular guidance tunnel areas were traced using Metamorph software from the photographs. The inability of MT1-MMP siRNA-treated cells to form tunnels is shown, with the bars representing average tunnel area per field ± SD (P < .01). (C) Western blots showing MT1-MMP expression demonstrate specific knockdown of the gene with siRNA directed to MT1-MMP compared with MT3-MMP and MMP-1 as well as the control siRNA directed to luciferase. Actin was used as a loading control. (D) Representative images of siRNA-transfected ECs seeded within FITC-labeled collagen type I 3D matrices are shown. Luciferase (Luc), MT1-MMP, MT3-MMP, and MMP-1 siRNA-transfected mRFP-ECs were allowed to form lumens and tube networks for 48 hours and data quantified (A). Bar equals 100 μm.
siRNAs directed to MT1-MMP block EC lumen formation and generation of vascular guidance tunnels in 3D collagen matrices. (A) The graph shows the average corresponding lumen area in micrometers2 per high-powered field for each siRNA treatment. Cultures were examined at either 24 or 48 hours of culture. The inability of MT1-MMP siRNA treated cells to form lumens is shown, with the bars representing the average lumenal area ± SD (P < .01; n = 3). (B) The graph shows the average corresponding vascular guidance tunnel area in micrometers2 per high-powered field for each siRNA treatment. Individual fields were photographed under fluorescence after immunostaining of gels (24-hour cultures) with anti-collagen type I antibodies. Vascular guidance tunnel areas were traced using Metamorph software from the photographs. The inability of MT1-MMP siRNA-treated cells to form tunnels is shown, with the bars representing average tunnel area per field ± SD (P < .01). (C) Western blots showing MT1-MMP expression demonstrate specific knockdown of the gene with siRNA directed to MT1-MMP compared with MT3-MMP and MMP-1 as well as the control siRNA directed to luciferase. Actin was used as a loading control. (D) Representative images of siRNA-transfected ECs seeded within FITC-labeled collagen type I 3D matrices are shown. Luciferase (Luc), MT1-MMP, MT3-MMP, and MMP-1 siRNA-transfected mRFP-ECs were allowed to form lumens and tube networks for 48 hours and data quantified (A). Bar equals 100 μm.
Additional experiments were performed to examine if similar results could be obtained using mouse ECs. Mouse bEnd3 cells in 3D collagen matrices formed lumens and tube networks, while 2 different siRNAs directed to mouse MT1-MMP markedly blocked mouse EC lumen formation compared with control siRNAs (supplemental Figure 2A-B). Thus, MT1-MMP is required for lumenogenesis and vascular guidance tunnel formation in a fibrillar collagen ECM environment for both human and mouse ECs. This latter finding is consistent with earlier reports showing a requirement for MT1-MMP in angiogenic sprouting assays using postnatal MT1-MMP knockout mice.21,39
MT1-MMP is required for motility of ECs in 3D collagen matrices but not on 2D collagen substrates
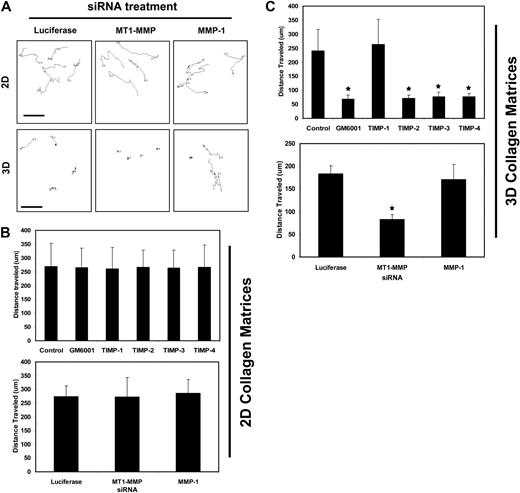
As shown in Figure 3, MT1-MMP is required for movement of ECs within 3D collagen matrices during morphogenic events but does not regulate movement on 2D collagen-coated surfaces (Figure 3A,C,D). Nuclear GFP-labeled ECs were cultured in 3D collagen matrices or on 2D collagen substrates and were either transfected with siRNAs or treated with MMP inhibitors, such as GM6001 or recombinant TIMPs from the beginning of the assay, to assess their effects on cell migratory activity (Figure 3 and supplemental Videos 5-10). Nuclear movement was tracked using MetaMorph software with tracings as well as quantification of cell migration speeds either in 3D or on 2D matrix environments shown (Figure 3). The effects of TIMPs implicate the involvement of MT1-MMP due to the ability of TIMP-2, 3, and 4 but not TIMP-1 to block EC motility in 3D matrices (Figure 3A,D and supplemental Videos 5-6,9-10). Interestingly, these MMP inhibitors had no effect on EC migration on 2D collagen substrates (Figure 3A,C and supplemental Videos 7-8). siRNA suppression of MT1-MMP recapitulates the influence of TIMPs 2-4 or GM6001 in these assays showing the importance of this proteinase in controlling EC invasion in 3D but not migration on 2D collagen matrices (supplemental Videos 9-10). Thus, the development of EC motility within 3D collagen matrices (a key step in EC morphogenesis and tube remodeling) depends on MT1-MMP activity.
