Abstract
The transdifferentiation of bone marrow cells (BMCs) into hepatocytes has created enormous interest in applying this process to the development of cellular medicine for degenerative and genetic diseases. Because the liver is the primary site of factor VIII (FVIII) synthesis, we hypothesized that the partial replacement of mutated liver cells by healthy cells in hemophilia A mice could manage the severity of the bleeding disorder. We perturbed the host liver with acetaminophen to facilitate the engraftment and hepatic differentiation of lineage-depleted enhanced green fluorescent protein-expressing BMCs. Immunohistochemistry experiments with the liver tissue showed that the donor-derived cells expressed the markers of both hepatocytes (albumin and cytokeratin-18) and endothelial cells (von Willebrand factor). The results of fluorescent in situ hybridization and immunocytochemistry experiments suggested that differentiation was direct in this model. The BMC-recipient mice expressed FVIII protein and survived in a tail clip challenge experiment. Furthermore, a coagulation assay confirmed that the plasma FVIII activity was maintained at 20.4% (± 3.6%) of normal pooled plasma activity for more than a year without forming its inhibitor. Overall, this report demonstrated that BMCs rescued the bleeding phenotype in hemophilia A mice, suggesting a potential therapy for this and other related disorders.
Introduction
Hemophilia A (HA) is an X-chromosome–linked recessive bleeding disorder with an incidence of 1 in 5000 males.1 Severe HA patients have 1% or less of normal plasma factor VIII (FVIII) activity and spontaneously bleed. Patients with 1% to 5% of normal activity have less severe bleeding, and patients with 5% to 25% of normal activity usually bleed only with surgery or trauma. The clinical manifestation of this disease is unpredictable, recurrent, and spontaneous bleeding in various areas, including soft tissues, major joints, and occasionally in internal organs. The standard treatment options for HA are either on-demand or prophylactic therapy with plasma-derived or recombinant human FVIII. The therapeutic use of this purified factor can be a potential biohazard because of blood-borne pathogens, and is ineffective because of the formation of inhibitors. Moreover, the lifelong requirement for replacement therapy can have a significant economic impact on patients. Gene therapy has the potential to provide lifelong correction of the bleeding disorder in animal models,2-5 but clinical trials have not conclusively shown long-term therapeutic benefits in treating hemophilia B.6,7 This was primarily the result of a cell-mediated immune response against the adeno-associated virus capsid protein that causes a decline in FIX activity.7 Another concern associated with the use of viral vectors is the transient elevation of liver transaminases in response to the vectors. Therefore, alternate therapeutic options need to be developed.
Several tissues, such as the spleen, lymph nodes, liver, and kidney, have the potential to express the FVIII gene.8 However, liver perfusion9,10 and orthotopic liver transplantation studies in both animals and humans11-13 suggest that the primary site of FVIII synthesis is the liver. Various lines of evidence indicate that, within the liver, hepatocytes are the major FVIII-producing cells.10,14,15 Thus, cell-based therapy using isolated hepatocytes has been proposed as an attractive approach to treat clotting disorders. The therapeutic effectiveness of human hepatocytes transplanted under the kidney capsules of mice has been demonstrated.16 In addition to hepatocytes, sinusoidal endothelial cells have been shown as a cellular site of FVIII synthesis in mice.17 A recent study demonstrated the therapeutic potential of liver sinusoidal endothelial cells (LSECs) in HA mice.18
Based on the previous studies, either hepatocytes or LSECs could be candidate cells for the therapeutic intervention of HA, but these treatments require cadavers or partially hepatectomized liver tissue from allogeneic sources. A cell-based therapy can become successful if the graft is easily obtained. Numerous studies have demonstrated that bone marrow (BM) stem cells differentiate into hepatocytes in rodents.19-23 Thus, we addressed the therapeutic potential of BM-derived liver cells in the phenotype correction of HA mice. Our study indicates that BM cells (BMCs) have potential clinical applications in managing bleeding disorders.
Methods
Animals
Six- to 8-week-old male HA mice (B6;129S4-F8tm1Kaz/J) and enhanced green fluorescent protein (eGFP)–expressing [C57BL/6-Tg(UBC-GFP)30Scha/J] female mice were used in this investigation. Mice were obtained from the Jackson Laboratories and maintained in the institute's experimental animal facility. Mice were kept in an isolator and fed with autoclaved acidified water and irradiated food ad libitum. All experiments were conducted as per procedures approved by the Institutional Animal Ethics Committee at the National Institute of Immunology, New Delhi, India.
Acute liver injury model
Acute liver injury was induced by the intraperitoneal injection of acetaminophen (500 mg/kg body weight) in male HA mice. Animals were killed at day 1, 2, or 3 after the administration of acetaminophen, and serum samples were collected for the analysis of alanine aminotransferase (ALT) level. The liver was dissected and fixed in 10% buffered formalin. Five-micron paraffin-embedded tissue sections were stained with hematoxylin and eosin for histologic examination. The liver functions of control (untransplanted) and transplanted mice were determined by ALT, and renal functions by creatinine and blood urea nitrogen (BUN) analyses. These assays were carried out using standard kits (Transasia Bio-Medicals).
Liver reconstitution
Lin− (CD5, CD11b, CD45R, 7-4, Gr-1, and Ter119-depleted) BMCs were isolated from eGFP transgenic female mice by a magnetic cell sorter (Miltenyi Biotec) following a negative selection method. A total of 250 000 sorted cells were transplanted in acetaminophen-treated (1 day) and control HA male mice through the tail vein.
Flow cytometry
Before staining, single cells from the liver were obtained by a 2-step enzymatic method.24 Details of the flow cytometry are given in the Supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Immunohistochemistry
Liver tissues were fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose solution for 24 hours at 4°C, and frozen in tissue freezing medium. Five-micron serial sections were treated with 0.15% Triton X-100 for 30 minutes at room temperature. The sections were then stained with anti-GFP, anti–mouse albumin (A90-134A; Bethyl), anti-CK-18 (sc-32329), and anti–von Willebrand (vWF) factor antibodies (sc-8086; Santa Cruz Biotechnology) for 1 hour at room temperature. The secondary antibodies were conjugated with AlexaFluor 488/594 (Invitrogen). The nuclei were stained with 4′,6-diamidino-2-phenylindole. Sections were imaged under an Olympus fluorescence microscope and a DP70 digital camera. The Olysia BioReport software was used for the image acquisition. The images were composed and edited in Photoshop 6.0 (Adobe).
