Abstract
Abstract 1560
Poster Board I-583
Hodgkin lymphoma is defined by Reed Stemberg (RS) tumor cells in a microenvironment composed of a deregulated cytokine network and diverse cell populations, associated with their respective extracellular matrix, defining an inflammatory microenvironment. COX-2 (cyclooxygenase 2) is an inflammatory induced enzyme involved in the pathogenesis and prognosis of several solid tumors (colorectal, breast, ovarian, lung, etc). Its role in hematological malignancies is being recognized and specifically its expression in Hodgkin lymphoma has been associated with higher proliferation and angiogenesis.
To investigate the prognostic value of COX-2 expression in a large uniformly treated population with Hodgkin lymphoma.
We examined tissue microarrays for COX-2 expression in 242 patients of the Hodgkin Lymphoma Spanish Network treated with ABVD with or without Radiotherapy (XRT) according to AA (Ann Arbor) staging. Tissue sections were stained with monoclonal antibodies to COX-2 and the expression was scored and validated by a pathology review. Cox-2 was dichotomized as either negative (no RS staining) or positive. Plots of Kaplan Meier were constructed for survival analysis and most recognized clinical variables (age, sex, presence of bulky disease, B symptoms, AA stage, International prognostic system for Hodgkin lymphoma (IPS), ECOG performance status (PS) were investigated for their prognostic importance along with COX-2 expression by univariate and multivariate analysis.
Median age was 33 (10 to 83). COX-2 was expressed in 89 pts (37%), 99 pts (41%) presented with AA stage III-IV, 98 pts presented B symptoms (40%) and 124 pts (51%) received XRT added to ABVD chemotherapy, 66% of patients with AA stage I-II and 30% of pts presenting with AA stage III-IV.
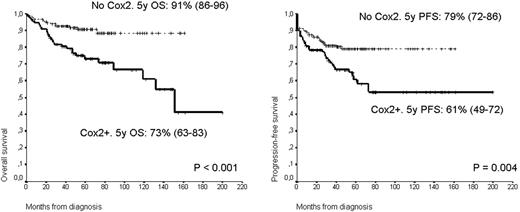
There were no differences in the distribution of clinical variables according to the COX-2 expression, except for a trend to increased positivity in nodular sclerosis and lymphocyte-depleted subtypes and higher AA stage. With a median follow up for alive patients of 59 months, the PFS at 5 years were 61% and 79% for COX-2 + and negative respectively (p=0.004). OS were 73% and 91% respectively (p<0.001) (Figure). By univariate analysis age higher than 45 years, presence of B symptoms, AA stage III-IV, PS >=2, IPS>2, no XRT treatment and COX-2 expression were associated with an unfavourable PFS and OS. By multivariate analysis only the IPS (HR 3.72) and COX-2 (HR 1.72) expression were independently associated with the outcome. Interestingly the major impact in the prognosis was observed in the favourable AA stage (I-II) and IPS (0-2) groups. In fact in these low risk groups the expression of COX-2 define a group of significant worse prognosis. Accordingly, the 46 patients (32%) of the 143 patients presenting an AA stage of I-II had a PFS at 5 years of 73% versus 86% of those COX-2 negative (p=0.031). Similarly the OS at 5 years of both groups also differ with 82 and 94% for COX + and negative respectively (p=0.004).
COX-2 is expressed in approximately a third of RS cells and is a major independent unfavourable prognostic factor. Of main relevance is the fact that in the favourable groups of the AA and IPS the expression of COX-2 identify a group of significant worse prognosis that need different approach. According to our data COX-2 can be a major prognostic variable in this disease and potentially could be a therapeutic target.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.