TIMPs 2, 3, and 4 and siRNA suppression of MT1-MMP block EC motility in 3D collagen matrices but not on 2D collagen substrates. (A-C) For 2D assays, nuclear GFP-labeled ECs were seeded on collagen coated plastic while for 3D assays, nuclear GFP-labeled ECs were placed into collagen gels. Time-lapse fluorescence microscopy was used to track cell motion using nuclei as a measure of EC migratory events. GM6001 was added to the culture media at 5 μM while the TIMPs were used at 5 μg/mL in the media. siRNA knockdown was performed in ECs for MT1-MMP, MMP-1, and Luciferase as control. n = 25 cells quantitated for each condition. P < .01 compared with control. (A) Representative overlays of tracking data are shown. The images show the movement of single cells after siRNA treatment on 2D collagen surfaces versus within 3D collagen matrices. Bar equals 100 μm.
TIMPs 2, 3, and 4 and siRNA suppression of MT1-MMP block EC motility in 3D collagen matrices but not on 2D collagen substrates. (A-C) For 2D assays, nuclear GFP-labeled ECs were seeded on collagen coated plastic while for 3D assays, nuclear GFP-labeled ECs were placed into collagen gels. Time-lapse fluorescence microscopy was used to track cell motion using nuclei as a measure of EC migratory events. GM6001 was added to the culture media at 5 μM while the TIMPs were used at 5 μg/mL in the media. siRNA knockdown was performed in ECs for MT1-MMP, MMP-1, and Luciferase as control. n = 25 cells quantitated for each condition. P < .01 compared with control. (A) Representative overlays of tracking data are shown. The images show the movement of single cells after siRNA treatment on 2D collagen surfaces versus within 3D collagen matrices. Bar equals 100 μm.
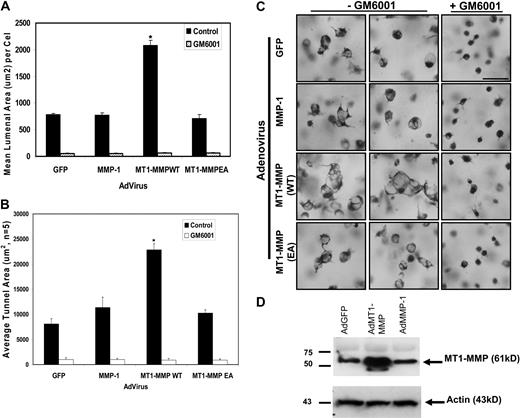
Increased expression of catalytically active MT1-MMP in ECs accelerates lumenogenesis in 3D collagen matrices
To further investigate the role of MT1-MMP in the EC lumen formation process, recombinant adenoviral vectors expressing wild type and catalytically inactive (E240A) MT1-MMP constructs were generated, with GFP and MMP-1 (a secreted collagenase) adenoviral vectors used as controls. Increasing the expression of wild type MT1-MMP but not catalytically inactive MT1-MMP strongly accelerates EC lumen formation resulting in a dramatic increase in both lumenal and vascular guidance tunnel areas (Figure 4). Importantly, GM6001 blocks these responses, demonstrating that the increased MT1-MMP is acting through its enzymatic activity. Increasing expression of MMP-1 does not produce this effect. Thus, siRNA suppression or blockade of MT1-MMP activity completely interferes with EC lumen and vascular guidance tunnel formation, while increasing expression of catalytically active MT1-MMP strongly accelerates lumen and vascular guidance tunnel formation. Overall, these results reveal a critical role for MT1-MMP in the molecular control of EC lumen and vascular guidance tunnel formation during vascular morphogenic events in 3D collagen matrices.