Immunostaining of FVIII
The tissue samples were fixed in 10% buffered formalin, embedded in paraffin, and sectioned (5 μm). Deparaffinized 5-μm sections were treated with 0.3% H2O2 to inactivate endogenous peroxidase. The sections were separately stained with anti-FVIII light chain–specific antibody (sc-33584; Santa Cruz Biotechnology) and a corresponding isotype control serum (S-5000; Vector Laboratories), followed by anti–rabbit IgG-horseradish peroxidase. Finally, the sections were reacted with 3,3′-diaminobenzidine substrate (Vector Laboratories), washed, and counterstained with Mayer hematoxylin. Sections were imaged using bright-field microscopy.
Sex chromosome FISH and immunocytochemistry
The same liver cells of HA-transplanted (HAT) mice were used for fluorescence in situ hybridization (FISH) and immunocytochemistry. The interphase FISH study was performed using whole-chromosome paint probes specific for chromosomes X and Y (RTU Mouse WCP fluorescein isothiocyanate chromosome X and RTU Mouse WCP Cy3 chromosome Y; Cambio Ltd). The cells were treated in a hypotonic (50 mmol KCl) solution for 30 minutes and fixed in a methanol and acetic acid mixture (3:1 ratio).A 25-μL cell suspension was used to prepare each slide. The slides were treated with 70% acetic acid for 1 minute and dehydrated in increasing concentrations of alcohol (70%, 90%, and 100%). The cells were further treated in pepsin solution (10 mg/L, pH 2.0) at 37°C for 20 minutes, washed in phosphate-buffered saline, further fixed in 1% paraformaldehyde at 4°C for 10 minutes, and finally dehydrated as before. A total of 10 μL of each labeled probe was used for FISH. Probes and nuclear DNA together were denatured at 75°C for 5 minutes in hybrite (Vysis Inc) and incubated in a humidified chamber at 37°C for 18 hours. Slides were washed using 0.03% and 0.01% NP40 at 72°C for 2 minutes each. The specimens were dehydrated in alcohol and mounted with antifade medium containing 4′,6-diamidino-2-phenylindole. The FISH analysis was carried out using an Olympus BX51 microscope with an epifluorescence attachment, and images were captured through the spectral imaging system.
For immunocytochemical analysis, cytospins were fixed and permeabilized as before. The cells were stained for albumin and CK-18 and examined under an Olympus fluorescence microscope.
Phenotypic correction
Phenotypic correction was assessed by the tail clip challenge test, as described in the literature.25 Tails of the transplanted mice were clipped at a length of 1.5 cm, without subsequent cauterization. Three groups of mice were subjected to tail clip challenge: wild-type (WT; positive control), HA (negative control), and HAT test mice. The clotting of blood and subsequent survival of mice were used to indicate the normal or corrected HA phenotype. The surviving mice were maintained for 3 to 6 months for additional studies. In another assay, the whole blood clotting times of the same 3 mice groups were determined.26 In brief, blood was withdrawn in a capillary tube (nonheparinized) from the eye. The loaded capillary tube was gently broken into 2 halves, with the ends slowly pulled apart to view the insoluble fibrin strands.
FVIII assays
The FVIII protein was measured using enzyme-linked immunosorbent assay (ELISA; see Supplemental methods). The amount of FVIII antigen in HAT plasma was determined relative to plasma from WT and HA mice run simultaneously. The FVIII activity was measured using COATEST FVIII kit (Instrument Laboratory Company).
Coagulation assay
The FVIII assay was performed as described in the literature,27 with minor modifications. Blood samples were collected in microfuge tubes containing sodium citrate. The samples were centrifuged and the plasma fractions were immediately stored at −70°C. The activated partial thromboplastin time (aPTT) assay was performed by incubating 100 μL of aPTT reagent (Diagnostica Stago) with 100 μL of plasma at 37°C for 5 minutes. Then, 100 μL of 25mM CaCl2 was added and the clotting time was determined. To establish the standard curve, pooled mouse and human plasma (separately) at dilutions of 1:10, 1:50, and 1:250 in Owren-Koller buffer was subjected to an aPTT assay. The clotting times of the diluted plasma were considered equivalent to that of 100%, 20%, and 4% normal plasma, respectively. For the test mice, 100 μL of diluted (1:10) mouse plasma, 100 μL of hFVIII-deficient plasma, and 100 μL of aPTT reagent were used, which was followed by 100 μL of CaCl2. For the human samples, the mouse plasma was replaced with human plasma. The plasma FVIII activities with respect to normal pooled plasma were determined by comparing clotting times from the standard curve.
FVIII inhibitor evaluation
The FVIII inhibitor was measured using a Bethesda assay,28 and the results were expressed as Bethesda units per milliliter. (One Bethesda unit is defined as the reciprocal of the dilution of test plasma that inhibits 50% of the total FVIII activity after 2 hours of incubation at 37°C.) For the standard curve, 200 μL of normal pooled mouse/human plasma and 200 μL of buffer were incubated at 37°C for 2 hours. For the samples, 200 μL of normal pooled mouse/human plasma were incubated with 200 μL of undiluted and diluted (1:1 to 1:64) test plasma at 37°C for 2 hours. The samples were further diluted to 1:10, 1:50, and 1:250 before the FVIII activity assay. The dilution of the sample at which 50% inhibition occurred was determined.
RT-PCR
Details of the total RNA extraction and the analysis of the amplified products are given in Supplemental methods. The primers and amplification conditions for PCR reactions are shown in Table 1.
Statistical values
The results of multiple experiments were reported as the mean plus or minus SEM. Tukey-Kramer multiple comparison tests were then used to compare between 2 means.
