Abstract
It has been reported that ectopically expressed interleukin-17 (IL-17) in tumor cells suppresses tumor progression through enhanced antitumor immunity in immune competent mice or promote tumor progression through an increase in inflammatory angiogenesis in immune-deficient mice. The role of endogenous IL-17 in tumor immunity remains undefined. Here we showed that tumor growth and lung metastasis were enhanced in IL-17–deficient mice, associated with decreased interferon-γ+ natural killer cells and tumor specific interferon-γ+ T cells in the tumor draining lymph nodes and tumors. Together with the published data showing that in vitro transforming growth factor-β and IL-6–polarized Th17 cells induce tumor regression, our work supports the notion that endogenous IL-17 or/and Th17 cells may play a protective role in tumor immunity.
Introduction
Interleukin-17 (IL-17) and Th17 cells play an active role in inflammation and autoimmune diseases in murine systems.1-9 Th17 cells are found in both mouse and human tumors.10-12 However, the role of endogenous IL-17 in tumor immunopathgenesis remains undefined. In this report, we evaluated tumor growth and metastasis in IL-17–deficient mice. We provide novel insight into the role of IL-17 in tumor immunity
Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory. IL-17–deficient C57BL/6 mice were previously described.13 MC38 cells were injected subcutaneously or intravenously (105) into IL-17–deficient and normal C57/BL6 mice. Subcutaneous tumor size was measured twice weekly using calipers fitted with a Vernier scale. Tumor volume was calculated based on 3 perpendicular measurements.14 At day 30 after intravenous tumor inoculation, mice were killed and lung metastatic foci were quantified as we described.15 The study was approved by the Committee on Use and Care of Animals at the University of Michigan.
Cell purification and staining
Single-cell suspensions were made from tumor tissues, spleens, non–tumor-draining lymph nodes, and tumor-draining lymph nodes. The phenotype and cytokine profile of each individual immune cell subset were determined by flow cytometric analysis (fluorescence-activated cell sorter).10,11 Natural killer (NK) and T cells were enriched using paramagnetic beads (StemCell Technologies).
ELISPOT assay
MultiScreen filtration plates (96-wells/plate; Millipore) were coated with 4 μg/mL anti–mouse interferon-γ (IFN-γ) monoclonal antibody (clone R4-6A2; BD Biosciences). Spleen NK cells and lymph node T cells were added (2 × 105 cells/well) and stimulated for 24 hours with or without irradiated (60 Gy) mouse MC38 or YAC-1 cells (5 × 104 cells) or with IL-2 (50 ng/mL), or phorbol-12-myristate-13-acetate (5 ng/mL) and ionomycin (500 ng/mL). Bonded IFN-γ was detected by biotinylated rat anti–mouse IFN-γ monoclonal antibody (clone XMG1.2; BD Biosciences) followed by antibiotin alkaline phosphatase. Spots were visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium alkaline phosphatase substrate and counted using the ImmunoSpot analyzer (Cellular Technology). Data are reported as an average number of spots per 105 responders plus or minus SEM of triplicate samples.
Statistical calculations
The Mann-Whitney U test was used to determine pairwise differences, and the χ2 test was used to determine differences between groups. A P value less than .05 was considered significant.
Results and discussion
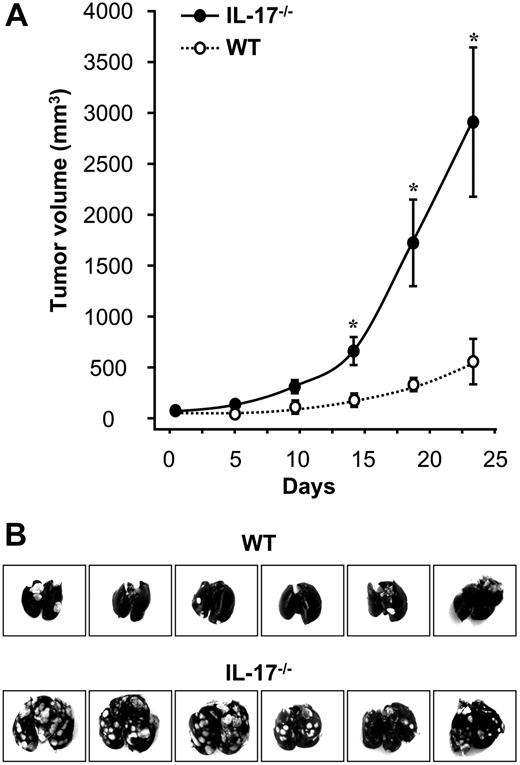
We studied the effect of IL-17 on tumor growth in IL-17–deficient mice. MC38, a murine colon cancer cell line, was subcutaneously inoculated into the wild-type and IL-17–deficient mice. The IL-17–deficient mice exhibited an accelerated tumor growth compared with the control mice (P < .01; Figure 1A). We further compared the metastatic potential of MC38 cells by intravenously inoculating cells into wild-type and IL-17–deficient mice. We observed more metastatic foci of tumors in the lungs of IL-17–deficient mice (59 ± 8) than in control mice (12 ± 5; P = .01; Figure 1B). These data suggest that endogenous IL-17 may play a protective role in tumor immunity.