Increased expression of catalytically active but not inactive MT1-MMP increases EC lumen and vascular guidance tunnel formation. (A) ECs transfected with the indicated adenovirus were cultured for 24 hours, fixed, stained, and photographed. Adenoviral vectors used were control GFP, MMP-1, wild-type MT1-MMP (MT1-MMPWT), and mutant MT1-MMP (E240A) to inactivate the catalytic activity of the enzyme (MT1-MMPEA). Mean lumenal area in micrometers2 was measured, with bars representing area ± SD (P < .01). Blockade of this response is seen in all cell types by the addition of 5 μM GM6001, a broad-spectrum chemical MMP inhibitor. (B) Mean vascular guidance tunnel area was measured in micrometers,2 with bars representing area ± SD (P < .01). Blockade of this response is seen in all cell treatments after the addition of 5 μM GM6001, a broad-spectrum chemical MMP inhibitor. (C) Increased expression of MT1-MMP but not other proteins leads to increased EC lumenal area. Cultures were fixed and stained after 24 hours and photographed. Bar equals 50 μm. (D) Western blot analysis demonstrates increased expression of MT1-MMP in ECs after adenoviral infection with AdMT1-MMP but not other adenoviruses.
Increased expression of catalytically active but not inactive MT1-MMP increases EC lumen and vascular guidance tunnel formation. (A) ECs transfected with the indicated adenovirus were cultured for 24 hours, fixed, stained, and photographed. Adenoviral vectors used were control GFP, MMP-1, wild-type MT1-MMP (MT1-MMPWT), and mutant MT1-MMP (E240A) to inactivate the catalytic activity of the enzyme (MT1-MMPEA). Mean lumenal area in micrometers2 was measured, with bars representing area ± SD (P < .01). Blockade of this response is seen in all cell types by the addition of 5 μM GM6001, a broad-spectrum chemical MMP inhibitor. (B) Mean vascular guidance tunnel area was measured in micrometers,2 with bars representing area ± SD (P < .01). Blockade of this response is seen in all cell treatments after the addition of 5 μM GM6001, a broad-spectrum chemical MMP inhibitor. (C) Increased expression of MT1-MMP but not other proteins leads to increased EC lumenal area. Cultures were fixed and stained after 24 hours and photographed. Bar equals 50 μm. (D) Western blot analysis demonstrates increased expression of MT1-MMP in ECs after adenoviral infection with AdMT1-MMP but not other adenoviruses.
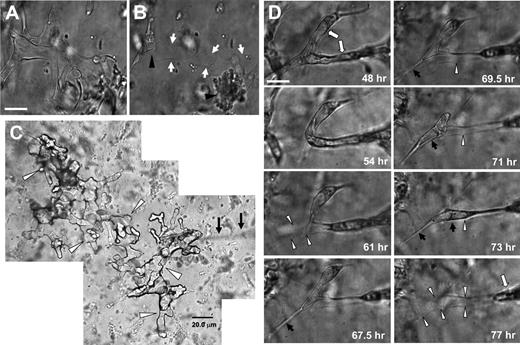
MT1-MMP is required for the creation of vascular guidance tunnels which are physical matrix conduits for vascular tube assembly and remodeling
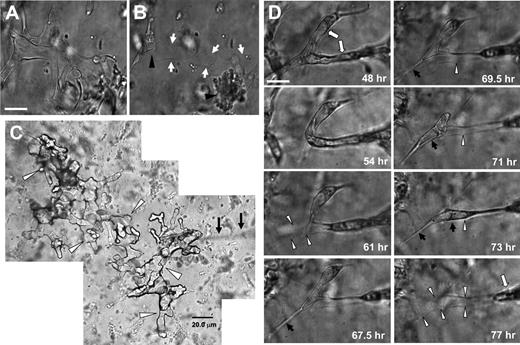
During the lumen formation process, ECs use MT1-MMP to degrade the 3D collagen matrix, leaving behind them matrix-free spaces, which we term vascular guidance tunnels. These vascular guidance tunnels do not collapse and close behind moving ECs, rather they remain as patent conduits for further EC movement and tube remodeling. EC tube networks were formed for 48 hours before further manipulation (Figure 5A). Rapid collapse of these tube structures by disrupting microtubules40 reveals an extensive underlying network of stable vascular guidance tunnels (borders indicated by arrows; Figure 5B), while the arrowheads denote EC aggregates derived from the collapsed tubes.
Vascular guidance tunnels are physical spaces in 3D collagen matrices that are generated by MT1-MMP-mediated proteolysis during EC tube morphogenesis, which support EC motility and vascular tube remodeling. (A) ECs were seeded within a 3D collagen matrix and allowed to form vascular tube networks for 48 hours. (B) Vascular networks were rapidly regressed for 10 minutes with the phosphatase inhibitor calyculin A. Arrows indicate borders of vascular guidance tunnels; arrowheads indicate collapsed EC aggregates. Bar equals 25 μm. (C) Silicone oil was injected into vascular guidance tunnels from a single injection site using a 2-μm diameter micropipette (arrows). Cultures were allowed to form (A), were collapsed (B) with calyculin A, and were microinjected. A montage of photographs are shown from this extensive vascular guidance tunnel network present within the 3D collagen matrices (arrowheads) as a result of EC lumen and tube morphogenesis. Bar equals 20 μm. (D) Photographs from time-lapse images showing EC tube remodeling (denoted by white arrows) whereby ECs and tube structures are observed moving through vascular guidance tunnels (outlined by white arrowheads). Black arrows indicate process extension and tube movement into a vacated vascular guidance tunnel space (white arrowheads). Bar equals 25 μm.