Results
Acetaminophen-induced acute liver injury in HA mice
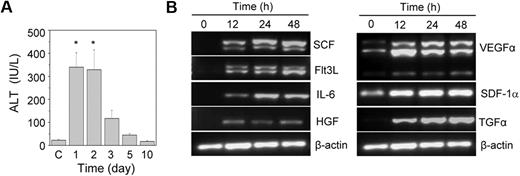
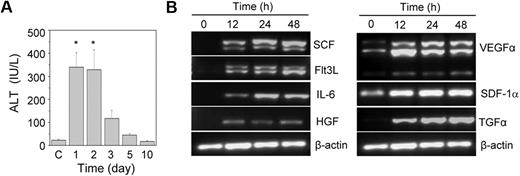
Acute liver injury was induced by the intraperitoneal injection of acetaminophen into HA mice. A significant increase in the serum ALT level was observed within one day of the acetaminophen injection compared with the control animals (Figure 1A). The ALT level was high up to the second day of treatment; after that it slowly normalized with time. A histopathologic examination revealed hemorrhagic centrilobular necrosis in the hepatic tissue on the first and second days of acetaminophen treatment (supplemental Figure 1). The liver of the control animal was histologically normal in its lobular architecture. The necrotic liver started regeneration after the third day of treatment (Figure 1A; supplemental Figure 1). Because the liver was completely restored within 10 days of the acetaminophen treatment, we predicted that the tissue microenvironment induced its regeneration. We analyzed the gene expression of several growth factors known to be involved in hematopoietic and hepatic activities. The results indicated no expression of the stem cell factor, fetal liver tyrosine kinase-3 ligand, interleukin-6, hepatocyte growth factor, and transforming growth factor-α (TGF-α) genes in the control liver tissue (Figure 1B). However, their expression was up-regulated within 12 hours of the acetaminophen treatment. The expression of the vascular endothelial growth factor α gene also increased in the damaged tissue, although basal level expression was observed in the control tissue (Figure 1B).
The effect of the acetaminophen treatment on liver function and cytokine gene expression. (A) Acetaminophen was administered to male HA mice by an intraperitoneal injection. At each time interval, the serum ALT level was estimated. Control mice “C” received normal saline (n = 6 for each group). *P < .001. (B) RT-PCR analysis of different growth factor transcripts. Acetaminophen was administered to several mice, and they were killed at 12, 24, and 48 hours. The control mice received saline. A representative gel picture shows the induction of growth factor transcripts in damaged liver tissue.
The effect of the acetaminophen treatment on liver function and cytokine gene expression. (A) Acetaminophen was administered to male HA mice by an intraperitoneal injection. At each time interval, the serum ALT level was estimated. Control mice “C” received normal saline (n = 6 for each group). *P < .001. (B) RT-PCR analysis of different growth factor transcripts. Acetaminophen was administered to several mice, and they were killed at 12, 24, and 48 hours. The control mice received saline. A representative gel picture shows the induction of growth factor transcripts in damaged liver tissue.
Lin−GFP+ BMCs engrafted in damaged HA mice liver
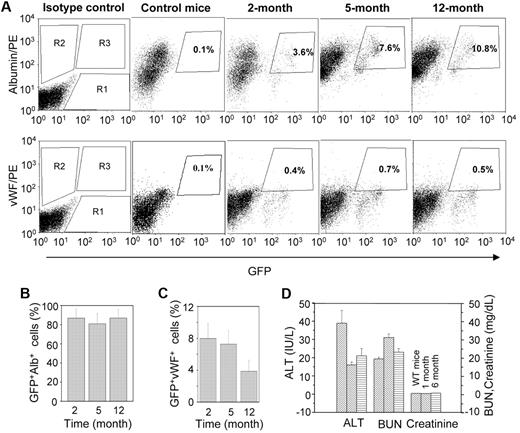
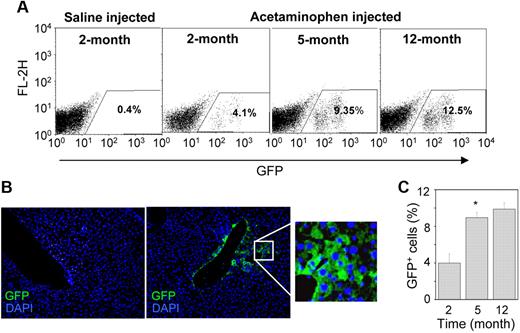
Twenty-four hours after the liver injury, 2.5 × 105 Lin−GFP+ BMCs were transplanted into HA mice. The number of engrafted cells in the recipient liver was determined by flow cytometric analysis. Representative dot-plots showed no engraftment of donor cells in the saline-injected control liver, whereas the cells were engrafted in the acetaminophen-treated liver (Figure 2A). The damaged liver tissue expressed the SDF-1α transcript (Figure 1B), the product of which is known to direct the migration of CXCR4-expressing BMCs.22 In the acetaminophen-treated mice, the donor cells were typically detected near the central vein, where severe tissue necrosis was observed (Figure 2B right). The number of donor-derived cells progressively increased in the HAT mice, and approximately 10.2% (± 2.3%) eGFP+ cells were detected in the recipient liver 12 months after transplantation (Figure 2A,C). Furthermore, we examined the biodistribution of donor cells in different organs, such as the heart, lung, kidney, spleen, and BM. Immunohistochemical analysis of serial tissue sections of the heart, lung, and kidney suggested that eGFP+ BMCs do not engraft in these organs (supplemental Figure 2A). Surprisingly, flow cytometric analysis of BM and spleen also revealed the absence of donor cells in these tissues (supplemental Figure 2B).
Engraftment of donor-derived cells in the liver. (A) A total of 250 000 Lin− cells were transplanted in HA mice after 1 day of the administration of normal saline (control) and acetaminophen. The presence of eGFP+ cells was determined by flow-cytometric analysis. Representative dot-plots show an increase in eGFP+ cells at different times after transplantation in acetaminophen-injected mice. (B) IHC analysis of liver sections of saline (left) and acetaminophen (right) injected mice after 5 months of transplantation. A representative photomicrograph shows the presence of eGFP+ cells near the central vein of acetaminophen-injected mice (original magnification ×20). (Inset) Magnified region of the section. (C) The percentage increase of eGFP+ cells in the whole liver at different times of transplantation. n = 4 for each time point. *P < .05.
Engraftment of donor-derived cells in the liver. (A) A total of 250 000 Lin− cells were transplanted in HA mice after 1 day of the administration of normal saline (control) and acetaminophen. The presence of eGFP+ cells was determined by flow-cytometric analysis. Representative dot-plots show an increase in eGFP+ cells at different times after transplantation in acetaminophen-injected mice. (B) IHC analysis of liver sections of saline (left) and acetaminophen (right) injected mice after 5 months of transplantation. A representative photomicrograph shows the presence of eGFP+ cells near the central vein of acetaminophen-injected mice (original magnification ×20). (Inset) Magnified region of the section. (C) The percentage increase of eGFP+ cells in the whole liver at different times of transplantation. n = 4 for each time point. *P < .05.