Enhanced tumor growth and metastasis in IL-17–deficient mice. MC38 cells were inoculated into IL-17–deficient mice and normal wild-type mice as we described. (A) Enhanced tumor growth in IL-17–deficient mice. After tumor inoculation, tumor volume was measured as described in “Methods.” Results are expressed as mean ± SEM; n = 5 per group. (B) Enhanced tumor lung metastasis in IL-17–deficient mice. Thirty days after tumor inoculation, tumor colonies in the lungs were photographed and quantified; n = 6 per group.
Enhanced tumor growth and metastasis in IL-17–deficient mice. MC38 cells were inoculated into IL-17–deficient mice and normal wild-type mice as we described. (A) Enhanced tumor growth in IL-17–deficient mice. After tumor inoculation, tumor volume was measured as described in “Methods.” Results are expressed as mean ± SEM; n = 5 per group. (B) Enhanced tumor lung metastasis in IL-17–deficient mice. Thirty days after tumor inoculation, tumor colonies in the lungs were photographed and quantified; n = 6 per group.
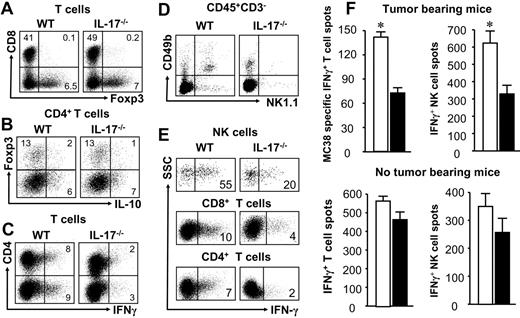
We next examined the phenotype and cytokine profile of tumor-infiltrating immune cells in tumor-bearing mice. We observed similar levels of CD4+FOXP3− T cells, CD8+ T cells, and FOXP3+CD4+ and IL-10+CD4+ T cells in the tumor microenvironments in both IL-17–deficient and wild-type mice (Figure 2A-B; supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, the levels of tumor-infiltrating IFN-γ+ T cells were significantly higher in wild-type mice than IL-17–deficient mice (Figure 2C). We also examined tumor-draining lymph nodes. Although the levels of CD8+ T cells and FOXP3+ T cells were comparable (supplemental Table 1), there were fewer NK cells (P < .01; Figure 2D), IFN-γ+ NK cells, IFN-γ+CD8+ T cells, and IFN-γ+CD4+ T cells in the tumor-draining lymph nodes in the IL-17–deficient mice than the control mice (P < .05; Figure 2E). We further evaluated MC38-specific effector T-cell responses and NK-cell activity against YAC-1 in these mice. ELISPOT assay demonstrated that tumor-specific IFN-γ+ T cells and IFN-γ+ NK cells were decreased in tumor-draining lymph node T cells in the IL-17–deficient mice (Figure 2F top panel; P < .001 for all). There were fewer than 4 nonspecific spots in NK-cell assay and fewer than 20 nonspecific spots in T-cell assay in the absence of specific stimuli. Altogether, the data indicate that both innate immunity and tumor specific T-cell immunity are weakened in the tumor-bearing IL-17–deficient mice.