Vascular guidance tunnels are physical spaces in 3D collagen matrices that are generated by MT1-MMP-mediated proteolysis during EC tube morphogenesis, which support EC motility and vascular tube remodeling. (A) ECs were seeded within a 3D collagen matrix and allowed to form vascular tube networks for 48 hours. (B) Vascular networks were rapidly regressed for 10 minutes with the phosphatase inhibitor calyculin A. Arrows indicate borders of vascular guidance tunnels; arrowheads indicate collapsed EC aggregates. Bar equals 25 μm. (C) Silicone oil was injected into vascular guidance tunnels from a single injection site using a 2-μm diameter micropipette (arrows). Cultures were allowed to form (A), were collapsed (B) with calyculin A, and were microinjected. A montage of photographs are shown from this extensive vascular guidance tunnel network present within the 3D collagen matrices (arrowheads) as a result of EC lumen and tube morphogenesis. Bar equals 20 μm. (D) Photographs from time-lapse images showing EC tube remodeling (denoted by white arrows) whereby ECs and tube structures are observed moving through vascular guidance tunnels (outlined by white arrowheads). Black arrows indicate process extension and tube movement into a vacated vascular guidance tunnel space (white arrowheads). Bar equals 25 μm.
To further illustrate that physical tunnel spaces exist in the ECM following the collapse of EC lined tubes, we microinjected these structures with silicone oil. Multiple interconnecting vascular guidance tunnel structures are visualized from a single injection site (arrowheads) using a micropipette (Figure 5C arrows). Injection of oil into matrices without any cells present or with cells in cultures that have been treated with GM6001 to block lumen and tube formation does not reveal such structures. These results dramatically demonstrate the presence of physical spaces within the ECM generated by MT1-MMP-mediated proteolysis during EC tube and network formation (Figure 5A-C).
ECs migrate within vascular guidance tunnels in 3D collagen matrices in an MMP-independent manner to control tube assembly and remodeling
Once formed, ECs can use the inner surface of vascular guidance tunnels (created by MT1-MMP–dependent proteolysis) as a 2D migratory surface within the 3D matrix environment. These tunnel spaces allow cells to move in an MMP-independent fashion by eliminating the need for proteolysis, promoting continuous movement of ECs and tube structures through these spaces (Figure 5D and supplemental Videos 11-12). Nuclear GFP-labeled ECs were seeded for 48 hours in collagen matrices to allowed to form lumen and tube networks. At this time, cultures were left untreated or GM6001 was added and time-lapse videos generated to assess EC motility under these 2 different conditions. As shown in Figure 6, EC migration within tunnel spaces is identical whether GM6001 was added or not (see supplemental Video 12 and supplemental Figure 5). The addition of TIMP-2 or TIMP-3 also has no influence on EC motility after tunnel formation has occurred (data not shown). These results should be contrasted with those shown previously in which GM6001, TIMP-2, and TIMP-3 completely block EC motility and tube morphogenesis when added from the beginning of culture (Figures 1 and 3C). This further supports the conclusion that MT1-MMP is required to create vascular guidance tunnel spaces during the lumen and tube formation process but is not required for EC motility on 2D collagen substrate surfaces (Figure 3B) or when ECs are located within vascular guidance tunnel spaces (Figure 6). These data demonstrate that ECs, by creating vascular guidance tunnels during lumen and tube formation, change their matrix contacts from an initial 3D relationship to a 2D relationship where ECs are attached through their abluminal surface to the matrix while their lumenal surface is exposed to fluid (Figure 7D). Thus, the ECs assume a 2D migratory phenotype within the 3D matrix environment during this later stage of tube morphogenesis, accounting for the inability of MMP inhibitors to block motility within preformed tunnel networks. However, MMP inhibitors will block any new attempts to sprout into the matrix from these tube networks.