Engrafted cells differentiate into hepatic and endothelial lineage
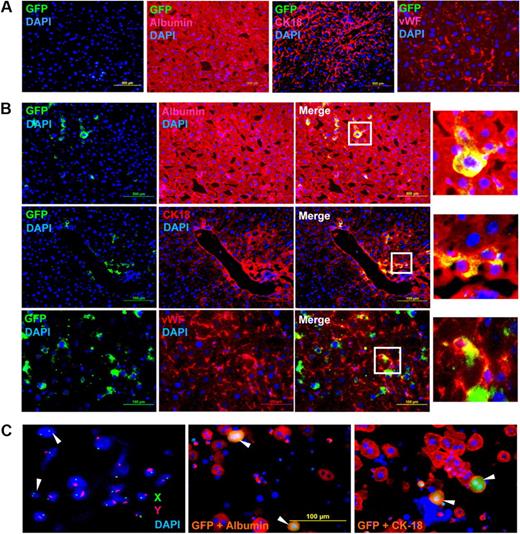
To determine whether engrafted cells differentiate into the target tissue, we analyzed serial liver sections by immunohistochemistry (IHC). The representative micrographs of 2 months after transplantation show that eGFP+ cells did not engraft in the control mice, and albumin, CK-18, and vWF were normally expressed in the host tissue (Figure 3A). IHC analyses of acetaminophen-treated mice not only revealed that the donor-derived cells engrafted in the liver but additionally showed that the eGFP+ cells were also expressing albumin, CK-18, and vWF (Figure 3B). To check for the expression of hematopoietic marker in the engrafted cells, we costained tissue sections with eGFP and CD45 antibodies. It was found that neither WT nor HAT mice liver tissue expressed the CD45 antigen (supplemental Figure 3). These results suggest that the donor cells might have differentiated into hepatocytes and endothelial-like cells.
IHC and FISH analyses of HAT mice. One day after normal saline (control) and acetaminophen administration, each mouse received a tail vein injection of donor cells. Two months after transplantation, mice were killed and liver cryosections were analyzed by IHC; 5-μm serial sections were stained with anti-GFP, antialbumin, anti-CK18, and anti-vWF antibodies. (A) Normal saline-injected mice. The merged picture shows that eGFP-expressing cells did not engraft in any of the sections examined. The sections were stained positive for albumin, CK-18, and vWF expressed by the host tissue (n = 4). (B) Acetaminophen-injected mice. The merged picture shows that donor-derived (eGFP+) cells were present in all the sections examined and expressed albumin (top panel), CK-18 (middle panel), and vWF (bottom panel; n = 4). (Insets) Magnified region of the tissue sections. As secondary antibody controls, the sections were also stained with respective conjugates (data not shown). (C) Sex chromosome FISH and immunocytochemistry. Liver cells of HAT mice (10 months of transplantation) were labeled with X (fluorescein isothiocyanate) and Y (Cy3) chromosome-specific probes. The fusions between donor (XX) and recipient (XY) cells were microscopically examined. White arrows represent donor-derived unfused cells (left). The same liver cells were immunostained for albumin and CK-18. The merge pictures show that donor-derived (eGFP+) cells express both albumin (middle) and CK-18 (right), marked by white arrows. Original magnifications: albumin and CK-18, ×20; vWF, ×40; and FISH, ×100.
IHC and FISH analyses of HAT mice. One day after normal saline (control) and acetaminophen administration, each mouse received a tail vein injection of donor cells. Two months after transplantation, mice were killed and liver cryosections were analyzed by IHC; 5-μm serial sections were stained with anti-GFP, antialbumin, anti-CK18, and anti-vWF antibodies. (A) Normal saline-injected mice. The merged picture shows that eGFP-expressing cells did not engraft in any of the sections examined. The sections were stained positive for albumin, CK-18, and vWF expressed by the host tissue (n = 4). (B) Acetaminophen-injected mice. The merged picture shows that donor-derived (eGFP+) cells were present in all the sections examined and expressed albumin (top panel), CK-18 (middle panel), and vWF (bottom panel; n = 4). (Insets) Magnified region of the tissue sections. As secondary antibody controls, the sections were also stained with respective conjugates (data not shown). (C) Sex chromosome FISH and immunocytochemistry. Liver cells of HAT mice (10 months of transplantation) were labeled with X (fluorescein isothiocyanate) and Y (Cy3) chromosome-specific probes. The fusions between donor (XX) and recipient (XY) cells were microscopically examined. White arrows represent donor-derived unfused cells (left). The same liver cells were immunostained for albumin and CK-18. The merge pictures show that donor-derived (eGFP+) cells express both albumin (middle) and CK-18 (right), marked by white arrows. Original magnifications: albumin and CK-18, ×20; vWF, ×40; and FISH, ×100.
To study the possibility of fusion between donor (XX) and recipient (XY) cells, we examined 9 slides from 3 HAT mice 10 months after transplantation by FISH. Before examining the fusion between donor and recipient cells, we evaluated the specificity of the probes on a BM metaphase preparation. The female control cells did not show the Y chromosome and only one X chromosome was detected in male control cells (supplemental Figure 4A), indicating that the FISH probes were specific. The cytogenetic analysis of 4454 nuclei suggests that discrete donor and recipient cells made up 9.45% and 90.35% of the tissue, respectively (Table 2). Furthermore, the fraction of cells containing XX and XY chromosomes was 0.2%, indicating that the probability of fusion between donor and recipient cells was negligibly low (Table 2). A representative micrograph of interphase FISH of the HAT mice liver cells show 2 distinct nuclei of donor cells (XX), which are not fused with the recipient cells (Figure 3C left). A potential fused cell (XXXY) and an aneuploid cell (XXY) are shown in supplemental Figure 4B. To prove that the eGFP+ cells present in the liver are hepatocytes, we separately analyzed the same cells by immunocytochemistry. It was observed that, like recipient hepatocytes, the donor eGFP+ cells also expressed the hepatic markers albumin and CK-18 (Figure 3C middle and right). A flow-cytometric analysis revealed that the majority of eGFP+ cells expressed albumin, further ensuring that they were directly differentiated from BMCs (supplemental Figure 4C). As further support of direct differentiation, we show 2 binucleated cells that are donor-derived hepatocytes (supplemental Figure 4D-E). It may be noted that both the nuclei in the cell are distinct, as in recipient hepatocytes. Together, the results of FISH and immunocytochemistry support the notion that BMCs directly differentiated into hepatocytes.
