Abstract
Abstract 1403
Poster Board I-425
Recombinant factor VIIa (rFVIIa) is a potent hemostatic agent licensed for treatment of bleeding in hemophiliac patients with inhibitors but has been increasingly used off-label to treat or prevent bleeding, despite sparse data to support its efficacy and a possible increase in thromboembolic events. While randomized controlled trials (RCTs) provide the best evidence regarding efficacy, they may not identify rare but important adverse events. Observational studies may capture the rate of such events in clinical practice more accurately.
To compare rates of thromboembolic events associated with off-label use of rFVIIa in RCTs and observational studies.
Studies of off-label application of rFVIIa were identified by review of 10 literature and clinical trial databases through February, 2009. The off-label indications examined were trauma, non-traumatic intracranial hemorrhage (ICH), and adult cardiac surgery. When an appropriate number and quality of studies were available, data from RCTs and higher quality comparative observational studies were combined via meta-analysis using the arc sine statistical method, a method that characterizes uncommon events more accurately than more conventional methods. To describe the absolute rate of thromboembolism for patients treated with rFVIIa in RCTs versus observational studies, we analyzed data from the interventional arms of RCTs and comparative observational studies, as well as data from non-comparative observational studies with 15 or more patients.
Included Studies
Our search identified 4 RCTs and 4 observational studies of rFVIIa use in ICH. Two RCTs and 5 observational studies were identified for trauma. For cardiac surgery, 2 RCTs, 2 higher quality comparative observational studies (included in the meta-analysis), and 8 additional observational studies were identified.
Meta-analyses
The meta-analysis of the RCTs investigating use of rFVIIa in ICH showed a trend toward increased thromboembolic risk with rFVIIa (arcsine summary effect size 0.100, 95% CI -0.072-0.272). For trauma, the two RCTs did not provide sufficient data to perform meta-analysis. Individually, they did not demonstrate significantly different incidences of thromboembolic events with rFVIIa compared to placebo but may have been underpowered to detect such differences. For cardiac surgery, the meta-analysis of the 4 studies showed a significant increase in thromboembolic events in the rFVIIa group (arcsine summary effect 0.14; 95% CI 0.038- 0.242).
Absolute rates of thromboembolic events in RCTs versus observational studies
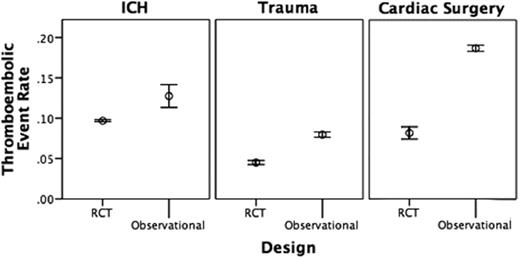
In ICH, thromboembolic event rates in the treatment arms of the RCTs ranged from 0.07-0.11, while those in the observational studies ranged from 0-0.20. In trauma, thromboembolic event rates in the RCT treatment arms ranged from 0.03-0.06, whereas those in the observational studies ranged from 0.02-0.11. In cardiac surgery, thromboembolic event rates in the RCTs ranged from 0.07-0.22 compared to a range of 0-0.25 in the observational studies. For all three indications, Figure 1 shows that the weighted mean thromboembolic event rates associated with rFVIIa use are higher in the observational studies than in the RCTs. Patients in the observational studies tended to be older and have a worse prognosis than those enrolled in the RCTs.
Weighted mean thromboembolic event rate (95% CI) by study design for each indication.
Weighted mean thromboembolic event rate (95% CI) by study design for each indication.
We identified a trend toward significantly higher rates of thromboembolic adverse events with off-label use of rFVIIa compared to placebo in our meta-analyses of ICH and cardiac surgery, but no similar pattern in trauma trials. For each of these indications, we identified a higher rate of thromboembolic adverse events associated with the use of rFVIIa in observational trials compared to RCTs. This finding suggests that patients receiving off-label rFVIIa for these off-label indications in real-world practice may be at higher risk of thromboembolic events than patients enrolled in clinical trials and may caution against widespread use, especially in the absence of convincing data on efficacy.
Off Label Use: This study examines the off-label use of rFVIIa in randomized controlled trials and observational studies of intracranial hemorrhage, trauma, and cardiac surgery.
Author notes
Asterisk with author names denotes non-ASH members.