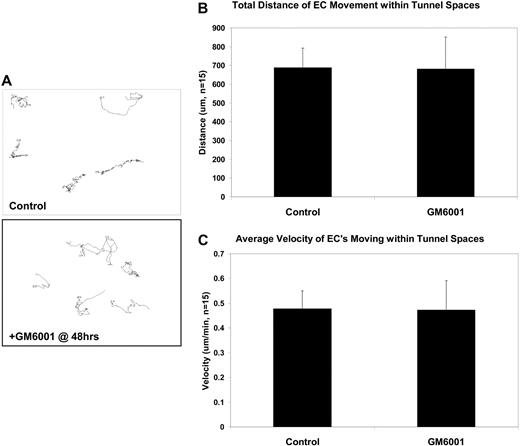
EC motility within vascular guidance tunnels is an MMP-independent process. Nuclear GFP-ECs were allowed to undergo morphogenesis in 3D collagen matrices for 48 hours to establish a network of tubes and vascular guidance tunnels. At 48 hours, GM6001 was either added or not to block further proteolysis and time-lapse imaging done. (A) Tracking of nuclei was done and representative tracings of cellular movement are shown. (B-C) Quantification of EC total distance traveled and average velocity are shown, demonstrating that EC migration within vascular guidance tunnels does not depend on MMP activity.
EC motility within vascular guidance tunnels is an MMP-independent process. Nuclear GFP-ECs were allowed to undergo morphogenesis in 3D collagen matrices for 48 hours to establish a network of tubes and vascular guidance tunnels. At 48 hours, GM6001 was either added or not to block further proteolysis and time-lapse imaging done. (A) Tracking of nuclei was done and representative tracings of cellular movement are shown. (B-C) Quantification of EC total distance traveled and average velocity are shown, demonstrating that EC migration within vascular guidance tunnels does not depend on MMP activity.
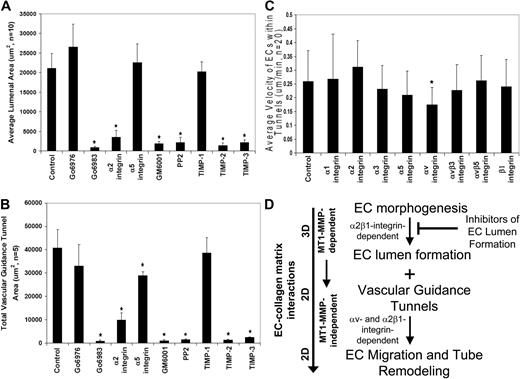
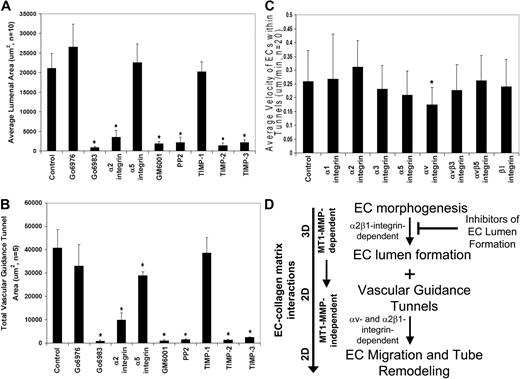
Inhibitors of EC lumen formation block vascular guidance tunnel formation, and differential role of integrins in controlling EC motility and adhesive interactions with vascular guidance tunnel matrices. ECs were seeded into collagen matrices, and various inhibitors of lumen formation were added from the beginning of the assay as indicated. The indicated drugs (Go6976, Go6983, PP2) were each added at 10 μM, the integrin blocking antibodies were added at 20 μg/mL, and the MMP inhibitors, GM6001, and the recombinant TIMPs were added at 5 μM and 5 μg/mL, respectively. (A) Average luminal area was measured in micrometers2 from 5 independent cultures, with bars representing area ± SD (P < .01) and quantitated from images obtained from stained cultures. (B) Cultures were immunostained for collagen type I and quantification of average vascular guidance tunnel formation measured in micrometers2 from 5 independent cultures, with bars representing tunnel area ± SD (P < .01). (C) Nuclear GFP-EC cultures were established for 48 hours, after which time integrin blocking antibodies were added at 20 μg/mL and real-time imaging was performed to assess EC motility within vascular guidance tunnels. Velocity of migration was quantitated from 20 independent cell motility tracings from triplicate cultures and is calculated as μm per minute. (D) Schematic diagram showing that EC morphogenic processes lead to both lumen formation and vascular guidance tunnel formation. The lumen formation mechanism depends on MT1-MMP-dependent proteolysis and the α2β1 integrin in a 3D matrix environment. ECs initially are completely surrounded by collagen matrix. Vascular guidance tunnels which form as a consequence of EC lumen formation are then used as 2D migratory matrix surfaces allowing EC motility and tube remodeling events that are MT1-MMP-independent. The ECs flatten out within these tunnel spaces and are interacting with collagen ECM on their abluminal surfaces while their luminal surfaces are exposed to fluid, thus, mimicking a 2D matrix environment. EC migratory events involve αv and α2β1 integrins that recognize the proteolytically altered vascular guidance tunnel matrix surface (containing both native and denatured collagen type I).