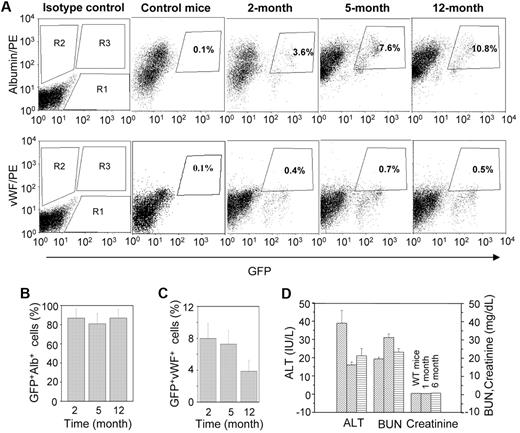
Quantitative analyses of donor-derived hepatic and endothelial cells were carried out by flow cytometry. Representative dot-plots showed that the number of albumin-expressing cells increased with time (Figure 4A). Approximately 85% of eGFP+ cells expressed albumin in all 3 time points examined (Figure 4B), whereas vWF was expressed in 4% to 8% of donor-derived cells (Figure 4C). Combined results of the IHC and flow-cytometric analyses suggest that the engrafted BMCs mostly differentiate into hepatocytes.
Quantitative analysis of GFP+albumin+ and GFP+vWF+ cells at different times of transplantation. A single-cell suspension of liver was analyzed by flow cytometry. (A) Representative dot-plots for GFP+albumin+ and GFP+vWF+ cells at different times: (top panel) R1, R2, and R3 regions for eGFP+, albumin+, and GFP+albumin+ cells, respectively; (bottom panel) R1, R2, and R3 regions for eGFP+, vWF+, and GFP+vWF+ cells, respectively. (B) The percentage of eGFP+ cells that express albumin at different times. The results show that more than 85% of donor-derived cells expressed albumin (n = 4, for each time point). (C) The percentage of eGFP+ cells that express vWF at different times. The results show that 4% to 8% of donor-derived cells expressed vWF (n = 4 for each time point). (D) The liver and renal function test in HAT mice. Mice at different times of transplantation were evaluated in terms of liver and renal functions. Serum ALT, plasma creatinine, and BUN levels of HAT mice were compared with those levels in WT mice.
Quantitative analysis of GFP+albumin+ and GFP+vWF+ cells at different times of transplantation. A single-cell suspension of liver was analyzed by flow cytometry. (A) Representative dot-plots for GFP+albumin+ and GFP+vWF+ cells at different times: (top panel) R1, R2, and R3 regions for eGFP+, albumin+, and GFP+albumin+ cells, respectively; (bottom panel) R1, R2, and R3 regions for eGFP+, vWF+, and GFP+vWF+ cells, respectively. (B) The percentage of eGFP+ cells that express albumin at different times. The results show that more than 85% of donor-derived cells expressed albumin (n = 4, for each time point). (C) The percentage of eGFP+ cells that express vWF at different times. The results show that 4% to 8% of donor-derived cells expressed vWF (n = 4 for each time point). (D) The liver and renal function test in HAT mice. Mice at different times of transplantation were evaluated in terms of liver and renal functions. Serum ALT, plasma creatinine, and BUN levels of HAT mice were compared with those levels in WT mice.
To study normal organ function, we determined the serum ALT level for the liver, and the plasma creatinine and BUN levels for the kidney of HAT mice, and compared these levels with those from WT mice. The biochemical parameters tested in 6 mice between 1 and 6 months of transplantation showed normal organ functions (Figure 4D). No macroscopic changes were noticed in the liver and kidney of these mice during the study (supplemental Figure 5). These results probably represent the normal physiologic functions of the liver and kidney in posttransplanted HA mice.
BMC transplantation resulted in FVIII light chain protein expression in HA mice
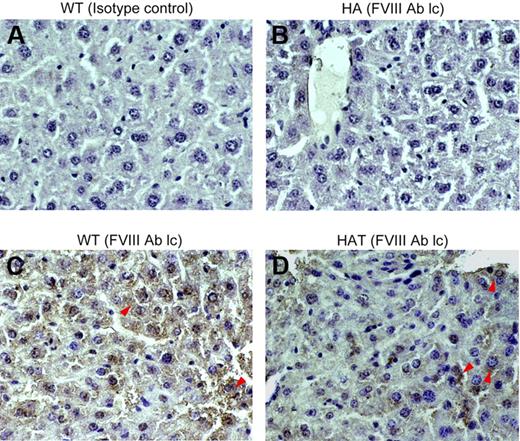
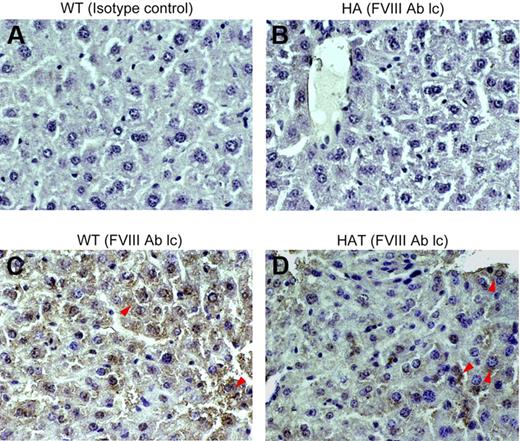
The expression of FVIII in the liver tissue was examined by IHC. The mutant mouse used in this investigation has a disrupted exon 16 within the A3 domain of the FVIII gene, and the intact light chain protein is thus not produced,29,30 whereas the heavy chain protein is expressed (data not shown). To determine whether HAT mice express the FVIII light chain protein, serial liver sections of control and transplanted mice were stained with either isotype control or specific antibodies. The WT mice showed expression of the FVIII-light chain throughout the section (Figure 5C). The same protein was not detected in the liver section of HA mice (Figure 5B). Interestingly, the FVIII-light chain protein expression was observed in HAT mice. This would not be possible unless BMCs differentiated into competent liver cells. The expression of the FVIII light chain protein was observed in the centrilobular and other parts of the liver section as well (Figure 5D; supplemental Figure 6A). No background staining was observed with the isotype control antibody, indicating that the aforementioned staining was specific.