Immune cells in the tumor and draining lymph nodes in IL-17–deficient mice. MC38 cells were inoculated into IL-17–deficient mice and normal wild-type mice as we described. Twenty-five days after tumor inoculation, single-cell (s.c.) suspensions were made from tumor tissues, tumor-draining lymph nodes (TDLN) and spleens, or non–tumor-bearing IL-17–deficient mice and wild-type mice. (A-E) Phenotype and cytokine profile of each immune cell subsets were analyzed by fluorescence-activated cell sorter. Results are the percentage of the targeted cell subset in a particular immune cell population. One representative of 5 experiments is shown in panels A to E (n = 13 mice per group in panels A-E). (A) T-cell subsets in tumor. Single-cell suspensions were made from tumor tissues. The levels of CD4+ and CD8+ T cells, and FOXP3+ T cells were quantified with LSR II by gating on CD3+ cells. The levels of these 3 T-cell subsets were comparable in IL-17–deficient mice and wild-type mice (P > .05 for all). (B) IL-10+ T cells and Treg cells in tumor. Single-cell suspensions were made from tumor tissues. The levels of IL-10+CD4+ T cells and CD4+FOXP3+ T cells were quantified with LSR II by gating on CD3+CD4+ cells. The levels of these 2 T-cell subsets were comparable in IL-17–deficient mice and wild-type mice (P > .05 for all). (C) IFN-γ+ T cells in tumor. Single-cell suspensions were made from tumor tissues. The levels of IFN-γ+CD4+ T cells and IFN-γ+CD8+ T cells were quantified with LSR II by gating on CD3+ cells. The levels of these 2 T-cell subsets were significantly lower in IL-17–deficient mice than wild-type mice (P < .01 for all). (D) NK cells in TDLNs. Single-cell suspensions were made from TDLNs. The levels of CD49b+NK1.1+ NK cells were quantified with LSR II by gating on CD3− cells. The levels of NK cells were significant lower in IL-17–deficient mice than wild-type mice (P < .01). (E) IFN-γ+ cells in TDLNs. Single-cell suspensions were made from TDLNs. The levels of IFN-γ+ NK cells, IFN-γ+CD4+ T cells, and IFN-γ+CD8+ T cells were quantified with LSR II by gating on NK cells and CD4+ and CD8+ T cells. The levels of these 3 IFN-γ+ cell subsets were significantly lower in IL-17–deficient mice than wild-type mice (P < .01 for all). (F) NK- and T-cell activities in tumor-bearing mice (top panels) and non–tumor-bearing mice (bottom panels). TDLN T cells were stimulated with irradiated MC38, and non–TDLN T cells were stimulated with phorbol-12-myristate-13-acetate and ionomycin. Spleen NK cells were stimulated with IL-2 and irradiated YAC-1 cells. The expression of IFN-γ was detected by ELISPOT assay in these stimulated T and NK cells. Results are mean ± SEM values of IFN-γ+ spots in triplicate wells. □ represent wild-type mice; and ■, IL-17–deficient mice. n = 5 mice per group. *P < .01. **P < .05.
Immune cells in the tumor and draining lymph nodes in IL-17–deficient mice. MC38 cells were inoculated into IL-17–deficient mice and normal wild-type mice as we described. Twenty-five days after tumor inoculation, single-cell (s.c.) suspensions were made from tumor tissues, tumor-draining lymph nodes (TDLN) and spleens, or non–tumor-bearing IL-17–deficient mice and wild-type mice. (A-E) Phenotype and cytokine profile of each immune cell subsets were analyzed by fluorescence-activated cell sorter. Results are the percentage of the targeted cell subset in a particular immune cell population. One representative of 5 experiments is shown in panels A to E (n = 13 mice per group in panels A-E). (A) T-cell subsets in tumor. Single-cell suspensions were made from tumor tissues. The levels of CD4+ and CD8+ T cells, and FOXP3+ T cells were quantified with LSR II by gating on CD3+ cells. The levels of these 3 T-cell subsets were comparable in IL-17–deficient mice and wild-type mice (P > .05 for all). (B) IL-10+ T cells and Treg cells in tumor. Single-cell suspensions were made from tumor tissues. The levels of IL-10+CD4+ T cells and CD4+FOXP3+ T cells were quantified with LSR II by gating on CD3+CD4+ cells. The levels of these 2 T-cell subsets were comparable in IL-17–deficient mice and wild-type mice (P > .05 for all). (C) IFN-γ+ T cells in tumor. Single-cell suspensions were made from tumor tissues. The levels of IFN-γ+CD4+ T cells and IFN-γ+CD8+ T cells were quantified with LSR II by gating on CD3+ cells. The levels of these 2 T-cell subsets were significantly lower in IL-17–deficient mice than wild-type mice (P < .01 for all). (D) NK cells in TDLNs. Single-cell suspensions were made from TDLNs. The levels of CD49b+NK1.1+ NK cells were quantified with LSR II by gating on CD3− cells. The levels of NK cells were significant lower in IL-17–deficient mice than wild-type mice (P < .01). (E) IFN-γ+ cells in TDLNs. Single-cell suspensions were made from TDLNs. The levels of IFN-γ+ NK cells, IFN-γ+CD4+ T cells, and IFN-γ+CD8+ T cells were quantified with LSR II by gating on NK cells and CD4+ and CD8+ T cells. The levels of these 3 IFN-γ+ cell subsets were significantly lower in IL-17–deficient mice than wild-type mice (P < .01 for all). (F) NK- and T-cell activities in tumor-bearing mice (top panels) and non–tumor-bearing mice (bottom panels). TDLN T cells were stimulated with irradiated MC38, and non–TDLN T cells were stimulated with phorbol-12-myristate-13-acetate and ionomycin. Spleen NK cells were stimulated with IL-2 and irradiated YAC-1 cells. The expression of IFN-γ was detected by ELISPOT assay in these stimulated T and NK cells. Results are mean ± SEM values of IFN-γ+ spots in triplicate wells. □ represent wild-type mice; and ■, IL-17–deficient mice. n = 5 mice per group. *P < .01. **P < .05.