Inhibitors of EC lumen formation block vascular guidance tunnel formation, and differential role of integrins in controlling EC motility and adhesive interactions with vascular guidance tunnel matrices. ECs were seeded into collagen matrices, and various inhibitors of lumen formation were added from the beginning of the assay as indicated. The indicated drugs (Go6976, Go6983, PP2) were each added at 10 μM, the integrin blocking antibodies were added at 20 μg/mL, and the MMP inhibitors, GM6001, and the recombinant TIMPs were added at 5 μM and 5 μg/mL, respectively. (A) Average luminal area was measured in micrometers2 from 5 independent cultures, with bars representing area ± SD (P < .01) and quantitated from images obtained from stained cultures. (B) Cultures were immunostained for collagen type I and quantification of average vascular guidance tunnel formation measured in micrometers2 from 5 independent cultures, with bars representing tunnel area ± SD (P < .01). (C) Nuclear GFP-EC cultures were established for 48 hours, after which time integrin blocking antibodies were added at 20 μg/mL and real-time imaging was performed to assess EC motility within vascular guidance tunnels. Velocity of migration was quantitated from 20 independent cell motility tracings from triplicate cultures and is calculated as μm per minute. (D) Schematic diagram showing that EC morphogenic processes lead to both lumen formation and vascular guidance tunnel formation. The lumen formation mechanism depends on MT1-MMP-dependent proteolysis and the α2β1 integrin in a 3D matrix environment. ECs initially are completely surrounded by collagen matrix. Vascular guidance tunnels which form as a consequence of EC lumen formation are then used as 2D migratory matrix surfaces allowing EC motility and tube remodeling events that are MT1-MMP-independent. The ECs flatten out within these tunnel spaces and are interacting with collagen ECM on their abluminal surfaces while their luminal surfaces are exposed to fluid, thus, mimicking a 2D matrix environment. EC migratory events involve αv and α2β1 integrins that recognize the proteolytically altered vascular guidance tunnel matrix surface (containing both native and denatured collagen type I).
To illustrate that tubes can regrow within preformed vascular guidance tunnels, a time-lapse experiment in which thrombin addition (to stimulate microtubule depolymerizing events) causes rapid collapse of EC-lined tubes is shown (supplemental Figure 6). ECs, however, are able to reform following addition of the thrombin antagonist, hirudin (supplemental Figure 6). The indicated tube structure collapses, leaving behind the vascular guidance tunnel (denoted with arrowheads), whereby ECs can move and reassemble into a lumen and tube structure (supplemental Figure 6 and supplemental Video 13). Collectively, these experiments show that vascular guidance tunnels are physical structures present within 3D collagen matrices that are generated by MT1-MMP proteolysis. The tunnel spaces allow for EC migration, tube migration and remodeling, and tube regrowth after collapse in a manner that does not depend on MMP activity.
Blockade of EC lumen formation in 3D collagen matrices also inhibits vascular guidance tunnel formation
Previous work in our laboratory has focused on several signaling molecules and pathways controlling EC lumen formation including α2β1 integrin, Cdc42, Pak2/4, PKCϵ, and Src family kinases.12,41 Here, we show a critical functional requirement for MT1-MMP and its coordination with these previously identified EC lumen formation regulatory signals. Our previous work revealed that anti-α2β1 integrin blocking antibodies31,38 as well as PKCϵ (ie, Go6983) or Src (ie, PP2) inhibitors markedly inhibit lumen formation.12,41 In support of our previous studies, anti-α2 integrin antibodies, as well as PKCϵ and Src inhibitors block EC lumen formation (Figure 7A) and, consequently, vascular guidance tunnel formation (Figure 7B). In addition, GM6001, TIMP-2, and TIMP-3, but not TIMP-1, block both processes similarly to the other lumen formation inhibitors (Figure 7A-B). The PKCα/β inhibitor, Go6976, had no influence on EC lumen or tunnel formation. Anti-α5 integrin antibodies have no effect on EC lumen formation (Figure 7A) but interestingly have a modest blocking influence on vascular guidance tunnel area (Figure 7B), suggesting that some matrix remodeling of tunnels is likely occurring during this process and is affecting tunnel formation. One intriguing issue is that ECs create twice as much vascular guidance tunnel area than they occupy after 24 to 48 hour of culture (as measured by EC lumen area; Figure 7A-B and supplemental Figure 1B). Thus, not all vascular guidance tunnels that are created are actually occupied by EC-lined tubes. A final point is that increased expression of wild-type MT1-MMP using adenoviral gene transfer does not overcome the lumen blocking influence of PKC, Src, or α2 integrin inhibitors during these assays (data not shown). Overall, these data indicate that molecules controlling EC lumen formation are coordinated with MT1-MMP–dependent proteolytic events leading to vascular guidance tunnel formation (Figure 7D). EC motility within vascular guidance tunnel spaces is dependent on αv integrins and not the collagen receptor, α2β1, while α2β1 controls maintenance of EC adhesive contacts with the vascular guidance tunnel wall and affects vascular remodeling events.