Expression of the FVIII light chain protein in liver. Paraffin-embedded serial liver sections of WT, HA, and HAT (5 months of transplantation) mice were stained and examined under a microscope (magnification ×40). (A) Isotype control in WT mice. (B) FVIII-light chain (lc) staining in HA mice. (C) FVIII-light chain staining in WT mice. (D) FVIII-light chain staining in HAT mice. Sections were also stained with secondary antibody alone (data not shown). Representative photomicrographs show the expression of the FVIII light chain protein in WT and HAT mice (red arrows). The light chain antibody staining was specific as no reaction was observed with isotype control antibody. n = 4 for each group.
Expression of the FVIII light chain protein in liver. Paraffin-embedded serial liver sections of WT, HA, and HAT (5 months of transplantation) mice were stained and examined under a microscope (magnification ×40). (A) Isotype control in WT mice. (B) FVIII-light chain (lc) staining in HA mice. (C) FVIII-light chain staining in WT mice. (D) FVIII-light chain staining in HAT mice. Sections were also stained with secondary antibody alone (data not shown). Representative photomicrographs show the expression of the FVIII light chain protein in WT and HAT mice (red arrows). The light chain antibody staining was specific as no reaction was observed with isotype control antibody. n = 4 for each group.
Bleeding disorder is corrected in HA mice
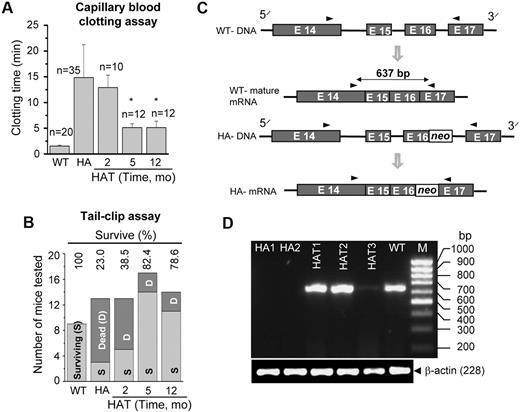
The phenotypic correction in the HA mice was determined by a blood clotting and tail-clip challenge experiment. The whole blood clotting time in WT mice was 1.9 (± 0.3) minutes (n = 20), which was increased to 14.9 (± 6.5) minutes (n = 35) in HA mice (Figure 6A). The whole blood clotting time was significantly (P < .01, n = 24) reduced after 5 months of transplantation (Figure 6A). Similarly, the survival rate in HAT mice increased from 23% to 80% in the tail-clip challenge experiment (Figure 6B). Twenty-five of 31 HAT mice (5 and 12 months of transplantation) that received Lin− BMCs achieved hemostasis at 4 hours or less and survived to the end of the study. The mice that did not stop bleeding died within 10 to 20 hours of the tail clip. It was found that the majority of HA mice did not survive more than 20 hours after the tail clip. The results of these functional assays suggest that BMCs not only produced FVIII protein in liver of HA mice, but also that the protein was secreted into the circulation.
Functional recovery and gene expression. (A) Whole blood clotting assay. Blood was withdrawn from the eye in a capillary tube. The clotting time was determined by visual observation of the clot formation. The results showed a significant drop in the blood clotting time in HAT mice after 5 months of transplantation compared with the HA mice. The number of mice tested (n) in each condition is mentioned in the figure. *P < .01. (B) Tail-clip challenge. The numbers of surviving and dead mice are shown after tail clip. In HAT mice, 80% were protected from death resulting from blood loss. The numbers of mice tested in each group are as follows: WT (9 mice), HA (13 mice), HAT-2 months (13 mice), HAT-5 months (17 mice), and HAT-12 months (14 mice). (C) The rationale for designing primers to amplify a sequence of the FVIII A3 domain. It is difficult to reverse transcribe across the neo sequence resulting from the presence of high G + C content in HA mice. (D) The RT-PCR analysis for the synthesis of the target amplicon. HAT1-3 mice show the synthesis of a 637-bp amplicon, the same as in WT mice. The same gene product was absent in both the knockout mice (HA1 and HA2).
Functional recovery and gene expression. (A) Whole blood clotting assay. Blood was withdrawn from the eye in a capillary tube. The clotting time was determined by visual observation of the clot formation. The results showed a significant drop in the blood clotting time in HAT mice after 5 months of transplantation compared with the HA mice. The number of mice tested (n) in each condition is mentioned in the figure. *P < .01. (B) Tail-clip challenge. The numbers of surviving and dead mice are shown after tail clip. In HAT mice, 80% were protected from death resulting from blood loss. The numbers of mice tested in each group are as follows: WT (9 mice), HA (13 mice), HAT-2 months (13 mice), HAT-5 months (17 mice), and HAT-12 months (14 mice). (C) The rationale for designing primers to amplify a sequence of the FVIII A3 domain. It is difficult to reverse transcribe across the neo sequence resulting from the presence of high G + C content in HA mice. (D) The RT-PCR analysis for the synthesis of the target amplicon. HAT1-3 mice show the synthesis of a 637-bp amplicon, the same as in WT mice. The same gene product was absent in both the knockout mice (HA1 and HA2).
To confirm the phenotypic rescue, we analyzed the gene expression of the FVIII light chain by reverse-transcribed polymerase chain reaction (RT-PCR), as the intact light chain is missing in HA mice. We designed a primer pair to amplify a specific segment of the light chain A3 domain where the gene disruption was introduced in the mutant mouse (Figure 6C). PCR amplification of the WT cDNA yielded a 637-bp product. However, because of the presence of the neo cassette sequence (high G + C content), it is difficult to reverse transcribe and amplify the corresponding product in HA mice.29 Our results showed that the same gene fragment was amplified in WT mice, but not in mutant (HA1 and HA2) mice (Figure 6D). Intriguingly, the target gene fragment was amplified in all 3 HAT mice (Figure 6D).