To determine whether the reduced NK- and T-cell responses in the tumor-bearing IL-17–deficient mice are specifically associated with tumor inoculation and development, we examined and compared the number and activities of NK and T cells in non–tumor-bearing IL-17–deficient mice and wild-type mice. The absolute number and the percentage of CD4+ and CD8+ T cells, NK cells, and CD4+FOXP3+ regulatory T cells (Tregs) were comparable in spleens and lymph nodes in non–tumor-bearing IL-17–deficient and wild-type mice (supplemental Table 1). The expression of IFN-γ was slightly reduced in mitogen-stimulated T cells (P > .05) and was moderately reduced in IL-2-activated NK cells in IL-17–deficient mice (P < .05), compared with wild-type mice (Figure 2F bottom panel). Altogether, the data suggest that the reduced function of NK and T cells is manifested upon in vivo tumor challenging in IL-17–deficient mice.
The role of IL-17 or Th17 cells in tumor pathogenesis is not well defined. Recombinant IL-17 has no significant effect on MC38 proliferation, cell cycle, and apoptosis (supplemental Figure 1). Forced overexpression of IL-17 ectopically in tumor cells can either suppress tumor progression through enhanced antitumor immunity in immune-competent mice16,17 or promote tumor progression through an increase in inflammatory angiogenesis in immune-deficient mice.18,19 These studies focus on the effects of exogenous IL-17 in established mouse tumor cell lines. Our data demonstrate that tumor growth in subcutaneous and lung metastasis are enhanced in IL-17–deficient mice and accompanied with reduced IFN-γ+ NK cells and tumor-specific IFN-γ+ T cells in the tumor-draining lymph nodes and tumors. It suggests that endogenous IL-17 positively impacts on tumor immunity. Along this line, IL-17 has been reported to be a potent activator of innate immunity.13,20
Notably, IL-17–deficient mice do not show a global immunodeficiency without appropriate challenging. T-cell proliferation and cytokine production are basically normal in response to in vitro mitogenic stimulation, and acute graft-versus-host responses remain intact in IL-17–deficient mice.13 However, in IL-17–deficient mice, T-cell responses are reduced in chronic immune responses, such as contact hypersensitivity, delayed-type hypersensitivity, and airway hypersensitivity response priming.13 On in vitro stimulation, the numbers of IFN-γ+ T cells are slightly and IFN-γ+ NK cells moderately reduced in non–tumor-bearing IL-17–deficient mice. It indicates a potential defect of NK- and T-cell function in IL-17–deficient mice. This defect is further amplified by MC38 tumor challenging. Although our data emphasize the importance of endogenous IL-17 in antitumor immunity in MC38 tumor-bearing mice, it remains to be determined whether endogenous IL-17 is involved in regulating tumor immunity in other tumor models, particularly multiple human epithelial carcinomas. Furthermore, it will be important to dissect how endogenous IL-17 affects the numbers of functional NK and effector T cells in tumor-draining lymph nodes and tumor environment.
Given the close relationship between endogenous IL-17 and Th17 cells, although our study does not focus on the role of Th17 cells in tumor immunity, it is probable that Th17 cells may affect tumor pathology in humans. For example, adoptively transferred transforming growth factor-β and IL-6–polarized murine transgenic T cells induce tumor regression.21 Thus, our work supports the notion that endogenous IL-17 and/or Th17 cells may promote protective tumor immunity.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Iwakura of the University of Tokyo for providing the IL-17–deficient mice.
This work was supported in part by the National Cancer Institute (grants CA123088, CA099985) (W.Z.).
National Institutes of Health
Authorship
Contribution: I.K., S.W., W.S., and L.V. performed research; and I.K., S.W., and W.Z. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Weiping Zou, University of Michigan School of Medicine, 1150 W Medical Center, MSRB II, C560, Ann Arbor, MI; e-mail: wzou@med.umich.edu.