Finally, we examined whether integrin requirements for EC motility within vascular guidance tunnel spaces were distinct from integrin requirements necessary to form lumens and tubes as well as vascular guidance tunnels (Figure 7A-B). Previous studies from our laboratory have shown a critical role for α2β1 but not αv integrins in either EC tube formation or invasion of 3D collagen matrices.31,38 We assessed, using real-time video analysis, whether integrin blocking antibodies affected tube remodeling events and their ability to maintain adhesive contact with the vascular guidance tunnel wall during this process. After 48 hours of culture, the motility of GFP nuclear-labeled ECs was assessed in the presence of blocking antibodies directed to the indicated integrins (Figure 7C). As shown in this figure, anti-αv blocking antibodies significantly block EC motility within tunnels, suggesting that proteolytic modification of collagen matrices has likely created matricryptic sites, such as RGD, within unfolded collagen molecules to regulate motility.42 In contrast, α2 integrin blocking antibodies do not block and may modestly accelerate motility within the tunnel spaces (Figure 7C). These data suggest that ECs respond in a unique manner through integrins to the proteolytically generated spaces after morphogenic events, in contrast to those events controlling the formation of tunnels (ie, anti-α2 integrin antibodies markedly block tunnel formation when added from the beginning of culture38 ; Figure 7B).
A different issue is how integrin blocking antibodies affect the ability of ECs to maintain cell adhesive contacts within vascular guidance tunnels and how this influences tube remodeling events. To address this question, we performed time-lapse experiments using preformed vascular tube networks in which anti-integrin blocking antibodies were added at 48 hours of culture. As shown in supplemental Videos 14 to 17, both anti-α2 (supplemental Video 15) and anti-β1 integrin antibodies (supplemental Video 16) affected vascular remodeling events, while anti-α5 integrin antibodies (supplemental Video 17) did not compared with control conditions (supplemental Video 14). The anti-β1 and anti-α2 antibodies promoted partial collapse of EC-lined tube networks from tunnel spaces and coalescence of vessels, while the other antibodies had no effect compared with control. In both cases, as vessels pull away from the tunnel wall in response to inhibition of α2β1 and then move centrally, vascular guidance tunnels become apparent. Thus, although anti-β1 and anti-α2 antibodies did not significantly affect motility rates within tunnel spaces (Figure 7C), they did appear to affect the ability of EC-lined tubes to maintain their adhesive contacts with the walls of the vascular guidance tunnels and thus have substantial influences on vascular tube remodeling events (supplemental Videos 14-15). These experiments reveal a unique ability of individual integrins to regulate distinct aspects of EC adhesive interactions with vascular guidance tunnel matrices during vascular remodeling events.
Discussion
EC lumenogenesis depends on MT1-MMP to form vascular guidance tunnels
Our work here, as well as previous studies from our laboratory, suggests that MT1-MMP is a dominant cell-surface proteinase required for EC tube morphogenesis and invasion in 3D collagen matrices.11 These results are consistent with data presented from other laboratories.21,28,30 In the present study, we show that MT1-MMP is required for the creation of vascular guidance tunnels, a previously unrecognized and required step in tube morphogenesis in 3D collagen matrices, and that the formation of vascular guidance tunnels is directly linked to the EC lumen formation process. Increased expression of catalytically active, but not inactive MT1-MMP, accelerates this process. Thus, the cell surface proteinase MT1-MMP locally degrades collagen type I during EC tubulogenesis to create a network of vascular guidance tunnels.
Vascular guidance tunnels control EC tube remodeling events
The creation of vascular guidance tunnels during EC tube morphogenesis allows for migration of ECs within physical spaces that are much like 2D matrix surfaces. Once vascular guidance tunnels are created, ECs are able to migrate in an MMP-independent manner in 3D matrices to facilitate remodeling and regrowth of tubes. A key function of vascular guidance tunnels could be to provide an environment whereby both ECs and support cells, such as pericytes or smooth muscle cells, could interact during maturation and stabilization of tubes. In work to be presented elsewhere, we have shown that pericytes are actively recruited to within EC-generated vascular guidance tunnel spaces during EC-pericyte tube coassembly events to promote tube stabilization (A.N.S., K.M. Malotte, R.D. Mahan, M.J.D., G.E.D, manuscript in preparation). Both cell types are able to move in an MMP-independent manner within vascular guidance tunnels during these events. Thus, vascular guidance tunnels appear to play a critical functional role not only in EC tube assembly and remodeling but also in further events critical to blood vessel assembly including the recruitment of perivascular supporting cells.