Plasma FVIII activity and inhibitor level
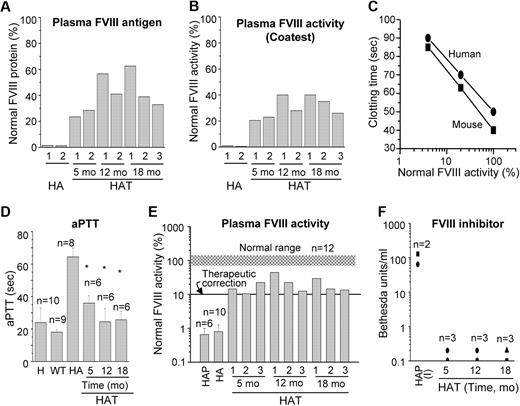
The results of the whole blood clotting and tail-clip challenge experiments in HAT mice suggest that the FVIII protein was present in the blood. We performed an ELISA to determine the relative FVIII antigen level in the plasma, which varied from mouse to mouse (Figure 7A). The average antigen levels during the study were 41.7% (± 5.4%; n = 7) of normal FVIII protein. Again, to evaluate the therapeutic effect of BMCs, the same plasma samples were analyzed by COATEST assay (Figure 7B). The average FVIII activities in plasma collected from 5 to 18 months after transplantation were 30.6% (± 3.0%; n = 7) of normal plasma. The phenotypic rescue in HAT mice was further examined by an aPTT assay. Because the aPTT assay reagent is customarily used for human samples, we initially tested both mouse and human pooled plasma and determined the correlation between these samples. It appears that the aPTT reagent responded similarly with mouse and human plasma FVIII (Figure 7C). There was a 3-fold increase in the aPTT time in HA mice compared with the WT mice (Figure 7D). The aPTT value was significantly (P < .01) decreased in HAT mice, supporting our tail-clip challenge results (Figures 6B, 7D). In WT mice, the plasma FVIII activity was 96% (± 15%; n = 12) compared with the normal pooled mouse plasma. The FVIII activity in HA mice was 0.7% (± 0.25%), which was increased to 15.7% (± 3.2%), 26.4% (± 9.4%), and 19.1% (± 5.1%) in HAT mice after 5, 12, and 18 months of transplantation, respectively (Figure 7E). A Tukey-Kramer multiple comparison analysis concluded that the plasma FVIII activity in HAT mice was significantly (HA to HAT5mo: P < .05; HA to HAT12mo: P < .001; HA to HAT18mo: P < .01) higher in all 3 test groups compared with the HA mice. These results indicated that the average FVIII activity from 5 to 18 months of study was stable at 20.4% (± 3.6%; n = 9; Figure 7E). Thus, the FVIII activity by COATEST assay was 1.5-fold higher than observed by aPTT assay. HA mice frequently develop inhibitors after FVIII protein or gene therapy. Therefore, the transplanted mice were tested to determine whether they developed inhibitors to FVIII. Nine of 9 HAT mice examined between 5 to 18 months of study confirmed that the BM cell therapy did not cause the formation of FVIII inhibitors (Figure 7F). Overall, these findings were in agreement with the maintenance of the secretory functions of BM-derived liver cells and the ability of these cells to supply active FVIII in HA mice.
Relative FVIII activity and inhibitor level in HAT mice. (A) The relative FVIII antigen expression in HAT mice. The FVIII protein level was determined by the ELISA method using a specific antibody. (B) The relative FVIII activity in HAT mice. The FVIII activity was determined by the COATEST assay according to the manufacturer's protocol. (C) The standard curve of normal pooled plasma FVIII activity versus clotting time in seconds. The results show that the response of the aPTT reagent to both mouse and human plasma are comparable. (D) The aPTT coagulation assay. The aPTT values of pooled human and WT, HA, and HAT mice are shown. The aPTT values of WT mice were 15.5 to 19.3 seconds, which was increased to 62.5 to 75.3 seconds in HA mice. The aPTT values were significantly lower (25.3-41.6 seconds) in HAT mice. *P < .01. (E) Relative FVIII activity. The relative percentage of FVIII activities in plasma samples from HAT mice and patients were determined. The normal range of mouse FVIII activity is indicated by the gray shaded region. (F) FVIII inhibitors in HAT mice and patients plasma. Mice and patient plasma samples, described in panel E, were tested for anti-FVIII activity by the Bethesda assay. The results show the absence of inhibitor in all the plasma samples of HAT mice. H indicates normal human; HA, hemophilic A mouse; HAP, hemophilic A patient; and HAP (I), hemophilic A patient-inhibitor.
Relative FVIII activity and inhibitor level in HAT mice. (A) The relative FVIII antigen expression in HAT mice. The FVIII protein level was determined by the ELISA method using a specific antibody. (B) The relative FVIII activity in HAT mice. The FVIII activity was determined by the COATEST assay according to the manufacturer's protocol. (C) The standard curve of normal pooled plasma FVIII activity versus clotting time in seconds. The results show that the response of the aPTT reagent to both mouse and human plasma are comparable. (D) The aPTT coagulation assay. The aPTT values of pooled human and WT, HA, and HAT mice are shown. The aPTT values of WT mice were 15.5 to 19.3 seconds, which was increased to 62.5 to 75.3 seconds in HA mice. The aPTT values were significantly lower (25.3-41.6 seconds) in HAT mice. *P < .01. (E) Relative FVIII activity. The relative percentage of FVIII activities in plasma samples from HAT mice and patients were determined. The normal range of mouse FVIII activity is indicated by the gray shaded region. (F) FVIII inhibitors in HAT mice and patients plasma. Mice and patient plasma samples, described in panel E, were tested for anti-FVIII activity by the Bethesda assay. The results show the absence of inhibitor in all the plasma samples of HAT mice. H indicates normal human; HA, hemophilic A mouse; HAP, hemophilic A patient; and HAP (I), hemophilic A patient-inhibitor.
Discussion
Hemophilia treatments are readily available in developed countries; however, it is estimated that approximately 70% of the people with this disease worldwide are undiagnosed or undertreated.31 Gene therapy appears promising in treating hemophilia as the disease is caused by a single-gene defect and a small increase in gene products could essentially transform a severe form of hemophilia into a mild one.32 Clinical studies show that approximately 20% of HA patients develop inhibitors to treatment33 and that these patients are difficult to treat.34 Cell-based therapies using isolated primary hepatocytes16,35 or LSECs17,18 are suggested to treat clotting disorders. In the past few years, a novel and exciting option to regenerate damaged liver from BM-derived cells has been proposed by many investigators.19-23,36-38 Although the contribution of BMCs to liver regeneration was found in the range from low to moderate, its significance has been widely recognized.