It is intriguing to consider the possible importance of vascular guidance tunnels in signaling events necessary to create vascular tissue boundaries either at the level of ECs, pericytes, or vascular smooth muscle cells with respect to formation of arteries, capillaries, and veins. For example, ephrin B2 and EphB4 signaling events during development occur through repulsive interactions and regulate arterial versus venous EC positions in blood vessels.43 Thus, vascular guidance tunnels, which allow for rapid motility of cells during morphogenesis, establish a matrix conduit and interface whereby repulsive cell-cell contacts could occur to create cellular boundaries within the vascular wall.
In this study, we have shown the ability of ECs to migrate within vascular guidance tunnel spaces, but it is also possible that other cell types could use such spaces to promote movement to vascular beds. One obvious example would be tumor cells, whose ability to metastasize may relate directly to their ability to be recruited to vascular guidance tunnels or to migrate within these physical spaces. Thus, a tumor cell with limited proteolytic capacity but possessing migratory activity may still move readily within 3D matrices through preformed tunnel spaces created by ECs or other proteolytically active cells during normal or pathologic events. Similar suggestions have been recently reported by others with regard to the influence of other cell types such as fibroblasts on tumor cell invasion.44,45
Another important issue regards the stability of vascular guidance tunnels as conduits for vascular cells to maintain vessel integrity. We have recently shown that pericyte recruitment to EC-lined tubes induces the pericyte expression of the MMP inhibitor, TIMP-3,11 and in conjunction with EC-derived TIMP-2 strongly contribute to the process of vascular tube stabilization by interfering with proteinases that regulate tube regression.11 Importantly, TIMP-2 and TIMP-3 completely block EC lumen and tube formation and markedly block EC movement in 3D matrices. In contrast, they do not restrict EC movement on 2D matrix surfaces or within vascular guidance tunnels. Thus, these TIMPs restrict EC movement to within the confines of preformed vascular guidance tunnels (by preventing invasive behavior) to control the maintenance of EC tube structures and the associated matrix tunnels themselves. Also, they are able to suppress proteinases such as ADAMs46 or MT-MMPs that regulate shedding of cell surface adhesion molecules such as VE-cadherin, N-cadherin, and VCAM-1 and, thus, contribute to the maintenance of cell-cell interactions (EC-EC and EC-pericyte) necessary for tube stabilization.47,48
Vascular guidance tunnels are matrix templates for vascular tube regrowth
A key regulatory function of vascular guidance tunnels is to serve as matrix conduits for the regrowth of vessels that have regressed as we have shown in our experiments using thrombin as a reversible collapsing agent. This finding is related to work presented by others showing that regressed tumor angiogenic vessels can regrow along preformed basement membrane matrix tracks after removal of the stimulus that caused vessel regression (eg, VEGF receptor 2 antagonism).49 Thus, there is strong precedent for the concept of that matrix tunnels exist within the ECM in vivo which can persist for long periods after vascular regression events to serve as conduits for vascular regrowth and remodeling.49,50 Overall, our work demonstrates that MT1-MMP generated vascular guidance tunnels serve a critical function during vascular morphogenic events to catalyze EC lumen and tube formation as well as serving as a matrix conduit for EC motility and tube remodeling to promote vascular wall assembly.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Steven Segal for the use of his Zeiss ACM microscope. We also thank Ms Anne Mayo, Ms Kristine Malotte, and Ms Jennifer Faske for technical help at various stages of this work.
This work was supported by National Institutes of Health (Bethesda, MD) grants HL79460, HL59373, and HL87308 to G.E.D.
National Institutes of Health
Authorship
Contribution: A.N.S. performed and designed research, analyzed data, and wrote the paper; W.B.S. performed and designed research and analyzed data; A.S., W.K., K.E.F., and D.C.Z. performed research and analyzed data; M.J.D. performed research, analyzed data, and wrote the paper; and G.E.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George E. Davis, MD, PhD, Margaret Proctor Mulligan Professor of Medical Research, Department of Medical Pharmacology and Physiology, School of Medicine, One Hospital Dr, MA415 Medical Sciences Bldg, University of Missouri-Columbia, Columbia, MO 65212; e-mail: davisgeo@health.missouri.edu.
References
Supplemental data
EC cultures were established with nuclear GFP-labeled ECs in 3D collagen matrices (after treatment with the control luciferase siRNA) and were imaged by time-lapse fluorescence and light microscopy over a 24 hr period. Frames were collected every 10 minutes, were overlaid, and are shown at 8 frames/sec.Additional videos can be found here.