In this study, we investigated the role of uncommitted BMCs in correcting a bleeding disorder in HA mice. Various in vivo studies demonstrated that HSCs fail to differentiate into hepatocytes in the absence of damaged liver tissue.21,37 Acetaminophen at high doses causes centrilobular necrosis, and we therefore used it to induce acute liver injury. The toxicity of acetaminophen is the result of its highly reactive metabolic product N-acetyl-p-benzoquinone imine, which prevents Ca2+ homeostasis inside cells by oxidizing thiol groups on proteins.39 Our earlier in vitro and in vivo studies showed that a subpopulation of BMCs migrate to the damaged tissue and are involved in liver regeneration.22 The expression of SDF-1α and growth factor genes in the liver of acetaminophen-treated mice suggested the potential homing, engraftment, and differentiation of BMCs into liver cells. Because the extracellular matrix scaffold remains unaltered in necrotic tissue, the complete restoration of the liver structure was possible. IHC results confirm that donor cells contributed to the regeneration of the tissue in the centrilobular and other regions of the liver. We transplanted 2.5 × 105 Lin− BMCs into each mouse, which corresponds to approximately 1.5 × 105 Lin−OSMRβ+CXCR4+ cells for potential hepatic differentiation.22 The gradual increase in donor-derived hepatocytes from 3.6% to 10.8% of an average of 60 × 106 total liver cells during the study suggested that there were several rounds of division in engrafted cells. Hepatocyte growth factor and TGF-α, 2 known strong hepatic mitogens,40,41 synthesized by the damaged liver tissue, might have triggered cellular proliferation. In contrast, BM-derived LSECs were much lower in number.
The transplantation study showed that BMCs not only differentiated into hepatic and liver sinusoidal endothelial cells, but also that they expressed the intact gene of the FVIII A3 domain, which was disrupted in HA mice. The IHC results confirmed that HAT mice synthesized the light chain FVIII, which is missing in HA mice. Flow-cytometric and IHC results combined with the morphology of the cells expressing the FVIII light chain protein suggested that the missing factor was produced by hepatocytes. We presumed that FVIII was produced in the liver, as donor cells were not detected in other organs, such as the heart, lung, etc. The absence of donor cells in these organs, specifically in BM, was the result of nonirradiated mice being used in this investigation. Further, we did not find eGFP+ cells in the peripheral blood. Our results do not exclude the possibility of LSECs18 as a source of FVIII. The specific cell type(s) responsible for the expression of active FVIII has remained elusive for the past few decades, as its mRNA is expressed in the liver, spleen, and peripheral lymphocytes in humans and other species.8,42,43 FVIII production by human lung microvascular endothelial cells has been reported.44 Orthotopic liver transplantation studies in both animals and humans11-13 suggest that the primary site of FVIII synthesis is liver. Again within the liver, hepatocytes were found to be the major source of FVIII.10,14,15 It was shown that transplanting human hepatocytes under the mouse kidney capsule elicits therapeutic effect through the secretion of FVIII.16 Recently, a therapeutic effect of hepatocytes in hemophilia B mice has been documented.35 The transplantation of BMCs into HA mice restored plasma FVIII activity well above the therapeutic correction level, which could eliminate spontaneous bleeding. Moreover, we did not observe the formation of FVIII inhibitors during this 18-month investigation. Thus, this report unequivocally establishes that BMC therapy in HA mice (liver injury model) results in phenotypic rescue and reduces mortality resulting from blood loss. This approach of BMC therapy for treating HA appears to be more efficient than the available treatment methods.33 The possible reasons for this efficient cell therapy in HA mice are as follows: (1) FVIII produced by BM-derived cells is structurally and functionally identical to the native molecule, and (2) no inhibitor is expected to form as in the case of adeno-associated virus vector delivery system.7 The tolerogenic properties of the liver seem to have an important role in maintaining the synthesis of FVIII by donor cells for a longer duration. The liver has a unique microenvironment, rich in anti-inflammatory cytokines and other molecules, such as interleukin-10, TGF-β, prostaglandin E2, and granulocyte-macrophage colony-stimulating factor.45 This microenvironment is thought to promote the immature/tolerogenic phenotype in the resident dendritic cells to maintain immunologic silence to some food antigenic materials.45,46 A similar phenomenon may be responsible for immunologic silencing of the FVIII antigen. In many gene therapy projects, either hematopoietic stem cells47 or diploid skin fibroblasts cells48 were used to deliver the desired gene. These cells homed into their respective niches and did not experience a tolerogenic environment like that of the liver. This might be an explanation for the formation of inhibitors of FVIII or other foreign proteins.
BMCs are shown to have a tendency to fuse with primary hepatocytes in vivo to attain the hepatic phenotype.49,50 Cell fusion occurs during normal mammalian development.51,52 In adult mammals, cell fusion may be associated with chromosomal abnormalities that can lead to oncogenic transformations.53 Thus, it is crucial to investigate the fusion between donor and recipient cells, if any. In the liver damage model, we and other groups have already shown that BMCs can directly differentiate into hepatocytes.21,22 The results of FISH experiments suggested that, as in previous reports,21,22 cell fusion unlikely occurred in the present model of the phenotypic rescue of HA mice. In other words, these results illustrate the possibility of the direct differentiation of BMCs into functional liver cells. However, it is crucial to mention that the methodology followed in this study may not definitively characterize the processes of cell fusion and direct differentiation. We believe that the extent of the damage, the nature of the disease (acute vs chronic), and the agent used to cause the liver damage have important roles in determining whether BM-derived cells will fuse with host cells or will directly differentiate into hepatocytes. The acetaminophen used in this investigation for liver damage has been shown to have a potential oncogenic risk. However, a relevant study showed no DNA adduct formation in the rat liver during week-long treatments of acetaminophen, and thus concluded that it has no tumor-initiating activity.54
In conclusion, BMC therapy might control the bleeding disorder in HA mice. The application of BM-derived hepatocytes in managing HA has distinct advantages over alternative approaches: (1) human leukocyte antigen-matched BMCs can be easily obtained through a BM donor registry, (2) there is no need to identify the effective sources of graft as in the case of LSECs, and (3) a lower amount of FVIII inhibitor is formed, which will make this therapy successful for the sustained correction of hemophilia A.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Department of Biotechnology, Government of India (N.Y., A.M.).
Authorship
Contribution: N.Y. designed and conducted major experiments, conducted assays, and analyzed data; S.K. conducted some experiments and assays; S.K. conducted FVIII assay; M.J. conducted FISH; A.H. analyzed FISH results; R.S. analyzed FVIII assay results; and A.M. designed and planned experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Asok Mukhopadhyay, Stem Cell Biology Laboratory, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi, 110067, India; e-mail: ashok@nii.res.in.